Introduction
Nasopharyngeal carcinoma (NPC) is a unique type of
head and neck cancer, which exhibits a distinct endemic
distribution, with a particularly high incidence in Southern China
and its surrounding regions (1).
Currently, radiotherapy (RT) is the standard treatment modality for
NPC, and advances in diagnostic imaging, radiotherapeutic
techniques and chemotherapy regimens have improved the treatment
outcomes of such patients (2,3).
However, numerous trials investigating the adoption of RT and
chemotherapy combination treatment strategies have not observed a
reduced incidence of distant metastasis, therefore, distant
metastasis continues to be an important reason for a poor prognosis
in NPC patients (4,5).
Currently, the most important prognostic factor for
NPC is the extent of the disease, which is defined using the
tumor-node-metastasis staging system (6). However, a number of additional
prognostic factors, which may significantly affect the prognosis,
appear to be directly or indirectly associated with the extent of
the NPC. Identification of these factors may provide novel
indicators to aid in the improvement of the prognosis of NPC
patients.
Platelets (PLTs) serve various roles in
physiological and pathological pathways, and were initially
associated with oncological processes, particularly the process of
tumor metastasis, in the nineteenth century (7). Tumor-associated thrombocytosis is
commonly observed in patients with solid cancer and has been
identified as an unfavorable prognostic factor in numerous types of
solid cancer, including oral squamous cell carcinoma (8), and esophageal (9), bronchial and lung (10), gastric (11) and breast (12) cancer. Furthermore, the percentage
and the prognostic effect of an increased PLT count appears to vary
depending on the disease and may change with the geological
location (13). Thrombocytopenia
may additionally be induced in patients with solid cancer, for
example, by chemotherapy and the cancer itself (14). Previously, a decreased PLT count was
identified as a prognostic factor for poor survival in esophageal
cancer patients (15), and Schwarz
and Keny (16) identified that a
low preoperative PLT count was associated with poor survival
following the resection of periampullary cancer; however, Domínguez
et al (17) reported that a
low PLT count was neither an adverse nor a favorable prognostic
factor in resected pancreatic ductal adenocarcinoma. Thus, limited
evidence is available to clarify the prognostic value of a
decreased PLT count in other types of solid cancer.
PLT count may serve as a prognostic factor for
cancer patients, however, there are few studies regarding its
prognostic value in NPC. Our previous study identified that a PLT
count of >300×109/l prior to RT is a predictor of
poor survival and distant metastasis in NPC patients (18); however, the prognostic value of a
decreased PLT count was not considered. In addition, the
administration of chemotherapeutic agents may have an impact on the
PLT count via the inhibition of marrow function; thus, neoadjuvant
chemotherapy may affect the PLT count prior to radiation treatment
and the prognostic value of the PLT count may differ in patients
receiving concurrent chemoradiotherapy (CCRT) (19). Therefore, in the present study, the
sample size was enlarged compared with our previous study (18) and NPC patients receiving CCRT or RT
alone were enrolled to investigate the prognostic significance of
different pretreatment PLT counts.
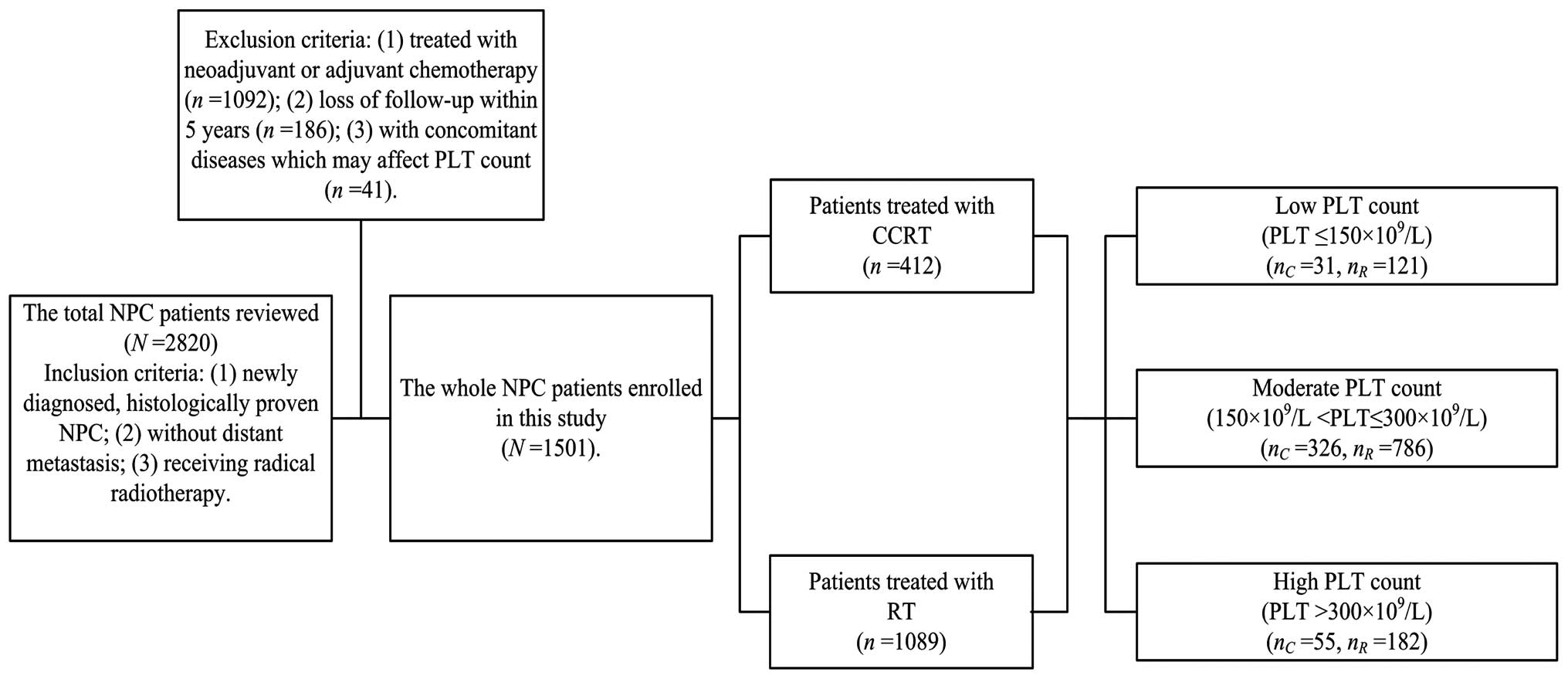
Patients and methods
Patients
A retrospective review of 2,820 newly diagnosed NPC
patients with no evidence of distant metastasis was conducted in
the Sun Yat-Sen University Cancer Center (SYSUCC) between November
2000 and December 2004. The inclusion criteria were as follows: i)
Newly diagnosed, histologically determined NPC; ii) no distant
metastasis; and iii) currently receiving radical RT. The exclusion
criteria were as follows: i) Treated with neoadjuvant or adjuvant
chemotherapy (n=1,092); ii) loss of follow-up within five years
(n=186); and iii) presence of concomitant diseases, which may
affect PLT count, including inflammation, autoimmune disease,
history of blood transfusion, liver cirrhosis, splenic disease and
severe hypertension (n=41). Thus, a total of 1,501 NPC patients
receiving CCRT or RT were enrolled in the present study. Computed
tomography and/or magnetic resonance imaging were essential for
disease staging prior to treatment, and all patients were restaged
according to the 2009 American Joint Committee on Cancer staging
system (20).
PLT measurement and grouping
Pretreatment PLT counts were measured at baseline
within seven days of the commencement of RT for all patients. In
accordance with a number of previous studies, including our
previous study, a PLT count of >300×109/l was
considered to be of prognostic significance (18,21,22). A
PLT count of <150×109/l is associated with a poor
treatment outcome in esophageal cancer patients (15) and indicates the requirement for a
change in the chemotherapy administration pattern in solid cancers
(14). Accordingly, the present
study used 150 and 300×109/l as the cut-off points and
the PLT count was categorized it into three groups: Low
(PLT≤150×109/l), moderate
(150×109/l<PLT≤300×109/l) and high
(PLT>300×109/l) (Fig.
1).
RT
Definitive-intent RT with high energy 6–8 MV X-ray
using a linear accelerator [Varian Clinac iX (Varian Medical
Systems, Inc., Palo Alto, CA, USA), Elekta Precise (Elekta,
Stockholm, Sweden) or Siemens Primus (Siemens Medical Solutions
USA, Inc., Malvern, PA, USA)] was used to treat all the patients;
1,362 (90.7%) patients were treated with two-dimensional conformal
RT (2D-CRT), 42 (2.8%) patients were treated with 3D-CRT and 97
(6.5%) patients were treated with intensity-modulated RT
(IMRT).
Opposing lateral facial-cervical fields were used in
the 2D-CRT to ensure that the nasopharynx and upper cervical
lymphatic drainage region were targeted, and one lower anterior
cervical field was used to cover the lower cervical region.
Following radiation at a dose of 36–40 Gy, opposing lateral
preauricular fields were used for the primary region and anterior
split neck fields were used for the cervical region. Furthermore,
the primary tumor was irradiated to a dose of 60–78 Gy. The
irradiation dose for patients undergoing 2D-CRT was 50–54 Gy to the
prophylactic areas; however, for 3D-CRT, the total prescribed dose
was 66–72 Gy to the gross tumor volume of the nasopharynx (GTVnx),
60–70 Gy to the region involved by the metastatic lymph nodes
(GTVnd), 60 Gy to the clinical target volume-1 (CTV-1), the GTVnx
and an additional 5–10-mm margin, and 50–54 Gy to the prophylactic
irradiating region (CTV-2). For IMRT, the target definition and
delineation were the same as the aforementioned values for 3D-CRT.
The prescription dose was 68 Gy to the GTVnx, 60–64 Gy to the GTVnd
of the neck, 60 Gy to the CTV-1 and 54 Gy to the CTV-2.
Chemotherapy
CCRT was received by 412 (27.4%) patients as
cisplatin/carboplatin plus 5-fluorouracil (FU) or cisplatin alone.
CCRT was mainly used for stage III–IV patients; the regimens for
CCRT were mainly cisplatin alone. The cisplatin/carboplatin plus
5-FU regimen consisted of 70–100 mg/m2 cisplatin or
300–400 mg/m2 carboplatin on day one, plus 500–1000
mg/m2/day 5-FU on days 1–5 every 3–4 weeks for 2–3
cycles. By contrast, the cisplatin alone regimen consisted of 30–40
mg/m2 cisplatin every week for 6–7 cycles. The dose
ranges were based on the conditions of the patients; if the side
effects were severe, then the doses were reduced accordingly.
Follow-up
Following the completion of treatment, patients were
followed up every three months for the first three years, with the
intervals gradually increasing to 6–12 months after three years.
The follow-up data was last reviewed in February 2011. The assessed
end-points included overall survival (OS), local-regional
recurrence-free survival (LRFS) and distant metastasis-free
survival (DMFS). OS was calculated as the time from the
commencement of RT to mortality by any cause, and LRFS and DMFS
were calculated as the time from the commencement of RT to the
initial occurrence of local-regional or distant failure,
respectively.
Statistical analysis
All analyses were performed using SPSS software
(version 19.0; IBM SPSS, Armonk, NY, USA). The χ2 test
was used to compare categorical variables between the three PLT
groups, and the rates of OS, LRFS and DMFS were estimated by means
of the Kaplan-Meier method, and were compared between subgroups
using the log-rank test. Multivariate analysis was performed by
using the Cox proportional hazards model to analyze the independent
significance of various variables in the CCRT and RT patients.
Two-sided P-values of <0.05 were considered to indicate a
statistically significant difference.
Results
Patient characteristics
The baseline characteristics of the 1,501 patients
analyzed in the present study are shown in Table I. The median patient age was 46
years (range, 11–78 years). In total, 1,375 (91.6%) patients
presented with undifferentiated non-keratinizing carcinoma, 117
(7.8%) with differentiated non-keratinizing carcinoma and nine
(0.6%) with other types of NPC. The median duration of follow-up
was 87 months (range, 2–125 months) and the pretreatment PLT count
range was 58–600×109/l, with a mean value of
232×109/l. Furthermore, the PLT count groups were
divided as follows: Low PLT count, 152 (10.1%) patients; moderate
PLT count, 1,112 (74.1%) patients; and high PLT count, 237 (15.8%)
patients.
 | Table IBaseline characterictics in the 1,501
nasopharyngeal carcinoma patients. |
Table I
Baseline characterictics in the 1,501
nasopharyngeal carcinoma patients.
| | CCRT patient PLT
count (n=412) | | RT patient PLT
count (n=1089) | |
|---|
| |
| |
| |
|---|
| Patient
characteristic | Total patients, n
(%)(n=1501) | Low, n (%) | Moderate, n
(%) | High, n (%) | P-valuea | Low, n (%) | Moderate, n
(%) | High, n (%) | P-valuea |
|---|
| Age, years | | | | | 0.326 | | | | 0.289 |
| ≤45 | 734 (48.9) | 13 (41.9) | 163 (50.0) | 32 (58.2) | | 52 (43.0) | 379 (48.2) | 95 (52.2) | |
| >45 | 767 (51.1) | 18 (58.1) | 163 (50.0) | 23 (41.8) | | 69 (57.0) | 407 (51.8) | 87 (47.8) | |
| Gender | | | | | 0.044 | | | | <0.001 |
| Male | 1158 (77.1) | 26 (83.9) | 271 (83.1) | 38 (69.1) | | 99 (81.8) | 613 (78.0) | 111 (61.0) | |
| Female | 343 (22.9) | 5 (16.1) | 55 (16.9) | 17 (30.9) | | 22 (18.2) | 173 (22.0) | 71 (39.0) | |
| Clinical
stageb | | | | | 0.237 | | | | 0.866 |
| I | 127 (8.5) | 2 (6.5) | 11 (3.4) | 2 (3.6) | | 10 (8.3) | 85 (10.8) | 17 (9.3) | |
| II | 583 (38.8) | 5 (16.1) | 93 (28.5) | 12 (21.8) | | 53 (43.8) | 344 (43.8) | 76 (41.8) | |
| III | 543 (36.2) | 17 (54.8) | 155 (47.5) | 22 (40.0) | | 43 (35.5) | 247 (31.4) | 59 (32.4) | |
| IV | 248 (16.5) | 7 (22.6) | 67 (20.6) | 19 (34.5) | | 15 (12.4) | 110 (14.0) | 30 (16.5) | |
| T stage | | | | | 0.101 | | | | 0.619 |
| T1 | 326 (21.7) | 5 (16.1) | 59 (18.1) | 7 (12.7) | | 24 (19.8) | 191 (24.3) | 40 (22.0) | |
| T2 | 600 (40.0) | 12 (38.7) | 112 (34.4) | 14 (25.5) | | 56 (46.3) | 333 (42.4) | 73 (40.1) | |
| T3 | 360 (24.0) | 9 (29.0) | 101 (31.0) | 15 (27.3) | | 26 (21.5) | 170 (21.6) | 39 (21.4) | |
| T4 | 215 (14.3) | 5 (16.1) | 54 (16.6) | 19 (34.5) | | 15 (12,4) | 92 (11.7) | 30 (16.5) | |
| N stage | | | | | 0.134 | | | | 0.111 |
| N0 | 466 (31.0) | 10 (32.3) | 52 (16.0) | 13 (23.6) | | 49 (40.5) | 283 (36.0) | 59 (32.4) | |
| N1 | 620 (41.3) | 8 (25.8) | 145 (44.5) | 20 (36.4) | | 45 (37.2) | 323 (41.1) | 79 (43.4) | |
| N2 | 376 (25.0) | 10 (32.3) | 114 (35.0) | 20 (36.4) | | 27 (22.3) | 161 (20.5) | 44 (24.2) | |
| N3 | 39 (2.6) | 3 (9.7) | 15 (4.6) | 2 (3.6) | | 0 (0.0) | 19 (2.4) | 0 (0.0) | |
| Treatment | | | | | | | | | |
| CCRT | 412 (27.4) | 31 (100.0) | 326 (100.0) | 55 (100.0) | | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| RT | 1089 (72.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | | 121 (100.0) | 786 (100.0) | 182 (100.0) | |
Within the cohort, 412 (27.4%) patients received
CCRT while 1,089 (72.6%) patients received RT. The pretreatment PLT
count was significantly correlated with gender in the CCRT
(P<0.001) and RT (P=0.044) patients (Table I), and overall, female patients
exhibited a higher PLT count. Furthermore, no significant
differences were identified in age, clinical stage, T stage and N
stage between the CCRT or RT PLT groups.
Treatment outcome of the PLT groups
Of the 1,501 patients in the present study, 250
(16.7%) developed local-regional failure, 132 (8.8%) developed
distant metastasis and 366 (24.4%) succumbed. The five-year OS,
LRFS and DMFS rates of the total cohort were 79.3, 84.9 and 91.6%,
respectively.
Table II indicates
the five-year OS, LRFS and DMFS rates of the three PLT groups in
the CCRT and RT patients. Among the PLT groups, joint analysis
identified significant differences in the OS and DMFS rates of
patients administered CCRT (P=0.005 and P=0.036, respectively) and
RT (P=0.005 and P<0.001, respectively; Table II). However, no significant
differences in LRFS were identified among the PLT groups in the
CCRT or RT patients (all P>0.05).
 | Table IITreatment outcome of the three
platelet groups in the 1,501 nasopharyngeal carcinoma patients. |
Table II
Treatment outcome of the three
platelet groups in the 1,501 nasopharyngeal carcinoma patients.
| Platelet count | Five-year OS,
% | P-valuea | Five-year LRFS,
% | P-valuea | Five-year DMFS,
% | P-valuea |
|---|
| CCRT patients
(n=412) | | 0.005 | | 0.153 | | 0.036 |
| Low | 57.9 | | 76.0 | | 82.5 | |
| Moderate | 76.7 | | 83.4 | | 89.5 | |
| High | 60.7 | | 72.4 | | 77.4 | |
| RT patients
(n=1089) | | 0.005 | | 0.224 | | <0.001 |
| Low | 79.3 | | 81.8 | | 93.5 | |
| Moderate | 83.2 | | 86.9 | | 94.9 | |
| High | 76.4 | | 85.5 | | 86.0 | |
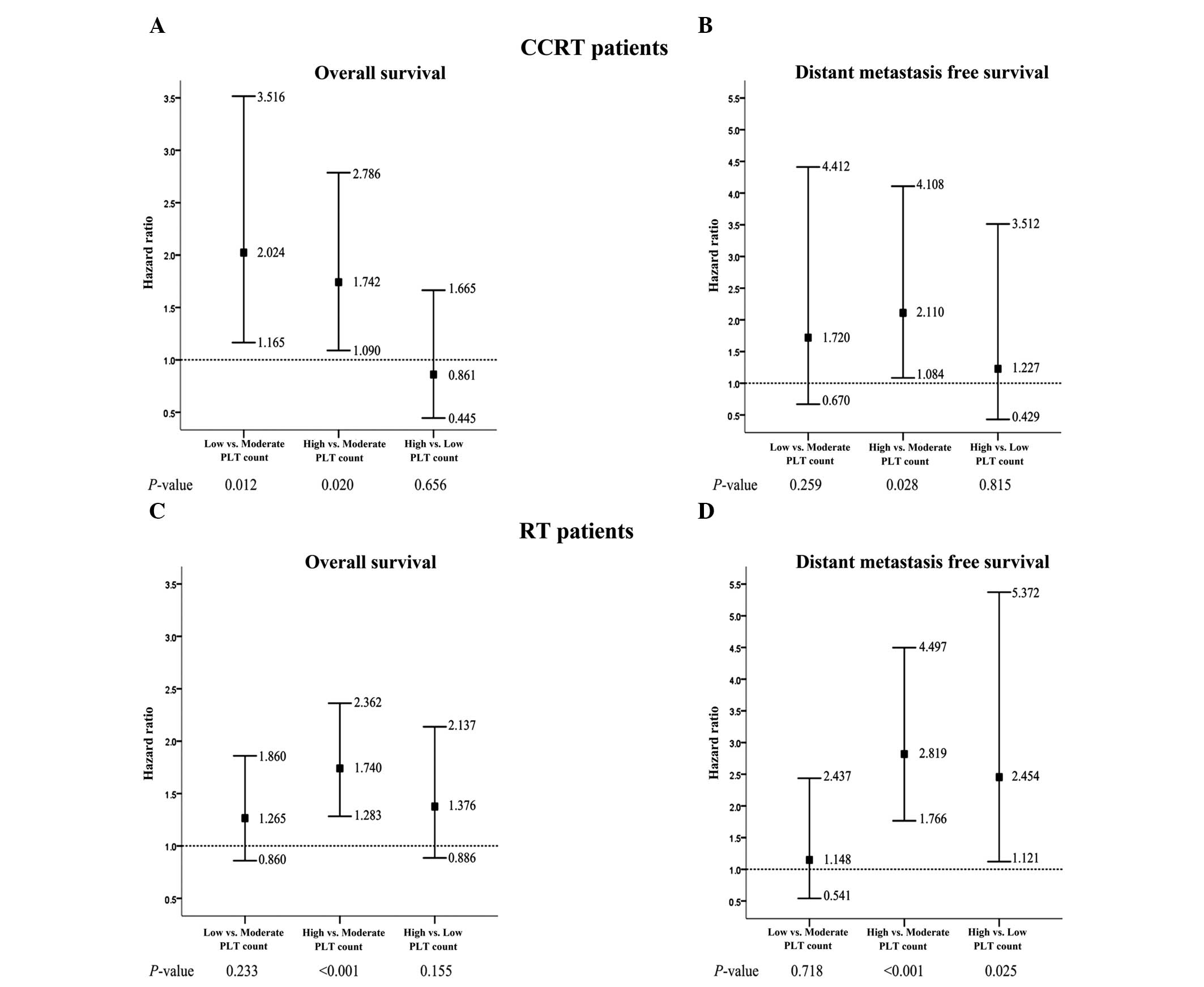
Additional analysis of the subgroups identified that
in the CCRT patients, the five-year OS rate in the low PLT group
was significantly lower compared with the moderate PLT group (56.9
vs. 76.7%; P=0.007; Table II;
Fig. 2A). Furthermore, the
five-year OS and DMFS rates in the high PLT group were
significantly lower compared with the moderate PLT group (OS: 60.7
vs. 76.7%; P=0.022; DMFS: 77.4 vs. 89.5%; P=0.012; Table II; Fig.
2A and B).
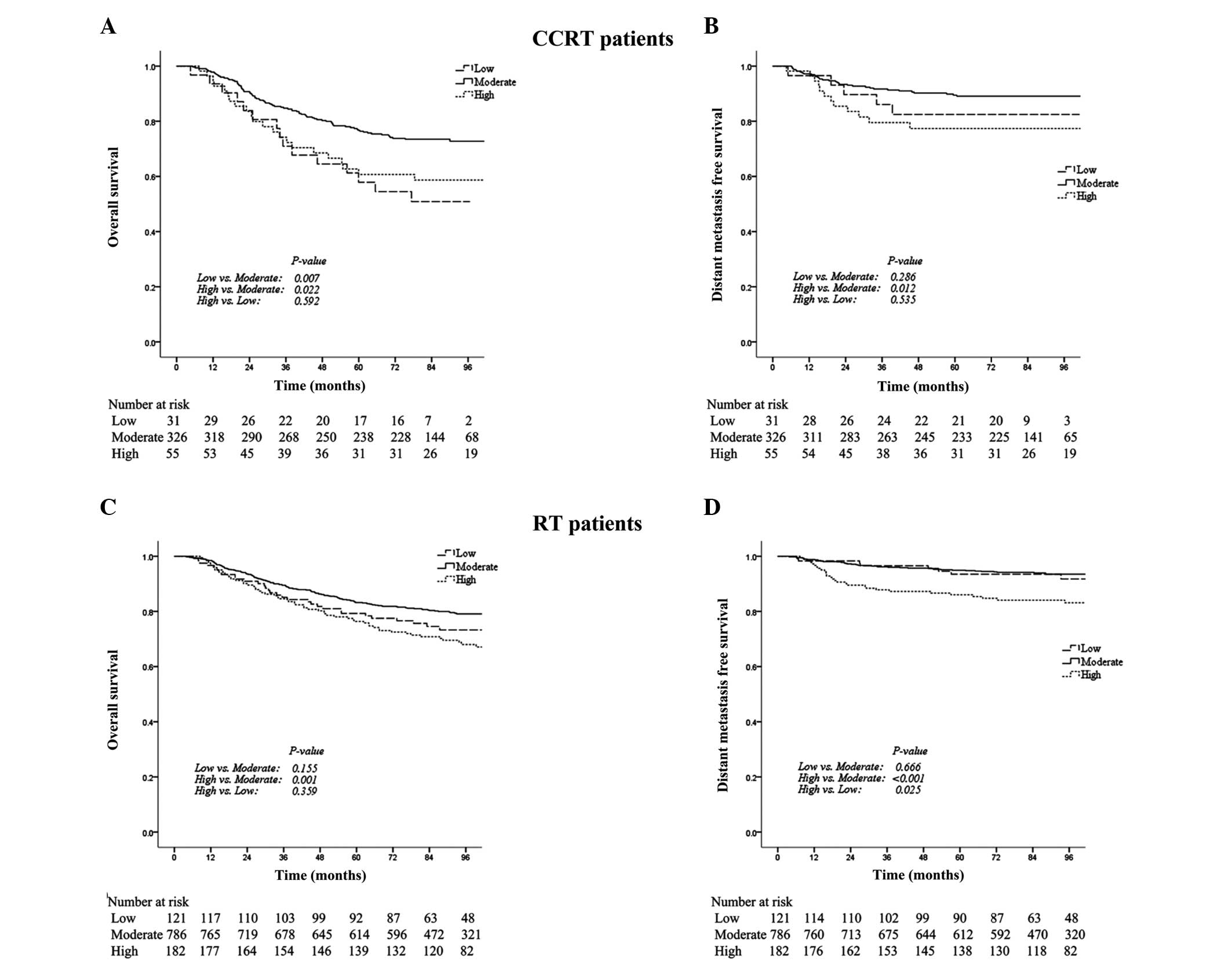 | Figure 2Kaplan-Meier curves indicating the
overall survival (OS) and distant metastasis free survival (DMFS)
rates in the low, moderate and high platelet (PLT) count groups,
among patients receiving CCRT or RT. P-values were determined using
the log-rank test for low vs. moderate, high vs. moderate and high
vs. low PLT counts. In the CCRT patients, (A) the five-year OS
rates in the low, moderate and high PLT groups were 57.9, 76.7 and
60.7% (P=0.007, P=0.022 and P=0.592), respectively and (B) the
five-year DMFS rates in the low, moderate and high PLT groups were
82.5, 89.5 and 77.4% (P=0.286, P=0.012 and P=0.535), respectively.
In the RT patients, (C) the five-year OS rates in the low, moderate
and high PLT groups were 79.3, 83.2 and 76.4% (P=0.155, P=0.001 and
P=0.359), respectively; and (D) the five-year DMFS rates in the
low, moderate and high PLT groups were 93.5, 94.9 and 86.0%
(P=0.666, P<0.001 and P=0.025), respectively. CCRT, concurrent
chemoradiotherapy; RT, radiotherapy alone. |
In the RT patients, the OS and DMFS rates were not
significantly different between the low and moderate PLT groups.
However, the five-year OS and DMFS rates in the high PLT group were
significantly lower than those in the moderate PLT group (OS: 76.4
vs. 83.2%; P=0.001; DMFS: 86.0 vs. 94.9%; P<0.001; Table II; Fig.
2C and D) and the five-year DMFS rate in the high PLT group was
significantly lower than that in the low PLT group (86.0 vs. 93.5%;
P=0.025; Table II; Fig. 2D).
Prognostic significance of the PLT
count
Table III
summarizes the univariate and multivariate analyses of relevant
prognostic factors in the CCRT and RT patients. Variables with
P-values >0.10 were excluded from the model. Univariate analysis
indicated that compared with a moderate PLT count, a low PLT count
was a significant predictor for a poor OS rate in the CCRT patients
only, while a high PLT count was a significant predictor for poor
OS and DMFS rates in the CCRT and RT patients (Table III). Additionally, a high PLT
count was significantly associated with a poor DMFS rate in the RT
patients in comparison with a low PLT count (P=0.025).
 | Table IIIUnivariate and multivariate analyses
of prognostic factors in the 1,501 nasopharyngeal carcinoma
patients. |
Table III
Univariate and multivariate analyses
of prognostic factors in the 1,501 nasopharyngeal carcinoma
patients.
| | Univariate |
Multivariatea |
|---|
| |
|
|
|---|
| Treatment
outcome | Variable | P-value | HR | 95% CI | P-value |
|---|
| CCRT patients
(n=412) |
| Overall
survival | Age (>45 vs. ≤45
years) | 0.033 | 1.576 | 1.096–2.264 | 0.014 |
| N status (N2–3 vs.
N0–1) | 0.079 | 1.508 | 1.055–2.154 | 0.024 |
| PLT count | | | | |
| Low vs.
moderate | 0.026 | 2.024 | 1.165–3.516 | 0.012 |
| High vs.
moderate | 0.022 | 1.742 | 1.090–2.786 | 0.020 |
| High vs. low | 0.857 | 0.861 | 0.445–1.665 | 0.656 |
| Local-regional
recurrence-free survival | Age (>45 vs. ≤45
years) | 0.057 | 1.550 | 0.983–2.444 | 0.059 |
| Distant
metastasis-free survival | T status (T3–4 vs.
T1–2) | 0.069 | 1.616 | 0.912–2.863 | 0.100 |
| N status (N2–3 vs.
N0–1) | 0.092 | 1.627 | 0.933–2.835 | 0.086 |
| PLT count | | | | |
| Low vs.
moderate | 0.286 | 1.720 | 0.670–4.412 | 0.259 |
| High vs.
moderate | 0.012 | 2.110 | 1.084–4.108 | 0.028 |
| High vs. low | 0.535 | 1.227 | 0.429–3.512 | 0.815 |
| RT patients
(n=1089) |
| Overall
survival | Age (>45 vs. ≤45
years) | 0.001 | 1.667 | 1.286–2.161 | <0.001 |
| Gender (female vs.
male) | 0.046 | 1.397 | 1.013–1.925 | 0.041 |
| N status (N2–3 vs.
N0–1) | 0.003 | 1.618 | 1.227–2.135 | 0.001 |
| PLT count | | | | |
| Low vs.
moderate | 0.149 | 1.265 | 0.860–1.860 | 0.233 |
| High vs.
moderate | 0.038 | 1.740 | 1.283–2.362 | <0.001 |
| High vs. low
PLT | 0.822 | 1.376 | 0.886–2.137 | 0.155 |
| Local-regional
recurrence-free survival | Age (>45 vs. ≤45
years) | 0.003 | 1.602 | 1.181–2.173 | 0.002 |
| N status (N2–3 vs.
N0–1) | 0.061 | 1.422 | 1.014–1.994 | 0.041 |
| Distant
metastasis-free survival | Age (>45 vs. ≤45
years) | 0.083 | 1.553 | 0.998–2.416 | 0.051 |
| N status (N2–3 vs.
N0–1) | 0.035 | 1.653 | 1.032–2.648 | 0.037 |
| PLT count | | | | |
| Low vs.
moderate | 0.666 | 1.148 | 0.541–2.437 | 0.718 |
| High vs.
moderate | <0.001 | 2.819 | 1.766–4.497 | <0.001 |
| High vs. low
PLT | 0.025 | 2.454 | 1.121–5.372 | 0.025 |
Furthermore, multivariate analysis identified that
in CCRT patients, a low PLT count was an independent negative
prognostic factor for OS rate [hazard ratio (HR), 2.024; 95%
confidence interval (CI), 1.165–3.516] and a high PLT count was an
independent negative prognostic factor for OS (HR, 1.742; 95% CI,
1.090–2.786) and DMFS (HR, 2.110; 95% CI, 1.084–4.108) rate
compared with a moderate PLT count (Table III; Fig. 3A and B). Furthermore, in terms of OS
rate, the negative effect of a low PLT count appeared to be greater
than the negative effect of a high PLT count (Fig. 3A).
In the RT patients, a low PLT count was not
determined to be a prognostic factor for OS or DMFS rate in
comparison with a moderate PLT count; however, a high PLT count was
identified to be an independent negative prognostic factor for OS
(HR, 1.740; 95% CI, 1.283–2.362) and DMFS (HR, 2.819; 95% CI,
1.766–4.497; Table III; Fig. 3C and D) rate in comparison with a
moderate PLT count. In addition, a high PLT count was independently
associated with a poor DMFS rate compared with a low PLT count (HR,
2.454; 95% CI, 1.121–5.372; Table
III; Fig. 3D).
Discussion
The present study evaluated the prognostic value of
low, moderate and high pretreatment PLT counts in NPC patients. The
study demonstrated that in comparison to a moderate PLT count, a
low PLT count was significantly and independently associated with a
poor OS rate in CCRT patients, and a high PLT count was
significantly and independently associated with poor OS and DMFS
rates in CCRT and RT patients. In CCRT patients, this negative
effect on OS rate was greater in the presence of a low PLT count
compared with a high PLT count. Furthermore, compared with a low
PLT count, a high PLT count was significantly and independently
associated with a poor DMFS rate in RT patients. These observations
highlight the importance of determining the pretreatment PLT count
and to the best of our knowledge, represents the first study to
address the prognostic value of different pretreatment PLT count
levels in NPC patients who have undergone radiation treatment.
In cancer patients, the administration of cytotoxic
agent chemotherapy is a common reason for a decreased PLT count,
while disseminated intravascular coagulation, which exhibits more
chronic and subclinical properties, is the most common
non-iatrogenic cause of a reduced PLT count (24,25).
Alidina et al (15)
identified that a PLT count of <150×109/l was
associated with poor survival in esophageal cancer (HR, 6.58;
P=0.001). These results are consistent with those of the present
study, which demonstrated that a low PLT count was an unfavorable
prognostic factor for OS in CCRT patients when compared with a
moderate PLT count. Furthermore, the negative effect of a low PLT
count was greater than that of a high PLT count. However, in
comparison to a high PLT count, a low PLT count was significantly
associated with reduced metastasis in RT but not CCRT patients,
which may be explained by the role of PLTs in tumor metastasis. A
large number of studies have indicated that an increased PLT count
may affect the metastatic potential of tumor cells by facilitating
immune evasion, promoting extravasation and impeding natural killer
cells (7,24). However, in CCRT patients in the
present study, the association between low or high PLT count and
DMFS was not significant, and a low PLT count was significantly
associated with a poor OS rate compared with a moderate PLT count,
despite exhibiting a greater negative effect on OS than a high PLT
count. This phenomenon indicates that the negative effect of a low
PLT count may only becomes apparent in CCRT patients. Furthermore,
Schwarz (26) proposed that a
decreased PLT count may reflect a poor performance status with
megakaryocyte inhibition in pancreatic cancer. In addition, a low
PLT count may contribute to an increase in the risk of developing
hemorrhagic complications (27),
invasive infection and chemotherapy intolerance (28), which all result in a poor prognosis.
Therefore, the present study proposes that a decreased PLT count
has a more apparent negative effect in patients receiving CCRT, as
CCRT may be tolerated less well than RT, resulting in the
development of more profound myelosuppression and causing patients
to become susceptible to complications associated with abnormal
coagulation, which ultimately results in inferior treatment
outcomes.
The cause of tumor-associated thrombocytosis remains
unclear, however, the tumor-associated production of
granulocyte-macrophage colony-stimulating factor or thrombopoietin
(TPO) mediated by interleukin-6 is considered to be responsible for
the increase in PLT count observed in cancer patients (29). In the present study, a high PLT
count was clearly demonstrated to be an unfavorable prognostic
factor for OS and DMFS rate in the CCRT and RT patients compared
with a moderate PLT count. As well as the aforementioned role of
PLTs in tumor metastasis, the possible roles of PLTs in tumor
growth and angiogenesis may explain this unfavorable prognostic
effect; for example, PLTs are able to secrete a number of
proangiogenic cytokines, including vascular endothelial growth
factor (30) and thymidine
phosphorylase (31). These
PLT-derived factors can affect hemostasis, as well as proliferative
and angiogenic activity, which may be associated with the depth of
tumor invasion and a poor response to CRT (32,33).
In addition to these proangiogenic cytokines, PLTs may promote
angiogenesis directly via integrins, which mediate cell-to-cell
adhesion (34). Furthermore, the
development of a hypercoagulable state in cancer patients can
increase the risk of thrombosis (35), and chemotherapy may additionally
potentiate this risk via endothelial cell damage, stimulation of
PLT aggregation and a reduction in anticoagulant synthesis
(36). Therefore, the present study
proposes that chemotherapy-associated thrombophilia may be an
important explanation for the poor outcome of the CCRT patients
with high PLT counts observed in the present study. This hypothesis
is supported by a previous lung cancer study conducted by Zecchina
et al (37), in which
thrombocytosis at the time of chemotherapy administration was found
to be involved in triggering thrombotic complications.
As the pretreatment PLT count appears to be an
independent prognostic factor affecting NPC treatment outcome,
corresponding active treatments should be considered prospectively.
For patients with a low PLT count, particularly those receiving
chemotherapy, PLT transfusion is a rapid and effective means of
controlling bleeding (38),
however, it is costly, may transfer infection and specific patients
may develop an immunoreaction or become refractory to the treatment
strategy. Thus, an alternative treatment strategy is TPO, which can
be administered in combination with chemotherapeutic agents to
prevent the occurrence of severe thrombocytopenia (19). Yang et al (39) reported that the use of uninterrupted
TPO support for the treatment of two cases of NPC with
thrombocytopenia was well-tolerated, and oprelvekin was identified
to be effective in the treatment of solid cancer patients with
chemotherapy-induced thrombocytopenia (40). However, these treatments strategies
may have an inherent oncological risk due to the aforementioned
pro-tumor effects of PLT; therefore, satisfactory optimization of
the therapeutic strategy is required. With respect to
thrombocytosis, anticoagulants have been successfully employed in
animal models to inhibit tumor metastasis and tumor-associated
thrombosis (41,42). A meta-analysis of 11 studies
demonstrated that anticoagulants significantly improved the OS rate
in cancer patients, despite increasing the risk of bleeding
complications (43); however,
anticoagulants lack selectivity, which affects hemostasis.
The retrospective nature of the present study and
the lack of detailed data collected regarding patient complications
following RT impedes further interpretation of the prognostic value
of pretreatment PLT counts. However, to the best of our knowledge,
the present study is the first to report the prognostic effect of
different pretreatment PLT count levels in NPC patients following
radiation treatment, and the first to propose its clinical
significance. In conclusion, the pretherapeutic period provides a
good opportunity to modify the treatment strategy of NPC patients
for an improved prognosis. Thus, pretreatment PLT count, which can
be easily and cheaply determined, represents an important
therapeutic tool in NPC. Additional studies are required to clarify
the effect of PLT levels in cancer and the benefits of
corresponding therapeutic strategies.
Acknowledgements
The present study was supported by grants from the
Hi-Tech Research and Development Program of China (grant no.
2006AA02Z4B4) and the National Natural Science Foundation of China
(grant nos. 30770641 and 31170805).
References
|
1
|
Jemal A and Bray F; Center MM300. Ferlay
J, Ward E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee N, Harris J, Garden AS, et al:
Intensity-modulated radiation therapy with or without chemotherapy
for nasopharyngeal carcinoma: radiation therapy oncology group
phase II trial 0225. J Clin Oncol. 27:3684–3690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen YP, Wang ZX, Chen L, et al: A
Bayesian network meta-analysis comparing concurrent
chemoradiotherapy followed by adjuvant chemotherapy, concurrent
chemoradiotherapy alone and radiotherapy alone in patients with
locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. Oct
29–2014.(Epub ahead of print). PubMed/NCBI
|
|
4
|
Fang FM, Tsai WL, Chien CY, et al:
Pretreatment quality of life as a predictor of distant metastasis
and survival for patients with nasopharyngeal carcinoma. J Clin
Oncol. 28:4384–4389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
OuYang PY, Xie C, Mao YP, et al:
Significant efficacies of neoadjuvant and adjuvant chemotherapy for
nasopharyngeal carcinoma by meta-analysis of published
literature-based randomized, controlled trials. Ann Oncol.
24:2136–2146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu LY, Pan J, Wu JX, et al: Factors
associated with overall survival in 1706 patients with
nasopharyngeal carcinoma: significance of intensive neoadjuvant
chemotherapy and radiation break. Radiother Oncol. 96:94–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buergy D, Wenz F, Groden C and Brockmann
MA: Tumor-platelet interaction in solid tumors. Int J Cancer.
130:2747–2760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu CC, Chang KW, Chou FC, et al:
Association of pretreatment thrombocytosis with disease progression
and survival in oral squamous cell carcinoma. Oral Oncol.
43:283–288. 2007. View Article : Google Scholar
|
|
9
|
Shimada H, Oohira G, Okazumi S, et al:
Thrombocytosis associated with poor prognosis in patients with
esophageal carcinoma. J Am Coll Surgeons. 198:737–741. 2004.
View Article : Google Scholar
|
|
10
|
Pedersen LM and Milman N: Prognostic
significance of thrombocytosis in patients with primary lung
cancer. Eur Respir J. 9:1826–1830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang SG, Kim KM, Cheong JH, et al: Impact
of pretreatment thrombocytosis on blood-borne metastasis and
prognosis of gastric cancer. Eur J Surg Oncol. 38:562–567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taucher S, Salat A, Gnant M, et al:
Austrian Breast and Colorectal Cancer Study Group: Impact of
pretreatment thrombocytosis on survival in primary breast cancer.
Thromb Haemost. 89:1098–1106. 2003.PubMed/NCBI
|
|
13
|
Qiu MZ, Xu RH, Ruan DY, et al: Incidence
of anemia, leukocytosis, and thrombocytosis in patients with solid
tumors in China. Tumor Biol. 31:633–641. 2010. View Article : Google Scholar
|
|
14
|
Hassan BA, Yusoff ZB, Hassali MA and Bin
Othman S: Treatment patterns and outcomes in management of solid
cancer patients suffering from thrombocytopenia in Penang hospital.
Asian Pac J Cancer Prev. 12:2841–2845. 2011.PubMed/NCBI
|
|
15
|
Alidina A, Gaffar A, Hussain F, et al:
Survival data and prognostic factors seen in Pakistani patients
with esophageal cancer. Ann Oncol. 15:118–122. 2004. View Article : Google Scholar
|
|
16
|
Schwarz RE and Keny H: Preoperative
platelet count predicts survival after resection of periampullary
adenocarcinoma. Hepatogastroenterology. 48:1493–1498.
2001.PubMed/NCBI
|
|
17
|
Domínguez I, Crippa S, Thayer SP, et al:
Preoperative platelet count and survival prognosis in resected
pancreatic ductal adenocarcinoma. World J Surg. 32:1051–1056. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao J, Zhang HY and Xia YF: Increased
platelet count is an indicator of metastasis in patients with
nasopharyngeal carcinoma. Tumour Biol. 34:39–45. 2013. View Article : Google Scholar
|
|
19
|
Vadhan-Raj S: Management of
chemotherapy-induced thrombocytopenia: current status of
thrombopoietic agents. Semin Hematol. 46(Suppl 2): S26–S32. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan J, Xu Y, Qiu S, et al: A comparison
between the Chinese 2008 and the 7th edition AJCC staging systems
for nasopharyngeal carcinoma. Am J Clin Oncol. Apr 19–2013.(Epub
ahead of print). View Article : Google Scholar
|
|
21
|
Brown KM, Domin C, Aranha GV, Yong S and
Shoup M: Increased preoperative platelet count is associated with
decreased survival after resection for adenocarcinorna of the
pancreas. Am J Surg. 189:278–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez GC, Clarke-Pearson DL, Soper JT,
et al: The negative prognostic implications of thrombocytosis in
women with stage IB cervical cancer. Obstet Gynecol. 83:445–448.
1994.PubMed/NCBI
|
|
23
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th edition. Springer; New York, NY:
2010
|
|
24
|
Nash GF, Turner LF, Scully MF and Kakkar
AK: Platelets and cancer. Lancet Oncol. 3:425–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levi M: Disseminated intravascular
coagulation in cancer patients. Best Pract Res Clin Haematol.
22:129–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schwarz RE: Platelet counts and prognosis
of pancreatic cancer. Lancet. 353:2158–2159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kantarjian H, Giles F, List A, et al: The
incidence and impact of thrombocytopenia in myelodysplastic
syndromes. Cancer. 109:1705–1714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santolaya ME, Alvarez AM, Becker A, et al:
Prospective, multicenter evaluation of risk factors associated with
invasive bacterial infection in children with cancer, neutropenia,
and fever. J Clin Oncol. 19:3415–3421. 2001.PubMed/NCBI
|
|
29
|
Kaser A, Brandacher G, Steurer W, et al:
Interleukin-6 stimulates thrombopoiesis through thrombopoietin:
role in inflammatory thrombocytosis. Blood. 98:2720–2725. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Möhle R, Green D, Moore MA, Nachman RL and
Rafii S: Constitutive production and thrombin-induced release of
vascular endothelial growth factor by human megakaryocytes and
platelets. Proc Natl Acad Sci USA. 94:663–668. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okamoto E, Osaki M, Kase S, Adachi H,
Kaibara N and Ito H: Thymidine phosphorylase expression causes both
the increase of intratumoral microvessels and decrease of apoptosis
in human esophageal carcinomas. Pathol Int. 51:158–164. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimada H, Hoshino T, Okazumi S, et al:
Expression of angiogenic factors predicts response to
chemoradiotherapy and prognosis of oesophageal squamous cell
carcinoma. Br J Cancer. 86:552–557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimada H, Takeda A, Shiratori T, et al:
Prognostic significance of serum thymidine phosphorylase
concentration in esophageal squamous cell carcinoma. Cancer.
94:1947–1954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pipili-Synetos E, Papadimitriou E and
Maragoudakis ME: Evidence that platelets promote tube formation by
endothelial cells on matrigel. Br J Pharmacol. 125:1252–1257. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Doormaal FF, Raskob GE, Davidson BL,
et al: Treatment of venous thromboembolism in patients with cancer:
Subgroup analysis of the Matisse clinical trials. Thromb Haemost.
101:762–769. 2009.PubMed/NCBI
|
|
36
|
Ahmed S, Shahid RK, Bhatt H, Lee-Ying R
and Lim J: Chemotherapy-related thrombocytosis: Does it increase
the risk of thromboembolism? Oncology. 82:327–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zecchina G, Ghio P, Bosio S, et al:
Reactive thrombocytosis might contribute to chemotherapy-related
thrombophilia in patients with lung cancer. Clin Lung Cancer.
8:264–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schiffer CA, Anderson KC, Bennett CL, et
al: American Society of Clinical Oncology: Platelet transfusion for
patients with cancer: clinical practice guidelines of the American
Society of Clinical Oncology. J Clin Oncol. 19:1519–1538.
2001.PubMed/NCBI
|
|
39
|
Yang KY, Zhang T, Chen J, et al: Immune
thrombocytopenia as a paraneoplastic syndrome in patients with
nasopharyngeal cancer. Head Neck. 34:127–130. 2012. View Article : Google Scholar
|
|
40
|
Lei W, Liang J, Chen WG, et al:
Effectiveness and safety of recombinant human interleukin-11 in the
treatment of chemotherapy-induced thrombocytopenia. Zhonghua Zhong
Liu Za Zhi. 28:542–544. 2006.(In Chinese). PubMed/NCBI
|
|
41
|
Amirkhosravi A, Mousa SA, Amaya M, et al:
Inhibition of tumor cell-induced platelet aggregation and lung
metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost.
90:549–554. 2003.PubMed/NCBI
|
|
42
|
Amirkhosravi A, Mousa SA, Amaya M and
Francis JL: Antimetastatic effect of tinzaparin, a
low-molecular-weight heparin. J Thromb Haemost. 1:1972–1976. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kuderer NM, Khorana AA, Lyman GH and
Francis CW: A meta-analysis and systematic review of the efficacy
and safety of anticoagulants as cancer treatment-Impact on survival
and bleeding complications. Cancer. 110:1149–1161. 2007. View Article : Google Scholar : PubMed/NCBI
|