Introduction
Phenolic compounds, a large family of natural
compounds with phenolic hydroxyl groups, are present in plants,
fruits, vegetables and teas, and have been demonstrated to exhibit
anticancer properties. The potential application of these phenolic
compounds in the development of therapeutic agents for cancer
treatment has gained increasing importance in the previous decade
and research in this field is currently expanding (1–3). For
example, Link et al (4)
reported that cancer chemoprevention using dietary phenolic
compounds may be important in the control of gene expression, for
example for inducing changes in DNA methylation, histone
modifications and non-coding RNAs. Furthermore, Mahbub et al
(5) proposed that phenolic
compounds may be potential therapeutic agents for the treatment of
leukemia by causing decreased cell viability and inducing cell
apoptosis. Additionally, previous studies have indicated that
phenolic compounds are possibly associated with a reduced risk of
liver (6), lung (7), cervical (8) and colorectal cancer (9).
Ovarian cancer is the second most lethal gynecologic
cancer amongst the female population in developed areas (10), with an estimated 22,240 new cases
and 14,030 mortalities due to ovarian cancer in 2013 (11). While the five-year survival rate of
ovarian cancer patients has improved following the development of
more effective treatment strategies with optimized therapeutic
agent treatment and more advanced surgical techniques, the overall
cure rate has remained ~30% over the previous two decades (12). An additional challenge for improving
the five-year survival rate of ovarian cancer is that only 20% of
ovarian cancers are diagnosed prior to the occurrence of metastasis
(12), as the symptoms do not
typically present until after the cancer has metastasized from the
ovaries to the surface of the peritoneal cavity. Once metastasis
has occurred, treatment of ovarian cancer is difficult as the
surgical removal of all of the lesions is no longer possible and
chemotherapy is the only remaining first-line treatment
strategy.
Although many female individuals may respond well to
initial first-line treatment, relapse frequently occurs with
chemotherapy-resistant disease, presenting a major obstacle in the
attempt to improve the prognosis of ovarian cancer patients
(13). Therefore, selecting novel
chemicals from natural compounds as cancer therapeutic agents
remains important for ovarian cancer research. In particular, the
discovery of novel natural compounds that meet or exceed the
effects of commonly used chemical agents or agents that may be
administered in combination with cisplatin to overcome the
resistance, are of great significance (14). Currently, platinum agents, such as
cisplatin, are one of the most active anticancer agents used in
clinical practice (13).
Traditional plant-based medicines are widely used in
developing countries for their primary healthcare requirement
(15). The European Prospective
Investigation cohort study into cancer and nutrition demonstrated
that consuming >6.2 g per day of nuts and seeds appear to reduce
a female individual’s risk of developing colorectal cancer
(16). In analyses of pecan extract
from various cultivars, (+)-catechin hydrate, ellagic acid,
(−)-epicatechin and gallic acid (GA) were identified to be the main
phenolic compounds (17,18). Furthermore, nobiletin, tangeretin,
baicalein and baicalin were the predominant flavonoids identified
in the common traditional Chinese medicines dried orange peel
(citrus) and Scutellaria, respectively (19,20).
These eight non-toxic dietary phenolic compounds have previously
demonstrated anticancer and chemopreventive properties in specific
types of cancer; however, little research has been conducted into
the chemopreventive effects of these eight phenolic compounds
against ovarian cancer.
In the present study, the effect of eight dietary
phenolic compounds on the cell proliferation and vascular
endothelial growth factor (VEGF) protein expression levels in human
ovarian cancer cells was investigated. The phenolic compounds
investigated were (+)-catechin hydrate, ellagic acid,
(−)-epicatechin, gallic acid, nobiletin, tangeretin, baicalein and
baicalin, the names and structures of which are indicated in
Fig. 1. Cisplatin, a commonly used
chemotherapeutic agent, was used as the positive control.
Materials and methods
Materials and cell culture
The eight phenolic compounds and cisplatin were
obtained from Sigma-Aldrich (St. Louis, MO, USA). The compounds
were dissolved in dimethyl sulfoxide (DMSO) to produce stock
solutions of 100 mM, and these stock solutions were subsequently
diluted to 20 or 40 μM with cell culture medium prior to use. An
equal amount of DMSO was included in the control solutions for
every experiment. The two ovarian cancer cell lines OVCAR-3 and
A2780/CP70 were provided by Dr Bing Hua Jiang at West Virginia
University (Morgantown, WV, USA) and were maintained in RPMI-1640
medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum
(Invitrogen Life Technologies, Grand Island, NY, USA) in a cell
culture incubator at 5% CO2 and a temperature of
37°C.
Cell viability assay
To determine cell viability, the two cell lines were
seeded in 96-well plates at a density of 1×104 cells per
well, and allowed to attach to the substrate and grown to log phase
overnight. Following incubation at 37°C, the culture medium was
removed and incubated with each of the nine compounds at a
concentration of 20 or 40 μM in RPMI-1640 medium for 24 h. Each
experiment was performed in triplicate. Following treatment, the
cells were washed twice with phosphate-buffered saline (PBS;
Invitrogen Life Technologies) and introduced with 100 μl freshly
prepared CellTiter 96® AQueous One solution (containing
MTS, a tetrazolium compound; Promega Corporation, Madison, WI, USA)
and 80 μl PBS. The cells were incubated for 1 h and the optical
density values were measured at an absorbance of 490 nm using an
ELISA plate reader (BioTek Instruments, Inc., Winooske, VT, USA).
Cell viability was expressed as a percentage of the control.
VEGF protein quantification
The effects of the nine compounds on VEGF protein
secretion were analyzed by performing an ELISA with a Quantikine
Human VEGF immunoassay kit (R&D Systems, Inc., Minneapolis, MN,
USA), targeting VEGF165 in the cell culture supernatant. Cells
(6×105) from the two cell lines were seeded in 60-mm
cell culture dishes and allowed to attach to the substrate and grow
for 16 h prior to treatment with 40 μM of each compound or without,
which served as a control, for an additional 24 h. The culture
supernatants were collected for the VEGF assay and the inhibition
of VEGF protein secretion was expressed as a percentage of the
control.
Western blot analysis
OVCAR-3 cancer cells were seeded and incubated
overnight prior to treatment with 0, 5, 10 and 20 μM GA. The cells
were double-washed with cold PBS, harvested with M-PER®
Mammalian Protein Extraction Reagent supplemented with Halt™
protease and phosphatase inhibitor cocktail, and the total protein
expression levels were assayed using a bicinchoninic acid protein
assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The cell
lysates (50 μg total protein) were separated by performing SDS-PAGE
and blotted onto a nitrocellulose membrane using the Mini-PROTEAN 3
system (Bio-Rad Laboratories, Hercules, CA, USA). For
immunodetection, mouse anti-human monoclonal HIF-1α (cat. no.
610959; dilution 1:500; BD Biosciences, Franklin Lakes, NJ, USA)
and mouse anti-human monoclonal GAPDH antibodies (cat. no.
sc-47724; dilution 1:500; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) were applied and the signals were visualized by
phycoerythrin-conjugated goat anti-mouse IgG polyclonal secondary
antibody (cat. no. 32230; dilution 1:2500; Pierce Biotechnology,
Inc.) and Supersignal West Pico Chemiluminescent substrate
application, followed by the utlilization of X-ray films (Pierce
Biotechnology, Inc.). The protein bands were quantified using
National Institutes of Health ImageJ software version 1.46
(Bethesda, MD, USA) and normalized by GAPDH bands for subsequent
analysis.
Statistical analysis
One-way and two-way analysis of variance (ANOVA)
followed by Student-Newman-Keuls tests were applied to compare the
effects of the nine compounds on cell viability and VEGF protein
expression levels. All statistical analyses were performed using
SAS software (SAS Institute, Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of the effects of various
natural compounds on cell viability in OVCAR-3 ovarian cancer
cells
The 24-h treatment of OVCAR-3 cells with 20 and 40
μM of the nine different compounds resulted in various effects on
OVCAR-3 cancer cell proliferation. As indicated in Fig. 2, (−)-epicatechin, (+)-catechin
hydrate and ellagic acid caused no significant inhibition of
OVCAR-3 cell proliferation; at 20 μM the cell viability ranged from
118.0±1.2 to 121.7±1.6% and at 40 μM from 119.7±2.8 to 127.0±3.8%.
By contrast, GA was identified to exhibit the lowest cell viability
value among all of the phenolic compounds, demonstrating an
inhibitory effect similar to that of cisplatin at 40 μM. These
results indicate that GA may have chemotherapeutic potential
against ovarian cancer. Baicalin, baicalein, nobiletin and
tangeretin exhibited a moderate inhibitory effect on cell
proliferation between the levels of 72.2 and 88.8% at 20 μM and
between 23.8 and 76.9% at 40 μM concentrations. According to the
mean values of the two-way ANOVA results from the 20- and 40-μM
treatments (Table I), the rank
order of cell viability inhibition in OVCAR-3 cancer cells was as
follows: Cisplatin > GA, baicalein > baicalin, nobiletin,
tangeretin > ellagic acid, (+)-catechin hydrate and
(−)-epicatechin (P<0.05).
 | Table IEffect of different compounds on
OVCAR-3 cell viability. |
Table I
Effect of different compounds on
OVCAR-3 cell viability.
| Cell viability,
% |
|---|
|
|
|---|
| Compound name | 20 μM OVCAR-3 | 40 μM OVCAR-3 | Mean |
|---|
| (−)-epicatechin |
118.00±1.15a |
127.01±3.79a |
122.50a |
| (+)-catechin
hydrate |
119.67±1.33a |
123.33±1.20a |
121.50a |
| Ellagic acid |
121.67±1.45a |
119.67±2.85a |
120.67a |
| Tangeretin |
88.80±1.54b |
76.90±2.29b |
82.85b |
| Nobiletin |
79.93±4.75b,c |
71.47±5.81b |
75.70b |
| Baicalin |
88.13±2.56b |
53.37±5.98c |
70.75b |
| Baicalein |
72.20±5.34c,d |
23.80±4.40d |
48.00c |
| Gallic acid |
64.53±6.72d |
2.43±0.34e |
33.48c |
| Cisplatin |
19.77±1.52e |
6.02±0.37e |
12.89d |
Comparison of the effects of natural
compounds on cell viability in A2780/CP70 ovarian cancer cells
The results of the cell viability assay indicated
that the nine different compounds at the two stated concentrations
exhibited varied effects on viability of ovarian cancer A2780/CP70
cells (Fig. 3). All of the phenolic
compounds demonstrated inhibitory effects on the A2780/CP70 cells,
with a cell viability range of 15.30±3.01 to 88.83±3.55% at 40 μM.
This inhibitory mechanism of phenolic compounds may be due to their
antioxidant activity, which would affect the cellular redox state
of the cancer cells (21). All of
the cell viability values of the investigated phenolic compounds
were smaller than the values of cisplatin at 20 and 40 μM
(P<0.05). Compared with the other phenolic compounds, GA and
baicalein exhibited the greatest inhibitory effects at 40 μM
(P<0.05); however, ellagic acid, baicalin, (−)-epicatechin and
(+)-catechin hydrate were identified to have a smaller inhibitory
effect on cell viability at 20 and 40 μM. The mean values of the
two-way ANOVA analysis results from the 20- and 40-μM treatments
indicated that the rank order of A2780/CP70 cell viability
inhibition was as follows: Cisplatin > baicalein > GA ≥
nobiletin, tangeretin ≥ ellagic acid, baicalin, (−)-epicatechin ≥
(+)-catechin hydrate (P<0.05) (Table II).
 | Table IIEffect of different compounds on
A2780/CP70 cell viability. |
Table II
Effect of different compounds on
A2780/CP70 cell viability.
| Cell viability,
% |
|---|
|
|
|---|
| Compound name | 20 μM
A2780/CP70 | 40 μM
A2780/CP70 | Mean |
|---|
| (+)-catechin
hydrate |
103.0±1.53a |
88.83±3.55a |
95.92a |
|
(−)-epicatechin |
102.8±3.03a |
73.37±5.76b |
88.1a,b |
| Baicalin |
94.87±1.25a |
70.43±5.31b |
82.65a,b |
| Ellagic acid |
96.90±2.11a |
64.43±2.38b |
80.67a,b |
| Tangeretin |
86.33±3.46a,b |
62.07±8.38b |
74.2b,c |
| Nobiletin |
80.27±2.48a,b |
63.08±4.10b |
71.68b,c |
| Gallic acid |
82.03±10.66a,b |
41.20±9.24c |
61.62c |
| Baicalein |
64.97±7.40b |
15.30±3.01d |
40.13d |
| Cisplatin |
25.34±10.76c |
1.92±0.54d |
13.63e |
Comparison of the effects of natural
compound on VEGF protein expression levels in the two ovarian
cancer cell lines
The suppressive effects of each compound were
measured by treating each cell line with 40 μM compounds for 24 h.
In the OVCAR-3 cell line, baicalein, GA, nobiletin, tangeretin and
baicalin all exhibited a moderate level of VEGF expression
inhibition, similar to that of cisplatin, ranging from 52.4±14.5 to
72.4±7.3% (Fig. 4). As with the
inhibitory effect on OVCAR-3 cancer cells (Fig. 5), GA, tangeretin, nobiletin,
baicalein and baicalin were all identified to exhibit a moderate
level of VEGF expression inhibition, ranging from 41.0±15.4 to
74.5±5.1%.
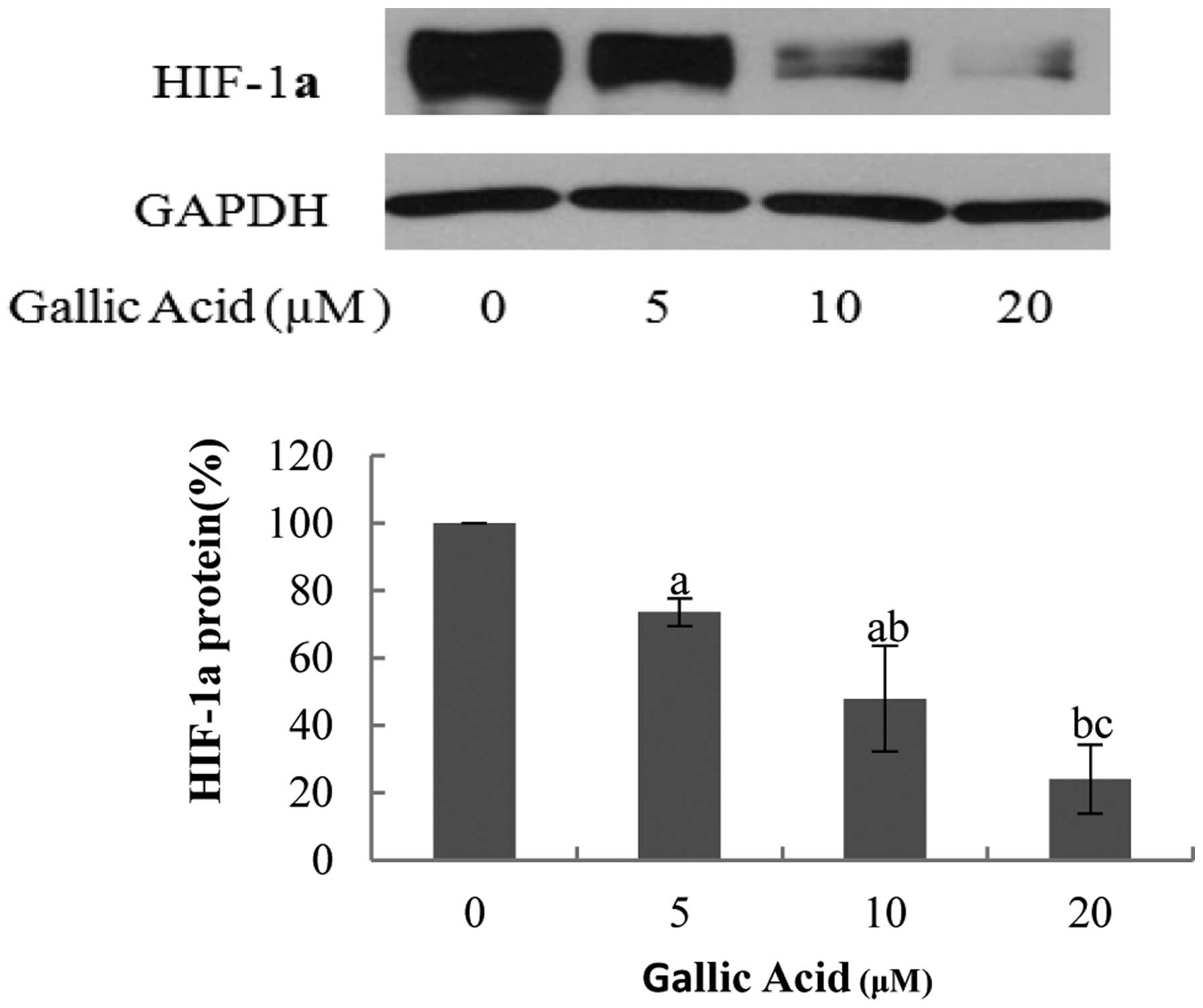
Effect of GA on HIF-1α protein expression
levels in OVCAR-3 cancer cells
GA, a type of phenolic acid, is present in a number
of plant sources, such as the gall nut, sumac, witch hazel and
various types of tea (22). Its
anticancer activity has previously been reported in human glioma
cells (22), as well as prostate
(23), lung (24), gastric, colon, breast, cervical and
esophageal cancer (25,26); however, the specific effect of GA on
human ovarian cancer cells has not previously been reported. The
present study demonstrated that GA markedly inhibits the
proliferation of OVCAR-3 cells and significantly suppresses VEGF
protein expression (P<0.05).
The transcription factor HIF-1α has previously been
reported to directly activate VEGF expression (27); therefore, the present study
investigated the effect of GA on HIF-1α expression. In the OVCAR-3
cancer cell line, the protein expression levels of HIF-1α were
significantly decreased (P<0.05) upon the administration of
higher concentrations of GA (Fig.
6). At 20 μM GA, the protein expression levels of HIF-1α were
24.07% of the control, indicating that GA may effectively decrease
the protein expression levels of HIF-1α in OVCAR-3 cells.
Discussion
The results of the present study demonstrated that
many of the compounds screened exhibit inhibitory effects on
OVCAR-3 and A2780/CP70 ovarian cancer cell lines. It was identified
that GA had the greatest inhibitory effect on OVCAR-3 cell
viability, compared with all of the phenolic compounds
investigated, with a similar viability to that of cisplatin at
concentrations of 40 μM. Furthermore, the bioavailability of GA is
high compared with the other polyphenols investigated (28), thus, GA is a potential agent for the
prevention and treatment of human ovarian cancer. To the best of
our knowledge, this is the first report to be conducted
investigating the inhibitory effects of GA on ovarian cancer
cells.
In the present study, all of the phenolic compounds
investigated demonstrated similar levels of proliferative
inhibition in the OVCAR-3 and A2780/CP70 cell lines, excluding GA.
The cell viability of A2780/CP70 cells treated with 40 μM GA was
markedly greater compared with the OVCAR-3 cells (P=0.014), which
may indicate that the A2780/CP70 cells have reduced functional DNA
mismatch repair, resulting in a decreased inhibitory effect of GA
on ovarian cancer cells (29).
Baicalein, nobiletin, tangeretin, baicalin,
(−)-epicatechin and (+)-catechin hydrate belong to a group of
compounds called flavonoids, a subclass of polyphenols. These
polyphenols all have a C6-C3-C6 structure with two benzene rings (A
and B rings). The results of the present study indicated that the
inhibitory effect of flavones (baicalein, nobiletin, tangeretin and
baicalin) on OVCAR-3 and A2780/CP70 cancer cell lines was greater
than that of flavanonols [(−)-epicatechin, (+)-catechin hydrate].
Of the flavones investigated, baicalein exhibited the greatest
inhibitory effect, possibly due to baicalein containing a greater
number of hydroxyls on the A ring compared with other flavones.
Typically, the majority of tumors grow to 1–2 mm, as
they lack blood vessels which provide oxygen and the essential
nutrients that are required for growth (30). Angiogenesis is a key process
required for the delivery of nutrients and oxygen to the tumor
nodule to allow the transition of a tumor from the dormant to
malignant state. VEGF is a common growth factor that induces blood
vessel growth in numerous types of cancer (31). Therefore, the identification of
compounds capable of inhibiting VEGF secretion would be useful in
preventing cancer growth. GA, tangeretin, nobiletin, baicalein and
baicalin were identified to exhibit a moderate level of inhibition
on the protein expression levels of VEGF, compared with the VEGF
expression levels following ellagic acid, (+)-catechin hydrate and
(−)-epicatechin treatment. These results may indicate that the
inhibitory effect of flavones on OVCAR-3 and A2780/CP70 cancer cell
lines is greater than the inhibitory effect of flavanonols.
Subsequent investigation identified that the protein
expression levels of HIF-1α were dramatically decreased in OVCAR-3
cells from 100% (control) to 24.07% at 20 μM GA. OVCAR-3 cells were
used as opposed to A2780/CP70 cells, as they exhibited lower
resistance to GA. The results indicated that GA inhibits VEGF
expression via the down-regulation of HIF-1α expression. In
agreement with this hypothesis, previous studies have indicated
that the inhibition of HIF-1α may be important in cancer therapy
(32,33).
In conclusion, the results of this study indicate
that GA exhibits an anti-angiogenic effect on ovarian cancer cells
and thus, may be applied for use in human ovarian cancer prevention
and therapy. However, future studies are required to explore the
molecular mechanisms underlying GA’s anti-angiogenic effects.
Acknowledgements
The authors thank Ms Rebekah Sine (Alderson Broaddus
University, Philippi, WV, USA) and Mr Zhaoliang Li (Alderson
Broaddus University) for their technical assistance. The present
study was supported by grants awarded to the West Virginia IDeA
Network of Biomedical Research Excellence by the National
Institutes of Health (grant nos. 5P20RR016477 and 8P20GM104434) and
the National Science Foundation (grant no. 1003907) to the West
Virginia Higher Education Policy Commission/Division of Science
Research. The authors also thank the financial support from the
Natural Science Foundation of Zhejiang province (grant no.
Y13C200053) and the Research and Development Fund of Zhejiang A
& F University (grant no. 2012FR024).
References
|
1
|
Henning SM, Wang P, Carpenter CL and Heber
D: Epigenetic effects of green tea polyphenols in cancer.
Epigenomics. 5:729–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang ZJ, Ohnaka K, Morita M, et al:
Dietary polyphenols and colorectal cancer risk: the Fukuoka
colorectal cancer study. World J Gastroenterol. 19:2683–2690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abbas A, Patterson W III and Georgel PT:
The epigenetic potentials of dietary polyphenols in prostate cancer
management. Biochem Cell Biol. 91:361–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Link A, Balaguer F and Goel A: Cancer
chemoprevention by dietary polyphenols: promising role for
epigenetics. Biochem Pharmacol. 80:1771–1792. 2012. View Article : Google Scholar
|
|
5
|
Mahbub AA, Le Maitre CL, Haywood-Small SL,
McDougall GJ, Cross NA and Jordan-Mahy N: Differential effects of
polyphenols on proliferation and apoptosis in human myeloid and
lymphoid leukemia cell lines. Anticancer Agents Med Chem.
13:1601–1613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stagos D, Amoutzias GD, Matakos A, Spyrou
A, Tsatsakis AM and Kouretas D: Chemoprevention of liver cancer by
plant polyphenols. Food Chem Toxicol. 50:2155–2170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee H, Kang C, Jung E, Kim JS and Kim E:
Antimetastatic activity of polyphenol-rich extract of Ecklonia cava
through the inhibition of the Akt pathway in A549 human lung cancer
cells. Food Chem. 127:1229–1236. 2012. View Article : Google Scholar
|
|
8
|
Di Domenico F, Foppoli C, Coccia R and
Perluigi M: Antioxidants in cervical cancer: chemopreventive and
chemotherapeutic effects of polyphenols. Biochim Biophys Acta.
1822:737–747. 2012. View Article : Google Scholar
|
|
9
|
Araújo JR, Gonçalves P and Martel F:
Chemopreventive effect of dietary polyphenols in colorectal cancer
cell lines. Nutr Res. 31:77–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: new opportunities for translation. Nat
Rev Cancer. 9:4152009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kigawa J: New strategy for overcoming
resistance to chemotherapy of ovarian cancer. Yonago Acta Med.
56:43–50. 2013.PubMed/NCBI
|
|
14
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akter R, Uddin SJ, Grice ID and Tiralongo
E: Cytotoxic activity screening of Bangladeshi medicinal plant
extracts. J Nat Med. 68:246–252. 2014. View Article : Google Scholar
|
|
16
|
Jenab M, Ferrari P, Slimani N, et al:
Association of nut and seedintake with colorectal cancer risk in
the European Prospective Investigation into Cancer and Nutrition.
Cancer Epidemiol Biomarkers Prev. 13:1595–1603. 2004.PubMed/NCBI
|
|
17
|
Villarreal-Lozoya JE, Lombardini L and
Cisneros-Zevallos L: Phytochemical constituents and antioxidant
capacity of different pecan [Carya illinoinensis (Wangenh.) K Koch]
cultivars. Food Chem. 102:1241–1249. 2007. View Article : Google Scholar
|
|
18
|
Wu X, Beecher GR, Holden JM, Haytowitz DB,
Gebhardt SE and Prior RL: Lipophilic and hydrophilic antioxidant
capacities of common foods in the United States. J Agr Food Chem.
52:4026–4037. 2004. View Article : Google Scholar
|
|
19
|
Mencherini T, Campone L, Piccinelli AL,
Mesa MG, Sánchez DM, Aquino RP and Rastrelli L: HPLC-PDA-MS and NMR
characterization of a hydroalcoholic extract of Citrus aurantium L.
var. amara peel with antiedematogenic activity. J Agric Food Chem.
61:1686–1693. 2013. View Article : Google Scholar
|
|
20
|
Yu MW, Lou SN, Chiu E and Ho CT:
Antioxidant activity and effective compounds of immature calamondin
peel. Food Chem. 136:1130–1135. 2013. View Article : Google Scholar
|
|
21
|
Mitjavila MT and Moreno JJ: The effects of
polyphenols on oxidative stress and the arachidonic acid cascade.
Implications for the prevention/treatment of high prevalence
diseases. Biochem Pharmacol. 84:1113–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu Y, Jiang F, Jiang H, Wu K, Zheng X, et
al: Gallic acid suppresses cell viability, proliferation, invasion
and angiogenesis in human glioma cells. Eur J Pharmacol.
641:102–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaur M, Velmurugan B, Rajamanickam S,
Agarwal R and Agarwal C: Gallic acid, an active constituent of
grape seed extract, exhibits anti-proliferative, pro-apoptotic and
anti-tumorigenic effects against prostate carcinoma xenograft
growth in nude mice. Pharm Res. 26:2133–2140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
You BR, Kim SZ, Kim SH and Park WH: Gallic
acid-induced lung cancer cell death is accompanied by ROS increase
and glutathione depletion. Mol Cell Biochem. 357:295–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You BR, Moon HJ, Han YH and Park WH:
Gallic acid inhibits the growth of HeLa cervical cancer cells via
apoptosis and/or necrosis. Food Chem Toxicol. 48:1334–1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Faried A, Kurnia D, Faried LS, Usman N,
Miyazaki T, Kato H and Kuwano H: Anticancer effects of gallic acid
isolated from Indonesian herbal medicine, Phaleria macrocarpa
(Scheff.) Boerl, on human cancer cell lines. Int J Oncol.
30:605–613. 2007.PubMed/NCBI
|
|
27
|
Luo H, Li B, Li Z, Cutler SJ, Rankin GO
and Chen YC: Chaetoglobosin K inhibits tumor angiogenesis through
downregulation of vascular epithelial growth factor-binding
hypoxia-inducible factor 1α. Anticancer Drugs. 24:715–724. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roberts AT, Martin CK, Liu Z, Amen RJ, et
al: The safety and efficacy of a dietary herbal supplement and
gallic acid for weight loss. J Med Food. 10:184–188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strathdee G, MacKean MJ, Illand M and
Brown R: A role for methylation of the hMLH1 promoter in loss of
hMLH1 expression and drug resistance in ovarian cancer. Oncogene.
18:2335–2341. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weng CJ and Yen GC: Chemopreventive
effects of dietary phytochemicals against cancer invasion and
metastasis: phenolic acids, monophenol, polyphenol, and their
derivatives. Cancer Treat Rev. 38:76–87. 2012. View Article : Google Scholar
|
|
32
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Semenza GL: HIF-1 Inhibitors for cancer
therapy: from gene expression to drug discovery. Curr Pharm Des.
15:3839–3843. 2009. View Article : Google Scholar : PubMed/NCBI
|