Introduction
Adenoid cystic carcinoma (ACC) is a malignant
neoplasm that frequently originates from the salivary glands of the
head and neck, but can also take place in the breast, skin, upper
digestive tract and lungs (1,2).
Formerly, it was referred to as a benign glandular neoplasm,
however, ACC is now regarded as a low-grade bronchial carcinoma
(3). Primary ACC of the lung (ACCL)
arising from the bronchial glands is a particularly rare disease
and accounts for only 0.04–0.2% of all primary lung tumors
(4,5). Although salivary gland-type tumors
generally have an indolent nature, ACCL has a notable tendency for
local recurrence and late hematogenous metastases. Due to its
rarity, primary ACCL has mainly been reported in small series or
case studies. Thus, its precisely clinical and pathological
features, clinical course, therapeutic strategy and survival data
have not been fully elucidated. The current study summarized 22
years of experience dealing with ACCL in a single institution
during the period between January 1993 and June 2014.
Materials and methods
Patients
In total, 36 patients who were admitted to the
Beijing Chest Hospital, Capital Medical University (Beijing, China)
and diagnosed with ACCL between January 1993 and June 2014 were
analyzed retrospectively for the present study. Two cases were
excluded from the series: One case presented with a sublingual
neoplasm concomitantly, except the main bronchus lesion was
excluded, and thus it was difficult to identify the primary lesion
at that time; and the other case underwent a submandibular tumor
resection four years prior to the present study. Therefore, 34
patients entered the final analysis. The medical records were
reviewed in order to extract data on demographic characteristics,
clinical and pathological features, tumor stage (American Joint
Committee on Cancer criteria), treatment and survival (6). The 34 patients were divided into
operable (n=26) and non-operable (n=8) groups. All cases underwent
bronchoscopy-guided biopsy, which was further corroborated
histologically in the surgical specimens. Immunohistochemical
staining was conducted in 17 cases to verify the diagnosis of ACCL.
The level of carcinoembryonic antigen (CEA) was determined by
chemiluminescence, and levels >5 ng/ml were defined as abnormal.
This study was approved by the Ethical committee of the Beijing
Chest Hospital, Capital Medical University.
Statistical analysis
Statistical analysis was performed using the SPSS 16
software (SPSS, Inc., Chicago, IL, USA). All categorical variables
are presented as counts and percentages. Continuous variables are
presented as the median and range. Overall survival time was
defined as the interval between primary surgery and mortality due
to lung cancer or the date of the last follow-up. The disease-free
survival time was defined as the interval between the date of
resection and the date of proven detection of recurrence or
metastases. Survival curves were generated using the Kaplan-Meier
method and the difference in survival was examined using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological features
Patient characteristics are summarized in Table I. The study consisted of 34 patients
with primary ACCL, including 16 males and 18 females, with a median
age of 46 years (range, 22–73 years). With respect to smoking
information, nearly one-third (32.4%) of patients were current or
former smokers, while 67.6% had never smoked. A total of 21 (61.8%)
primary lesions were located in the trachea, main bronchus or
truncus intermedius, 11 (32.3%) tumors were below the lobar
bronchus and 2 (5.9%) tumors were located in the pulmonary
parenchyma. Of the 22 patients whose CEA levels were recorded, one
patient exhibited an abnormal level. A total of 30 patients were
symptomatic when they sought treatment; the most frequent symptom
was coughing (n=26; 76.5%), followed by hemoptysis (n=13; 38.2%),
shortness of breath (n=10; 29.4%), stridor (n=5; 14.7%), fever
(n=3; 8.8%) and chest pain (n=2; 5.9%). The majority of these
patients had two or more symptoms. Several patients were
incorrectly diagnosed with bronchitis or asthma prior to their
visit to the Beijing Chest Hospital, Capital Medical University.
Four patients without subjective symptoms visited their doctors for
a shadow that was incidentally detected on X-ray or computed
tomography (CT) scans of the chest. The median duration between the
appearance of the symptoms and the time of diagnosis was 4 months
(2 weeks to 42 months). In the operable group, the greatest
diameter of the lesions ranged between 1.0 and 7.0 cm, with a
median of 2.5 cm. Involvement of the lymph nodes were observed in
five patients (N1=3; N2=2). Three of the 26 samples showed
infringement of the whole bronchus wall. A total of 16 patients
achieved an R0 resection, which meant that the surgical margin was
histologically free of disease at the final pathological
examination (Table I). Of the 10
patients with microscopically residual tumor at the transected
ends, nine patients received post-operative radiotherapy (50–70 Gy,
5–7 weeks) and one succumbed perioperatively.
 | Table IClinicopathological characteristics of
patients (n=34)with primary adenoid cystic carcinoma of the
lung. |
Table I
Clinicopathological characteristics of
patients (n=34)with primary adenoid cystic carcinoma of the
lung.
| Variables | Value |
|---|
| Median age (range),
years | 46 (22–73) |
| Gender, n (%) |
| Male | 16 (47.1) |
| Female | 18 (52.9) |
| Smoking status, n
(%) |
| Current or ever | 11 (32.4) |
| Never | 23 (67.6) |
| CEA level (n=22), n
(%) |
| ≥5 ng/ml | 1 (4.5) |
| <5 ng/ml | 21 (95.5) |
| Tumor location, n
(%) |
| Trachea, main
bronchus | 17 (50.0) |
| Truncus
intermedius | 4 (11.8) |
| Lobar bronchus | 8 (23.5) |
| Segmental
bronchus | 3 (8.8) |
| Periphery | 2 (5.9) |
| Operable group
(n=26) |
| Node invasion, n
(%) |
| N0 | 21 (80.8) |
| N1 | 3 (11.5) |
| N2 | 2 (7.7) |
| Resection margin, n
(%) |
| Negative | 16 (61.5) |
| Positive | 10 (38.5) |
| Pathological stage,
n (%) |
| I | 11 (42.3) |
| II | 6 (23.1) |
| IIIA | 7 (26.9) |
| IIIB | 2 (7.7) |
| Median tumor size
(range), cm | 2.5 (1.0–7.0) |
| Surgical procedure,
n (%) |
| Tracheal
resection | 4 (15.4) |
| Carinal resection
and reconstruction | 3 (11.5) |
| (Bi)lobectomy | 9 (34.6) |
| Sleeve
lobectomy | 5 (19.2) |
| Pneumonectomy | 5 (19.2) |
| Adjuvant therapy
(n=25), n (%) |
| No | 9 (36.0) |
| Chemotherapy and
radiotherapy | 6 (24.0) |
| Chemotherapy
only | 3 (12.0) |
| Radiotherapy
only | 7 (28.0) |
| Non-operable group
(n=8) |
| Clinical stage, n
(%) |
| III | 5 (62.5) |
| IV | 3 (37.5) |
Immunohistochemical features
Immunohistochemical examination was conducted in 17
cases. Antibodies to wide-spectrum keratin, actin, vimentin (Vim),
S-100 protein, CEA, glial fibrillary acidic protein (GFAP), smooth
muscle actin (SMA), cluster of differentiation 56 (CD56), thyroid
transcription factor-1 (TTF-1), p63, chromogranin A (CgA),
synaptophysin (Syn), cytokeratin (CK)20), CK7, leukocyte common
antigen (LCA), caudal-type homeobox 2 (CDX-2), neuron-specific
enolase (NSE) and CK5/6 were chosen in different combinational
panels to confirm the diagnosis of primary ACCL according to the
respective condition of the patient. The positive staining counts
of this antigen spectrum was present as follows: Wide-spectrum
keratin in 17/17 patients; p63 in 11/12 patients; SMA in 6/9
patients; S-100 in 7/8 patients; Vim in 10/12 patients; CK7 in
11/11 patients; GFAP in 1/3 patients; CEA in 2/9 patients; actin in
2/2 patients; CK5/6 in 1/1 patient; and NSE in 1/1 patient. One
sample was positively-stained with the periodic acid-Schiff stain
(PAS) method (n=1). Staining was absent for Syn in seven patients,
CD56 in seven patients, CK20 in four patients, CgA in four
patients, CDX-2 in one patient, TTF-1 in 14 patients and LCA in one
patient.
Treatment of ACC
In the operable group (n=26), all patients
selectively underwent plain CT, contrast-enhanced CT, radioisotope
bone scans, ultrasound scanning, brain magnetic resonance imaging,
blood examinations and lung functional examinations prior to
surgery to determine clinical stage and evaluate the feasibility of
the procedure. The surgical procedures included tracheal resection
(n=4; 15.4%), carinal resection and reconstruction (n=3; 11.5%),
(bi)lobectomy (n=9; 34.6%), sleeve lobectomy (n=5; 19.2%) and
pneumonectomy (n=5; 19.2%). One patient succumbed to a cardiac
arrest within 30 days of a carinal resection and reconstruction. A
total of 13 cases received adjuvant radiotherapy (40–70 Gy, 4–7
weeks), while nine cases received neither radiotherapy nor
chemotherapy. In the cases with recurrence or metastasis, local or
systemic treatment was administered according to the respective
condition of the patient, including palliative radiotherapy,
systemic chemotherapy, cryotherapy and Chinese herbal medicine.
Nine of the 10 patients with positive margins received adjuvant
radiotherapy (50–70 Gy, 5–7 weeks). Local recurrence/metastasis
occurred during the follow-up of the nine patients; of these
patients, cases 3, 5, 8 and 9 received the initial chemotherapy in
the Beijing Chest Hospital, Capital Medical University (Table II). Case 3 was administered
paclitaxel and cis-platinum (paclitaxel, 175 mg/m2, day
1; cis-platinum, 75 mg/m2, days 1–3), but the metastatic
lesions in each lung progressed subsequent to the first cycle. One
cycle of gemcitabine (1,250 mg/m2, days 1 and 8) and
cis-platinum (75 mg/m2, days 1–3) was administered to
case 5, which was followed by continuous tumor enlargement in the
lung and pleura. Single-drug chemotherapy with pemetrexed (500
mg/m2) was attempted as second-line treatment, but also
failed. Case 8 was administered one cycle of navelbine (25
mg/m2, days 1 and 8), with cis-platinum (75
mg/m2, days 1–3) as the first-line treatment and one
cycle of paclitaxel (175 mg/m2, day 1) and cis-platinum
(75 mg/m2, day 1) as second-line treatment. Although no
response was observed throughout the chemotherapy regimen, the
patient remains alive at the time of writing this study. Case 9
received two cycles of paclitaxel (175 mg/m2, day 1) and
cis-platinum (75 mg/m2, days 1–3) treatment, and the
lesion was markedly reduced in size. However, six months later, new
metastasis appeared. The use of other chemotherapeutic agents
(gemcitabine and carboplatin) was attempted in the patient’s
district hospital.
 | Table IISummarized information on tumor
recurrence/metastasis and mortality in the operable group. |
Table II
Summarized information on tumor
recurrence/metastasis and mortality in the operable group.
| Case no. | Recurrence/metastasis
(site) | Resection margin | Status | DFS, months | Follow-up,
months |
|---|
| 1 | Bronchial resection
margin | Positive | Alive | 3 | 120 |
| 2 | Brain, lung,
bone | Negative | Succumbed | 83 | 96 |
| 3 | Kidney, lung, bone,
bronchial resection margin | Negative | Succumbed | 197 | 225 |
| 4 | Lung, liver | Negative | Succumbed | 128 | 135 |
| 5 | Lung, pleura | Positive | Alive | 7 | 21 |
| 6 | Bronchial resection
margin | Positive | Alive | 13 | 28 |
| 7 | Lung, pleura | Positive | Succumbed | 16 | 26 |
| 8 | Bronchial resection
margin | Positive | Alive | 2 | 127 |
| 9 | Lung, mediastinal
lymph nodes, liver | Negative | Succumbed | 78 | 87 |
In the non-operable group (n=8), three patients were
diagnosed with stage IV disease, with multiple lung metastases, and
five patients were diagnosed with stage IIIB disease due to the
advanced local situation. Palliative treatments, including
radiotherapy, systemic chemotherapy, tracheal stent implantation,
interventional therapy, high-frequency electrocautery through
bronchoscopy and Chinese herbal medicine, were administered to
these patients at different time-points in the clinical course. Two
stage III patients with lesional invasion into the pulmonary artery
were administered primary radiotherapy (the schedued dose was 40
Gy) in the Beijing Chest Hospital, Capital Medical University. The
lesions remained unchanged following radiotherapy, so the
possibility of surgery was not achieved. One stage III patient was
firstly administered a tracheal stent implantation to alleviate
airway stenosis; this patient was the only patient alive in the
non-operable group after 44 months. One stage III patient was
forced to give up subsequent radiotherapy due to aggravation of the
symptom of stridor following the initial 2 Gy fraction. Another
stage III patient received palliative radiotherapy in a local
hospital following the final diagnosis in the Beijing Chest
Hospital, Capital Medical University, but the effect was poor. The
patient succumbed to respiratory failure one year later. Two stage
IV patients received primary chemotherapy in the Beijing Chest
Hospital, Capital Medical University; one was administered with
gemcitabine (1,250 mg/m2, days 1 and 8) and cis-platinum
(75 mg/m2, day 1), but developed PD due to enlargement
of the primary lesion and mediastinal lymph nodes subsequent to two
cycles. The other case was treated with navelbine (25
mg/m2, days 1 and 8) and cis-platinum (75
mg/m2, days 1–3); the subjective symptom was relieved
marginally and the lesion remained unchanged. Additionally, one
patient insisted on receiving the epidermal growth factor
receptor-tyrosine kinase inhibitor (EGFR-TKI), erlotinib, although
the patient was aware of the fact that there would probably be no
benefit due to a unknown EGFR mutation status. The primary tumor
was stable after one month, but the mediastinal lymph nodes became
enlarged three months later. It is notable that the EGFR mutation
status (exons 18, 19, 20 and 21) from two tumor samples were
examined by sequencing method, including a young female who had
never smoked, but no mutations were detected.
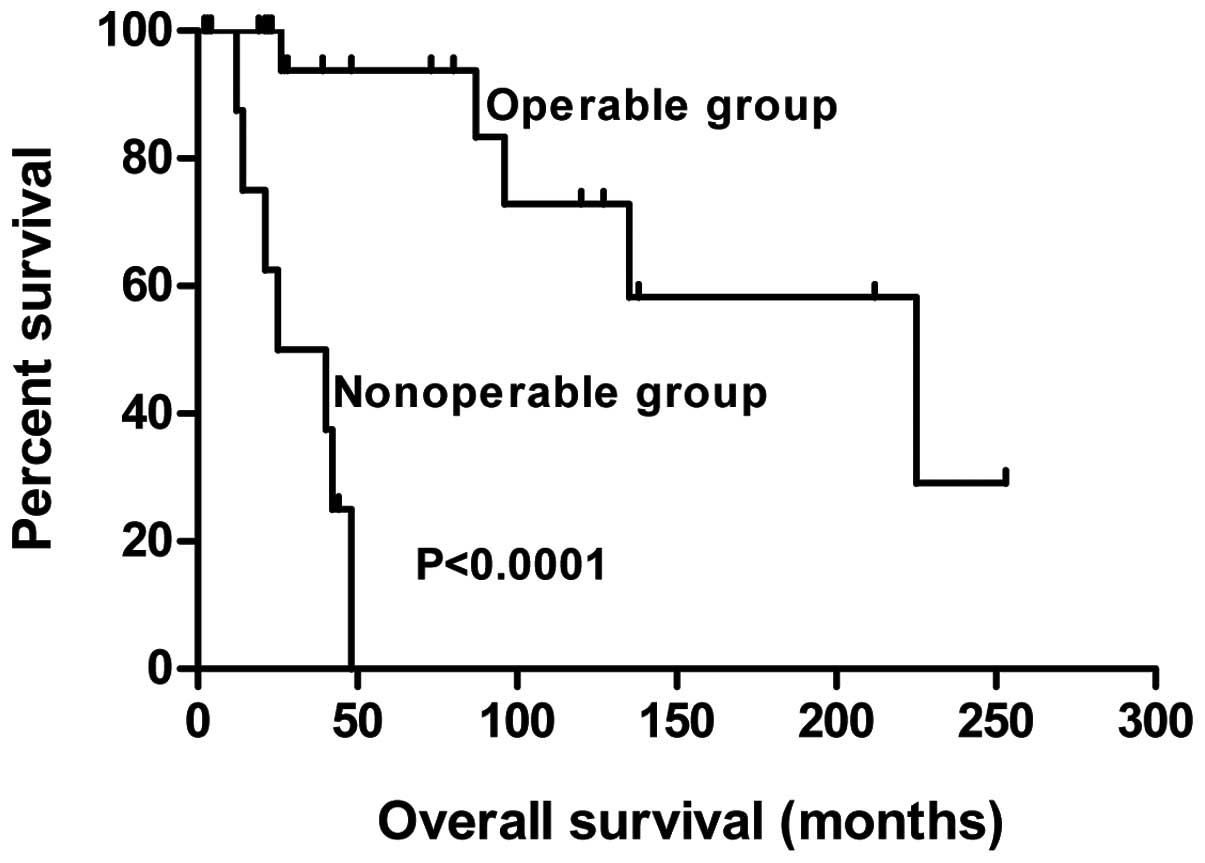
Survival
Operable group
For the survival analysis, the single case of
perioperative mortality was ruled out (n=25). Tumor
recurrence/metastasis was identified in nine patients, five of who
exhibited residual tumor at the resection margin at the time of the
primary surgery (Table II). The
local recurrence/metastasis rate was 55.6% (5/9) in the group with
positive resection margins, while in the group with negative
resection margins, the rate was 25.0% (4/16). The three-, five- and
10-year disease-free survival rates of the operable group were
68.8, 64.3 and 38.5%, respectively. Five mortalities due to ACCL
occurred during the follow-up period and these patients survived
for 26, 87, 96, 135 and 225 months post-surgery, respectively. For
the remaining 20 patients in the operable group, the survival time
ranged between two and 253 months. The three-, five- and 10-year
overall survival rates of the operable group were 92.9, 91.7 and
70.0% (Table III).
 | Table IIISurvival information for the operable
(n=26) and non-operable (n=9) groups. |
Table III
Survival information for the operable
(n=26) and non-operable (n=9) groups.
| Overall survival
rate, % | Disease-free
survival rate, % |
|---|
|
|
|
|---|
| Group | 3-year | 5-year | 10-year | 3-year | 5-year | 10-year |
|---|
| Operable | 92.9 | 91.7 | 70.0 | 68.8 | 64.3 | 38.5 |
| Non-operable | 50.0 | 0.0 | 0.0 | - | - | - |
Although the presence of a positive margin appeared
to be associated with recurrence/metastasis, it did not decrease
long-term survival. The addition of adjuvant therapy to R1
resection resulted in long-term survival results equivalent to
those of an R0 resection. Eight out of the nine patients with
positive resection margins who received post-operative radiotherapy
were alive at the time of writing this study (Fig. 1; P=0.76). Similarly, the presence of
lymph node invasion did not correlate with overall survival time
(Table IV). In the subgroup
analysis, post-operative radiotherapy did not correlate with local
recurrence/metastasis or mortality in the R0 resection group
(Table V).
 | Table IVEffect of lymph node involvement on
the survival time of patients with primary adenoid cystic carcinoma
of the lung in the operable group. |
Table IV
Effect of lymph node involvement on
the survival time of patients with primary adenoid cystic carcinoma
of the lung in the operable group.
| Group | Patients, n |
Recurrence/metastasis, n | Mortality, n | Follow-up,
months |
|---|
| Positive | 4 | 2 | 1 | 21, 22, 39,
87a |
| Negative | 21 | 7 | 4 | 48 (2–253)b
26a, 96a, 135a, 225a |
 | Table VEffect of adjuvant radiotherapy on
the survival of patients with primary adenoid cystic carcinoma of
the lung who underwent an R0 resection. |
Table V
Effect of adjuvant radiotherapy on
the survival of patients with primary adenoid cystic carcinoma of
the lung who underwent an R0 resection.
| Group | Patients, n |
Recurrence/metastasis, n | Mortality, n | Follow-up,
months |
|---|
| Radiotherapy | 4 | 1 | 1 | 2, 19, 87a, 253 |
| Without
radiotherapy | 12 | 3 | 3 | 4, 22, 22, 23, 27,
48, 73, 80, 96a, 135a,138, 225a |
Non-operable group
In the non-operable group, eight patients succumbed.
Only one patient survived for 44 months post-diagnosis; stent
implantation was administered to this patient to alleviate local
stenosis. The overall survival period of the patients who succumbed
ranged between 12 and 48 months, with a median of 23 months. The
three- and five-year overall survival rates for the non-operable
group were 50.0 and 0.0%, respectively (Table III).
The overall survival curves of the operable and
non-operable groups are shown in Fig.
2.
Discussion
Primary ACCL is a rare salivary gland-type malignant
neoplasm that is distributed along the submucosa of the major
airways, therefore, a prolonged period is required in order to
accumulate a number of cases. In the present study, the clinical
features, histopathology and immunohistochemical characters,
treatment strategy and follow-up data of 34 patients treated at the
Beijing Chest Hospital, Capital Medical University, between 1993
and 2014, was investigated in order to enhance our understanding of
primary ACCL.
Compared with other primary lung cancers, ACCL is
quite different in terms of its demographic characteristics. ACCL
tends to occur in younger patients, with an approximate male/female
ratio of 1:1 (7,8). However, there is a marked predominance
of males in the incidence of other lung cancers (8,9). All
smoking information was collected in the present study, and the
majority of the cohort (67.6%) were found to be never smokers. This
was generally accordant with other studies (7,10).
Therefore, smoking does not appear to be associated with the
etiology of ACCL. In the present series, coughing was the most
common symptom of ACCL, followed by hemoptysis and shortness of
breath. Several patients were previously wrongly treated for asthma
and bronchitis. Occasionally, patients were asymptomatic, until
detected in imaging examination, particularly in those patients
with peripheral ACCL (11).
ACCL presents with particular histopathological
features; in the present study, lymphatic metastases were
relatively uncommon, occurring in only five patients undergoing
resection. However, due to the extensive spread along the major
axis of the trachea at the time of diagnosis, residual tumors at
the resection margin are not rare. However, resection with upper
and lower margins of 1 cm from the macroscopic tumor, cannot
guarantee an R0 resection. In such cases, post-operative
radiotherapy is indispensable (12). In total, >90% of ACCLs arise in
the central airway, thus accounting for the small number of
reported cases in the peripheral lung (13). ACC of the peripheral lung must
therefore be distinguished from metastatic lung tumors (13,14).
In the present study, two cases of peripheral ACCL, without
bronchoscopic evidence, were confirmed. These patients did not
exhibit salivary gland neoplasms five years prior to or following
surgery, and the diagnosis was supported by immunohistochemistry in
the post-operative tumor tissues.
Although hematoxylin-eosin staining remains the
principal method used for the diagnosis of ACCL, it can
occasionally be misdiagnosed as carcinoid or mucinous
adenocarcinoma. In these cases, immunohistochemistry is a useful
tool to enhance the accuracy of histological diagnosis (15). To the best of our knowledge, the
number of studies investigating the immunohistochemistry
characteristics of ACCL is sparse. In the present study,
immunostaining of wide-spectrum keratin (17/17), CK7 (11/11) and
actin (2/2) was positive in all samples examined for these markers,
and the vast majority showed positivity for p63 (11/12), S-100
(7/8), Vim (10/12) and SMA (6/9). These immunohistochemical
findings were comparable to the study by Moran et al
(16). No staining for TTF-1
(0/14), Syn (0/7), CD56 (0/7), CK20 (0/4) or CgA (0/4) was
observed. Only partial staining was present for CEA (2/9) and GFAP
(1/3) antibodies. The expression of TTF-1, a transcription factor
specifically expressed in the thyroid gland and lungs, is found in
60–70% of pulmonary adenocarcinomas. An et al (17) reported 12 cases of primary ACCL
without TTF-1 staining, which was comparable with our result, but a
case of peripheral ACCL with positive TTF-1 has been reported
(14).
The optimal management for ACCL is surgical
resection whenever feasible. in the present study, 26 patients
comprised the operable group. The three-, five- and 10-year overall
survival rates were 92.9, 91.7 and 70.0%, respectively. Patients
with positive margins were all administered adjuvant radiotherapy
(n=9). No difference was found in overall survival time between
those who underwent R0 and R1 resections (Fig. 1). The longest survival time in the
operable group was 253 months, without recurrence or metastasis.
The combination treatment of surgery and radiotherapy in the
patients who underwent R1 resection resulted in long-term survival
that was comparable that of patient who underwent R0 resection,
although positive cases were prone to recur locally. Eight patients
with positive resection margins were alive at the time of writing
this study, and three patients survived for 120, 127 and 212
months, respectively, post-surgery. It is our belief that surgery
should be the first therapeutic option, but that the security of
the patient and the healing of the anastomosis is more important
than more tumor-free margins (18–20). A
survival analysis was also performed in the patients who underwent
an R0 resection (n=16) in the present study. In four cases
receiving radiotherapy, one patient experienced
recurrence/metastasis and succumbed at 87 months post-surgery. In
the 12 cases without radiotherapy, three patients experienced
recurrence/metastasis and succumbed 96, 135 and 225 months
post-surgery, respectively. In the R0 resection group, adjuvant
radiotherapy was not found to correlate with overall survival. The
possible benefit of post-operative radiotherapy on overall survival
in R1 resection only was not analyzed, as all patients were
administered radiotherapy for prudential reasons. Based on our
observations, it appeared to be controversial to recommend
post-operative radiotherapy for all patients with ACCL undergoing
resection. To date, concrete data on the benefit of adjuvant
radiotherapy in R0 resection has not been reported, but the
conclusions of the present study also agreed with another larger
sample study (n=64) (19).
Similarly, the presence of positive lymph nodes did not seem to
decrease survival times, as reported by Regnard et al
(19) and Maziak et al
(20). However, as ACCL is likely
to grow along the major axis of the trachea and more extensive
invasion than is found in the pre-operative evaluation is often
found intraoperation, sophisticated skills are required for
surgical treatment.
With regard to advanced ACCL, palliative
chemotherapy has seldom been described in the literature. One case
sensitive to uracil-tegafur and cisplatin plus radiotherapy has
been reported (21). In the present
study, in the five cases receiving palliative chemotherapy, only
one showed sensitivity to paclitaxel and cis-platinum treatment.
Four patients benefited marginally from platinum-based double
chemotherapy, which is the most frequently chosen first-line
therapy for advanced non-small cell lung cancer (NSCLC). In
addition, four cases in the non-operable group responded poorly to
palliative irradiation. EGFR-TKIs are recommended as the first-line
targeted therapy in cases of advanced NSCLC with an activating EGFR
mutation. EGFR kinase domain mutations are rare in salivary gland
carcinomas (22,23). In the present retrospective study,
two cases provided samples for examination, including a young,
non-smoking female, who was regarded as more prone to an EGFR
mutation. Another non-smoking female unaware of the EGFR mutational
status insisted in being administered erlotinib and exhibited a
stable lesion for one month, prior to progression of the local
lymph nodes after three months. Taking these data into
consideration, a novel therapeutic strategy must be sought for
advanced ACCL. The use of imatinib has been reported for one
advanced case and achieved a satisfactory effect (24).
In conclusion, the present study has described the
demographic characteristic, clinicopathological and
immunohistochemical features, clinical treatment and long-term
survival of 34 patients with ACCL. As a retrospective study, the
collection and review of these data clearly has limitations.
Additionally, the study lacked statistical power due to the
relatively small number of patients, although the cases were
accumulated over a 22-year time span. Furthermore, three different
subtypes of this tumor, tubular, cribriform and solid, have been
described by pathologists, with the solid subtype assumed to be
associated with a more aggressive phenotype (16). Another shortcoming of the present
study was that subtype identification was not performed.
References
|
1
|
Chaudhry AP, Leifer C, Cutler LS, et al:
Histogenesis of adenoid cystic carcinoma of the salivary glands.
Light and electronmicroscopic study. Cancer. 58:72–82. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence JB and Mazur MT: Adenoid cystic
carcinoma: a comparative pathologic study of tumors in salivary
gland, breast, lung, and cervix. Hum Pathol. 13:916–924. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spencer H: Pathology of the Lung. 4th
edition. Pergamon; Oxford: pp. 968–969. 1985
|
|
4
|
Travis WD, Travis LB and Devesa SS: Lung
cancer. Cancer. 75(1 suppl): 191–202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawashima O, Hirai T, Kamiyoshihara M,
Ishikawa S and Morishita Y: Primary adenoid cystic carcinoma in the
lung: report of two cases and therapeutic considerations. Lung
Cancer. 19:211–217. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsim S, O’Dowd CA, Milroy R and Davidson
S: Staging of non-small cell lung cancer (NSCLC): a review. Respir
Med. 104:1767–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu F, Liu Z, Hou Y, et al: Primary
salivary gland-type lung cancer: clinicopathological analysis of 88
cases from China. J Thorac Oncol. 8:1578–1584. 2013. View Article : Google Scholar
|
|
8
|
Grillo HC and Mathisen DJ: Primary
tracheal tumors: treatment and results. Ann Thorac Surg. 49:69–77.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Debieuvre D, Locher C, Neidhardt AC, et
al: Ten-year evolution in non-small-cell lung cancer according to
sex. Results of the KBP-2010-CPHG study by the College of General
Hospital Respiratory Physicians. Rev Mal Respir. 31:805–816.
2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanematsu T, Yohena T, Uehara T, et al:
Treatment outcome of resected and nonresected primary adenoid
cystic carcinoma of the lung. Ann Thorac Cardiovasc Surg. 8:74–77.
2002.PubMed/NCBI
|
|
11
|
Kurul IC, Demiroz SM, Celik A, et al:
Primary pulmonary adenoid cystic carcinoma: report of two cases.
Asian Cardiovasc Thorac Ann. 20:604–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu J, Oda M, Matsumoto I, et al:
Clinicopathological study of surgically treated cases of
tracheobronchial adenoid cystic carcinoma. Gen Thorac Cardiovasc
Surg. 58:82–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue H, Iwashita A, Kanegae H, et al:
Peripheral pulmonary adenoid cystic carcinoma with substantial
submucosal extension to the proximal bronchus. Thorax. 46:147–148.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitada M, Ozawa K, Sato K, et al: Adenoid
cystic carcinoma of the peripheral lung: a case report. World J
Surg Oncol. 8:742010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagao T, Sato E, Inoue R, et al:
Immunohistochemical analysis of salivary gland tumors: application
for surgical pathology practice. Acta Histochem Cytochem.
45:269–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moran CA, Suster S and Koss MN: Primary
adenoid cystic carcinoma of the lung. A clinicopathologic and
immunohistochemical study of 16 cases. Cancer. 73:1390–1397. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
An J, Park S, Sung SH, et al: Unusual
expression of thyroid transcription factor 1 and napsin A in
metastatic adenoid cystic carcinoma of extrapulmonary origin in the
lung. Am J Clin Pathol. 141:712–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prommegger R and Salzer GM: Long-term
results of surgery for adenoid cystic carcinoma of the trachea and
bronchi. Eur J Surg Oncol. 24:440–444. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Regnard JF, Fourquier P and Levasseur P:
Results and prognostic factors in resections of primary tracheal
tumors: a multicenter retrospective study. The French Society of
Cardiovascular Surgery. J Thorac Cardiovasc Surg. 111:808–814.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maziak DE, Todd TR, Keshavjee SH, et al:
Adenoid cystic carcinoma of the airway: thirty-two-year experience.
J Thorac Cardiovasc Surg. 112:1522–1532. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suemitsu R, Okamoto T, Maruyama R, et al:
A long-term survivor after aggressive treatment for tracheal
adenoid cystic carcinoma: a case report. Ann Thorac Cardiovasc
Surg. 13:335–337. 2007.PubMed/NCBI
|
|
22
|
Dahse R, Driemel O, Schwarz S, et al:
Epidermal growth factor receptor kinase domain mutations are rare
in salivary gland carcinomas. Br J Cancer. 100:623–625. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Macarenco RS, Uphoff TS, Gilmer HF, et al:
Salivary gland-type lung carcinomas: an EGFR immunohistochemical,
molecular genetic, and mutational analysis study. Mod Pathol.
21:1168–1175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhattacharyya T, Bahl A, Kapoor R, et al:
Primary adenoid cystic carcinoma of lung: a case report and review
of the literature. J Cancer Res Ther. 9:302–304. 2013. View Article : Google Scholar : PubMed/NCBI
|