Introduction
Prostate cancer is the most common form of cancer
and the second leading cause of cancer-related mortality among
males in the United States (1). In
2010, there was an incidence of ~217,000 new prostate cancer cases
and 32,000 mortalities due to prostate cancer in the United States
(1). Advanced-stage prostate cancer
is characterized by tropism to bone, with skeletal involvement
present in ~90% of patients with metastatic disease (2,3).
Metastatic disease to bone is commonly associated with
skeletal-related events, which are associated with decreased
quality of life, increased pain and worsened survival (4). A bone scan is the standard method for
detecting bone metastases from prostate cancer. In certain cases,
additional tests, including computed tomography scan, magnetic
resonance imaging (MRI) scan or bone biopsy, may be needed to
diagnose bone metastases. Local external beam radiotherapy,
systemic radioisotope therapy, endocrine therapy, chemotherapy, and
bisphosphonates and denosumab are the mainstays of treatment, as
well as painkillers and other usual classical interventions
(5).
Polycythemia vera (PV), a type of myeloproliferative
neoplasm, is a clonal disorder characterized by unwarranted
production of red blood cells, and associated with JAK2 mutations
(V617F or exon 12) in almost all cases (6). It is more common in the elderly and
may be symptomatic or asymptomatic. Typical signs and symptoms
include itching (pruritus) and severe burning pain in the hands or
feet that is frequently accompanied by a red/blue coloration of the
skin. Other disease features include leukocytosis, splenomegaly,
thrombohemorrhagic complications, vasomotor disturbances and a
small risk of disease progression into acute myeloid leukemia (AML)
or myelofibrosis (MF) (7).
Diagnosis of PV is currently according to World Health Organization
criteria and based on a composite assessment of clinical and
laboratory features, including two major criteria (increased
hemoglobin levels and presence of JAK2 mutation) and three minor
criteria (trilineage myeloproliferation in bone marrow biopsy,
increased serum erythropoietin levels and endogenous erythroid
colony formation in vitro) (8). The aims of therapy are to prevent the
occurrence and recurrence of thrombosis; to delay (if feasible)
evolution into MF or AML; and to control disease-associated
symptoms (9). The treatment
strategy in PV patients comprises a combination of modification of
cardiovascular risk factors, antiplatelet therapy, phlebotomy and
cytoreduction (9). Both of the
diseases can involve bone or bone marrow, but each disease has
unique changes in bone structure. As treatment options are
fundamentally different between metastatic prostate cancer and PV
and it is therefore important to distinguish the differences using
different techniques, such as CT, MRI and bone scans. We report a
case of prostate cancer and PV and our approach to differential
diagnosis for these diseases using radiological imaging techniques.
The patient provided written informed consent.
Case report
A 60-year-old male was admitted to The First
Hospital, Jilin University (Changchun, China) in October 2012
complaining of redness on the hands and face that had been apparent
for three months. The patient exhibited no feelings of discomfort,
and reported that the skin problems appeared to be insidious. A
review of the medical history revealed that the patient had
experienced a lacunar infarct eight years previously, and had
suffered with hypertension for >30 years, for which
antihypertensive drugs had been taken for ~20 years. The patient
did not smoke or regularly consume alcohol. Immediate family
members exhibited no history of any neurological or hematological
diseases.
A physical examination revealed that the patient was
afebrile, with an Eastern Cooperative Oncology Group score of 0
(10), a normal heart rate and a
blood pressure of 145/90 mmHg. The face, neck and hands appeared
red, but no pain was reported. There was no evidence of edema or
blistering of the skin. Lymphadenopathy, hepatosplenomegaly or an
abnormal physical mass in the abdomen were not apparent. Digital
rectal examination did not reveal any masses or enlargement of the
prostate.
Hematological analysis revealed slightly elevated
readings for hemoglobin (Hb; 20.4 g/dl; normal range, 12.0–16.0
g/dl) and the hematocrit (Hct; 0.610 l/l; normal range, 0.400–0.500
l/l), and also evidence of leukocytosis [white blood cell (WBC)
count, 12.89×109/l; normal range,
4.0–10.0×109/l]. The WBC count consisted of 80%
neutrophils (normal range, 50–70%), but normal levels of platelets
(253×109/l; normal range, 100–300×109/l). The
concentration of alkaline phosphatase (ALP) was slightly high
(115.0 U/l; normal range, 15.0–112.0 U/l), but liver function tests
appeared normal. The serum total prostate-specific antigen (TPSA)
level was 10.94 ng/ml (normal range, 0–4 ng/ml), and the serum free
PSA (FPSA) level was 0.98 ng/ml. As a result, the percentage of
FPSA in TPSA was 9%, which was less than the normal value of
19%.
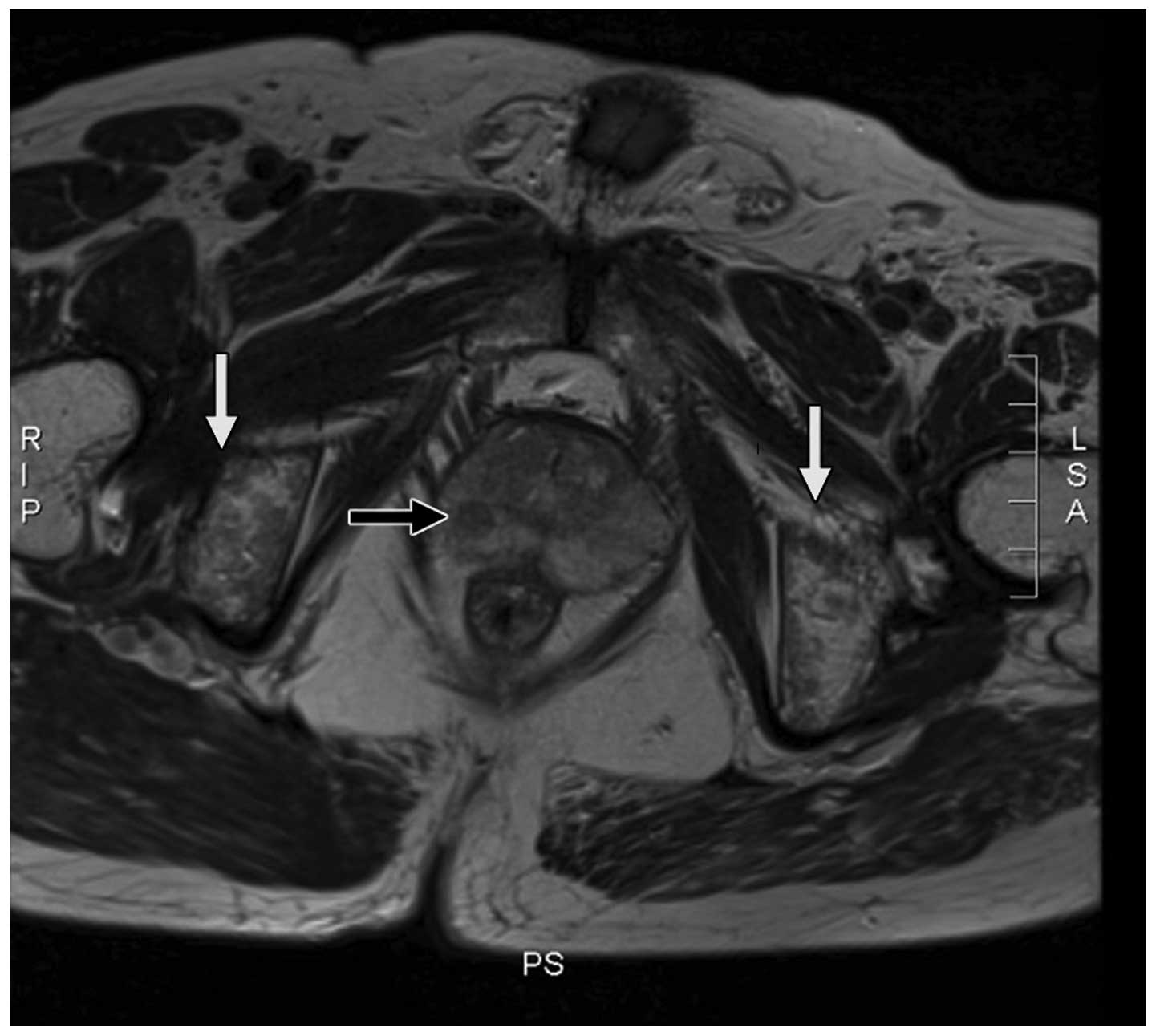
MRI of the prostate (Fig. 1) revealed a small nodule measuring
5×5 mm, which exhibited abnormal intensity signals in the right
prostatic peripheral zone. The capsule of the prostate gland was
continuous and complete, and no evidently enlarged surrounding
lymph nodes were observed. The signal intensity of the pelvic bone
marrow was diffusely inhomogeneous, but the structure of the
cortical region was intact. A bone scan indicated slightly elevated
radioactivity within the left ankle and right knee, but without any
other abnormalities. A computed tomography scan of the lung
appeared normal. A transrectal ultrasound-guided biopsy of the
prostate initially revealed a well-differentiated adenocarcinoma
measuring <0.5×0.1 cm, with a Gleason score of 3+3 (11). The bone marrow aspiration and biopsy
revealed erythrocytosis and leukocytosis consisting of 12%
eosinophils (normal range, 0–8%). The genetic chromosome analysis
indicated the presence of a JAK2-V617F mutation, but no other
abnormalities.
A diagnosis of stage IIA prostate adenocarcinoma
(TNM stage, cT1aN0M0) and PV was established (12). The patient was subjected to
phlebotomy procedures to remove 400 ml of blood twice weekly for
two weeks, and was treated with 100 mg aspirin daily. Prior to
surgery, the blood Hb level dropped to 14.1 g/dl, and the Hct to
0.47 l/l. In November 2012, a radical prostatectomy was performed
by laparoscopy. Pathological examination of the surgical specimen
revealed an adenocarcinoma with negative margins, but with no
evidence of seminal vesicle invasion or extra-capsular extension.
The tumor volume occupied ~3% of the total prostate volume. The
patient was discharged seven days after the surgery. Following
surgery, the Hb level and Hct values gradually increased. The
patient was therefore continually treated with 300×104
units of interferon-α2b (Beijing Kawin Bio-Tech Co., Ltd., Beijing,
China) twice-weekly, and 100 mg aspirin daily.
The patient was continually followed up, and the
serum TPSA levels were monitored every three months. Abnormal
levels of serum TPSA were not reported during the one-year
follow-up period. At six months post-surgery, MRI analysis of the
prostate indicated no evident abnormalities. However, there was no
marked change in the atypical signal intensity of the pelvic bone
marrow that was detected during the initial MRI scan. At present,
the blood Hb, Hct, platelet and WBC counts remain within the normal
ranges.
Discussion
Prostate cancer is the most frequently diagnosed
non-cutaneous type of cancer, and the second most common cause of
cancer-related mortality affecting males in the United States. In
2010, there were an estimated 217,730 cases of newly-diagnosed
prostate cancer, and at present, the disease has an estimated
lifetime disease incidence in males of 20% (1). The majority of patients with advanced
prostate cancer also present with bone metastases (BMs) (13–15).
Patients with metastatic prostatic cancer are treated with
different therapeutic strategies to those without. Therefore, it is
critical to rule out the presence of metastases, such as BMs, prior
to the initiation of treatment. Patients with localized prostate
cancer, which is most often curable, usually benefit from radical
treatment. By contrast, patients with prostate cancer and BMs are
not recommended for surgery, and are usually treated with systemic
chemotherapies. Several risk factors, including high levels of
serum PSA (16–20), the presence of locally advanced
tumors (16,20,21),
high levels of serum ALP (16,22,23)
and high Gleason scores (20), are
believed to be associated with BMs.
In the present study, the results of the MRI scan
made the diagnosis challenging. The diffusely inhomogeneous signals
in the bone marrow of the pelvis, together with a small mass in the
prostatic tissue, should indicate the presence of BMs. The patient
exhibited histological evidence of adenocarcinoma with a Gleason
score of 6, a serum PSA level of 10.94 ng/ml, a slightly high level
of serum ALP and a small mass in the prostatic tissue, with a
continuous and complete capsule and no evidence of lymph node
enlargement. Therefore, a diagnosis of localized, early-stage
prostate cancer, rather than a local BM, was proposed. Previous
studies have identified that prostate cancer-related BMs usually
invade the cortical bone, and rarely affect the bone marrow
(24,25). Furthermore, BMs are
characteristically accompanied by cytopenia, which ruled out the
likelihood of BMs in the present case. Due to the importance of
identifying the presence of BMs for correct diagnosis, a bone
biopsy was performed in the present study, which identified no
prostate cancer-related BMs. In addition, the bone scan did not
reveal any evidence of BMs. Previous studies have demonstrated the
presence of diffusely inhomogeneous signals in the bone marrow of
patients with PV (26,27). In the present study, the results of
the laboratory tests revealed an abnormal WBC count, percentage of
neutrophils, and blood Hb and Hct, and the presence of a JAK-2V617F
mutation. Therefore, the patient was primarily diagnosed with PV
and treated with aspirin, which significantly reduced the levels of
blood Hb and Hct prior to the radical prostatectomy.
According to the 2013 NCCN Guidelines for Prostate
Cancer, the tumor recurrence risk is intermediate, and there are no
adverse features (5). Therefore,
the patient was not recommended for further aggressive
post-surgical therapies. Despite no significant changes identified
in the signal intensity of the pelvic bone marrow during the
one-year follow-up period, abnormal levels of serum PSA were not
detected, which indicated no recurrence of the prostate cancer.
Upon MRI analysis, prostate cancer-related BMs are often difficult
to distinguish from prostate cancer cases occurring with PV.
Therefore, it is important for physicians to consider these rare
diseases in clinical practice. The use of bone biopsies and
systemic bone scans should allow for discrimination between the
two. In addition, careful analysis of MRI, with close attention to
the bone structure, together with a hematological examination,
should aid in the diagnosis of PV. Empiric therapies, which in the
present study included the phlebotomy procedure and aspirin
treatment, are valuable as they have the potential to improve
clinical symptoms, restore the hematological status and improve the
condition of the body for subsequent surgical treatments. The
surgical removal of prostate tumors should improve the clinical
symptoms of patients with prostate cancer-related PV, however, in
the present study, increased values of Hb and Hct were observed
post-surgery, which indicated an incomplete response to phlebotomy
and aspirin treatment. The patient was subsequently treated with
interferon-α2b, which effectively controlled the symptoms of PV.
Therefore, if available, interferon-α2b should be administered to
patients with PV. However, the precise mechanism underlying the
therapeutic action of interferon-α2b requires further
investigation.
The present study described a case of prostate
cancer occurring with PV. The simultaneous presence of these
diseases should be recognized, and careful analysis of diagnostic
imaging, such as MRI, should be performed. Furthermore, risk
factors for BMs should be considered following the detection of
abnormal intensity signals in the pelvis of patients with prostate
cancer. To the best of our knowledge, this was the first case study
to describe the systemic medical history and treatment of a patient
with prostate cancer occurring with PV. The findings of the present
study may aid in the future diagnosis and treatment of patients
with prostate cancer and PV.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scher HI, Morris MJ, Kelly WK, Schwartz LH
and Heller G: Prostate cancer clinical trial end points:
‘RECIST’ing a step backwards. Clin Cancer Res. 11:5223–5232. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacobs SC: Spread of prostatic cancer to
bone. Urology. 21:337–344. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DePuy V, Anstrom KJ, Castel LD, Schulman
KA, Weinfurt KP and Saad F: Effects of skeletal morbidities on
longitudinal patient-reported outcomes and survival in patients
with metastatic prostate cancer. Support Care Cancer. 15:869–876.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National comprehensive cancer network.
NCCN Clinical Practice Guidelines in Oncology: Prostate cancer.
http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Accessed May 23, 2013
|
|
6
|
Tefferi A: Polycythemia vera and essential
thrombocythemia: 2013 update on diagnosis, risk-stratification, and
management. Am J Hematol. 88:507–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Passamonti F: How I treat polycythemia
vera. Blood. 120:275–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spivak JL and Silver RT: The revised World
Health Organization diagnostic criteria for polycythemia vera,
essential thrombocytosis, and primary myelofibrosis: an alternative
proposal. Blood. 112:231–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbui T, Barosi G, Birgegard G, et al:
Philadelphia-negative classical myeloproliferative neoplasms:
critical concepts and management recommendations from European
LeukemiaNet. J Clin Oncol. 29:761–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gleason DF and Mellinger GT: Prediction of
prognosis for prostatic adenocarcinoma by combined histological
grading and clinical staging. J Urol. 111:58–64. 1974.PubMed/NCBI
|
|
12
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: Prostate. AJCC Cancer Staging Manual. 7th
edition. Springer; New York: pp. 457–468. 2010
|
|
13
|
Tombal B and Lecouvet F: Modern detection
of prostate cancer’s bone metastasis: Is the bone scan era over?
Adv Urol. 2012:8931932012. View Article : Google Scholar
|
|
14
|
Nørgaard M, Jensen AØ, Jacobsen JB, Cetin
K, Fryzek JP and Sørensen HT: Skeletal related events, bone
metastasis and survival of prostate cancer: a population based
cohort study in Denmark (1999 to 2007). J Urol. 184:162–167. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carlin BI and Andriole GL: The natural
history, skeletal complications, and management of bone metastases
in patients with prostate carcinoma. Cancer. 88(Suppl 12):
2989–2994. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Briganti A, Passoni N, Ferrari M, et al:
When to perform bone scan in patients with newly diagnosed prostate
cancer: external validation of the currently available guidelines
and proposal of a novel risk stratification tool. Eur Urol.
57:551–558. 2010. View Article : Google Scholar
|
|
17
|
Lai MH, Luk WH and Chan JC: Predicting
bone scan findings using sPSA in patients newly diagnosed of
prostate cancer: feasibility in Asian population. Urol Oncol.
29:275–279. 2011. View Article : Google Scholar
|
|
18
|
Ho CC, Seong PK, Zainuddin ZM, Abdul Manaf
MR, Parameswaran M and Razack AH: Retrospective study of predictors
of bone metastasis in prostate cancer cases. Asian Pac J Cancer
Prev. 14:3289–3292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thompson I, Thrasher JB, Aus G, et al: AUA
Prostate Cancer Clinical Guideline Update Panel: Guideline for the
management of clinically localized prostate cancer: 2007 update. J
Urol. 177:2106–2131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heidenreich A, Aus G, Bolla M, et al:
European Association of Urology: EAU guidelines on prostate cancer.
Eur Urol. 53:68–80. 2008. View Article : Google Scholar
|
|
21
|
Koutsopoulos AV, Dambaki KI, Datseris G,
Giannikaki E, Froudarakis M and Stathopoulos E: A novel combination
of multiple primary carcinomas: urinary bladder transitional cell
carcinoma, prostate adenocarcinoma and small cell lung carcinoma -
report of a case and review of the literature. World J Surg Oncol.
3:512005. View Article : Google Scholar
|
|
22
|
Têtu B, Ro JY, Ayala AG, Johnson DE,
Logothetis CJ and Ordonez NG: Small cell carcinoma of the prostate.
Part I A clinicopathologic study of 20 cases. Cancer. 59:1803–1809.
1987. View Article : Google Scholar
|
|
23
|
Rubenstein JH, Katin MJ, Mangano MM,
Dauphin J, Salenius SA, Dosoretz DE and Blitzer PH: Small cell
anaplastic carcinoma of the prostate: seven new cases, review of
the literature, and discussion of a therapeutic strategy. Am J Clin
Oncol. 20:376–380. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seo Y, Shuke N, Yamamoto W, Usui K and
Aburano T: Sub-super bone scan caused by bone marrow involvement of
prostate cancer. Ann Nucl Med. 13:351–354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tachev S, Ormanov I and Slavov CH: Cancer
of the prostate with metastasis to the bone marrow. Khirurgiia
(Sofiia). 39:96–97. 1986.(In Bulgarian).
|
|
26
|
Steiner RM, Mitchell DG, Rao VM, et al:
Magnetic resonance imaging of bone marrow: diagnostic value in
diffuse hematologic disorders. Magn Reson Q. 6:17–34.
1990.PubMed/NCBI
|
|
27
|
Birchall JD and O’Connor RA: Prostatic
metastases versus polycythemia on bone imaging. Clin Nucl Med.
29:375–377. 2004. View Article : Google Scholar : PubMed/NCBI
|