Introduction
Hepatocellular carcinomas (HCCs) are potentially
life-threatening (1), whose
prognosis is dependent on the tumor stage at the time of diagnosis
and the possibility of providing radical treatment. Thanks to the
development of clinically based staging systems such as the
Barcelona Clinical Liver Cancer (BCLC) classification, which takes
into account parameters as liver functionality (often as a
consequence of underlying liver cirrhosis; Child-Pugh score), tumor
burden (number and invasiveness), clinical performance and divides
patients into very early/early, intermediate, advanced, and
end-stage, the life expectancy of HCC patients can be reliably
predicted and appropriate treatment may be identified (2). According to the classification system,
some of the treatments including surgical resection, liver
transplantation, percutaneous local radio frequency ablation,
transarterial chemoembolization, palliative care are chosen
(2). Recently the multikinase
inhibitor sorafinib has been approved for use in patients with
advanced HCC, for whom no therapy was previously available
(3).
Spontaneous necrosis of a malignant tumor is rare,
with an incidence of one case per 6,000–100,000 individuals
(4). In cases with large HCC,
spontaneous massive necrosis is common. It has been reported that
cases of advanced necrosis that exhibit a necrosis rate of ≥90%
within the HCC lesion account for ~2% of all HCCs (5). However, only 12 cases have been
reported to not develop necrosis due to partial hepatectomy, or the
pathological anatomy revealed that the patients did not possess HCC
(1,6–16).
Spontaneously necrotized HCC is considered to be a rare condition.
Several mechanisms of spontaneous necrosis of HCC have been
proposed, including a reduced blood supply to the cancer (such as
capsule formation and arterial thrombosis), rapid tumor growth,
immunological reaction (10),
abstinence from drinking (17) and
the use of herbal medicine (18).
The present study reports the case of a patient with a
spontaneously necrotized HCC, including its pathogenic
mechanism.
Case report
The present study reports the case of a 68-year-old
male with alcoholic liver disease who was regularly treated at
Tokyo Rosai Hospital (Tokyo, Japan). A 30 mm tumor was observed in
the S2 liver segment upon abdominal contrast-enhanced computed
tomography (CT), which was performed prior to admission. In
addition, dysphagia had commenced three months prior and a weight
loss of ~3 kg was observed. Therefore, the patient was admitted to
Tokyo Rosai Hospital due to the presence of a hepatic tumor and in
order to examine the cause of the tumor.
The medical history of the patient included the
implantation of a pacemaker, due to sick sinus syndrome, in 2011.
The patient had consumed 1800 cc of Japanese sake per day (200 g
ethanol/day) for 45 years, but had completely stopped this intake
three years prior to the current presentation. The patient was 159
cm in height and weighed 48.3 kg, with a body mass index of 19.1,
blood pressure of 109/69 mmHg, pulse rate of 79 beats/min and a
body temperature of 37.3°C. Examination of the palpebral
conjunctiva did not reveal anemia, and jaundice was not observed in
the bulbar conjunctiva. Neither cardiac nor pulmonary murmurs were
observed in the chest. The abdomen was flat and soft, with no pain
upon the administration of pressure, and bowel sounds were normal.
The liver and spleen were not palpated. There was no edema in the
lower extremities and the superficial lymph nodes were not
palpated. Spider angioma and palmar erythema were not observed.
Upon blood testing, the blood platelet count was found to be
13.0×104/μl (normal range, 14.0–37.9×104/μl)
and a low Na+ level of 133 mEq/l (normal range, 135–147
mEq/l) was identified, while the level of protein induced by
vitamin K absence or antagonists II (PIVKA-II) was elevated, with a
value of 427 milli-arbitrary units (mAu)/ml (normal level, <40
mAu/ml). No elevation in the levels of hepatic-cystic system
enzymes or α-fetoprotein (AFP; 1.5 ng/ml; normal level, <10
ng/ml) were observed. No inflammatory or coagulating system
abnormalities were observed, and no abnormalities were identified
in the hepatitis B virus surface antigen (HBs-Ag) or hepatitis C
virus antibody (HCV-Ab) levels (Table
I). A tumor 30 mm in size was observed in the S2 liver segment
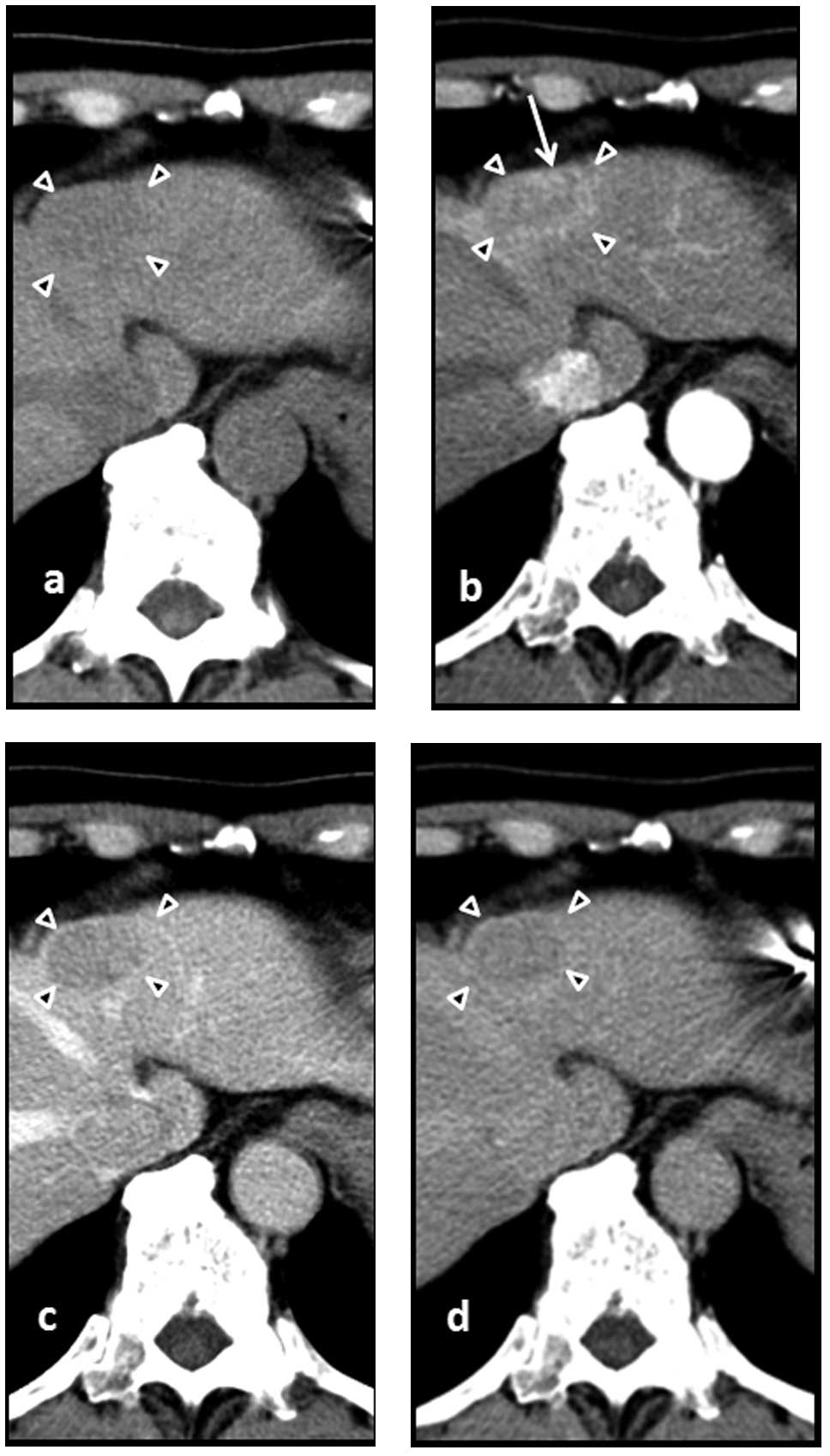
upon abdominal contrast-enhanced CT, performed at the time of
admission, which indicated a high density in the arterial phase and
a low density in the portal and delayed phases (Fig. 1). Upon abdominal ultrasound, the
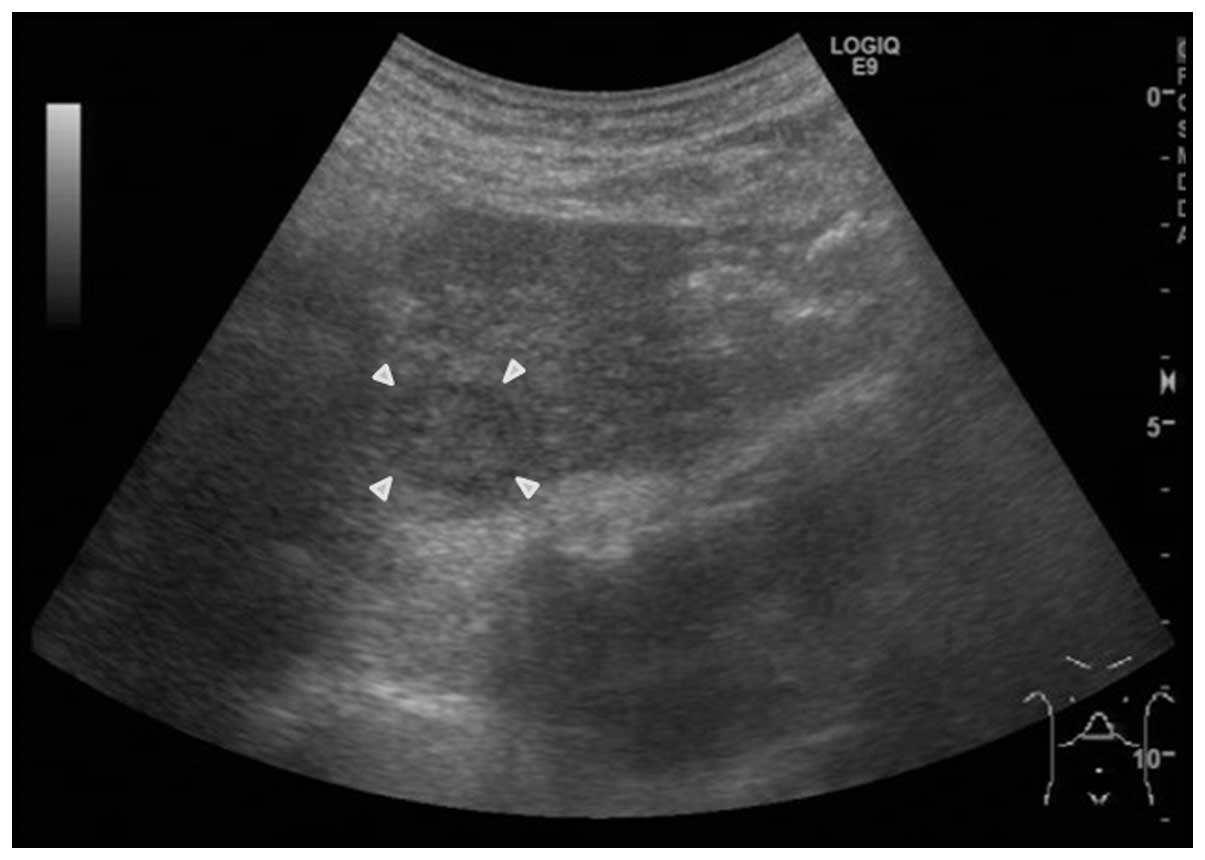
tumor was found to be slightly poorly defined and was a 30 mm low
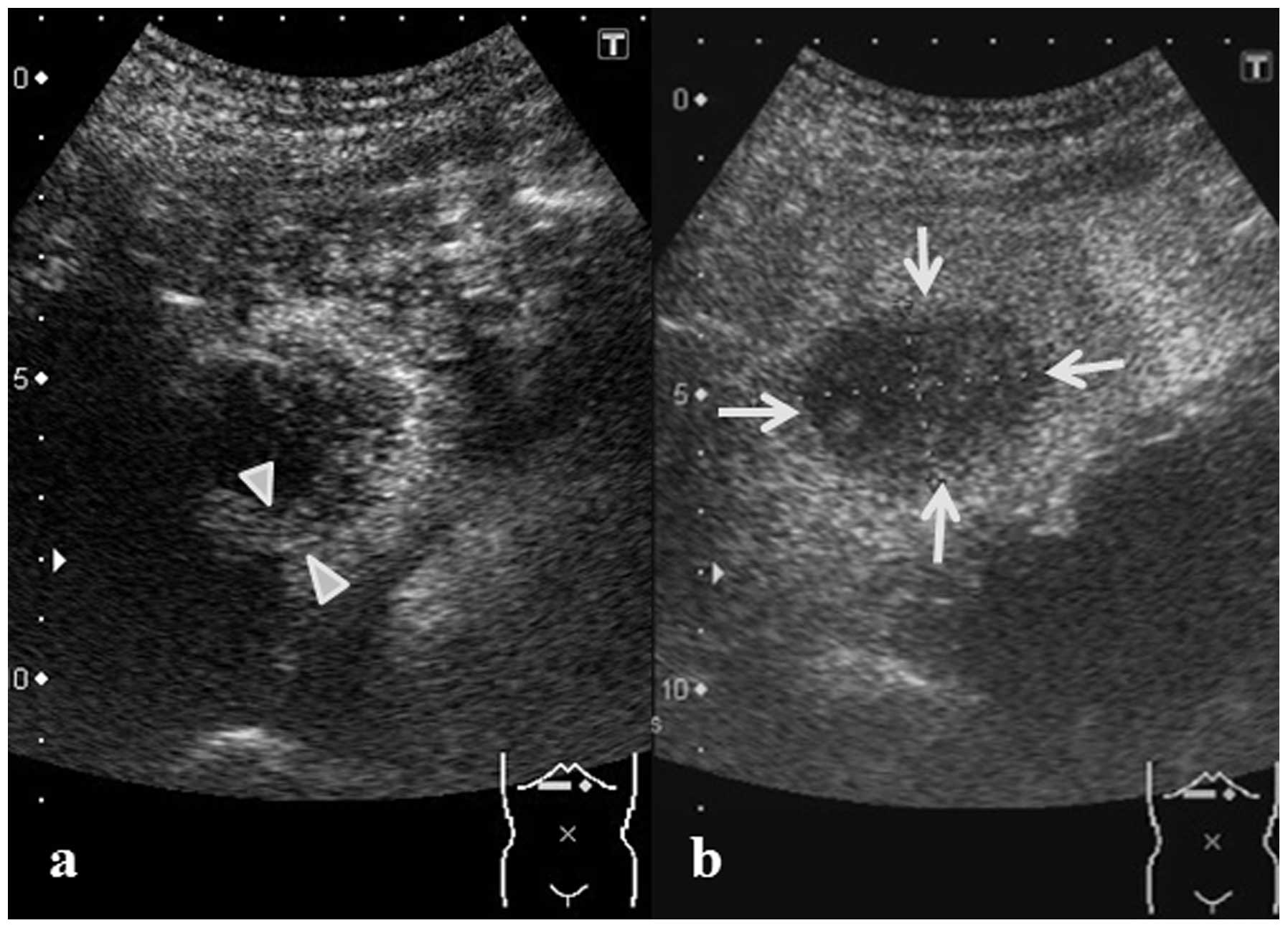
echoic nodule that was internally heterogeneous (Fig. 2). A 5-mm thick region of enhancement
was observed in the tumor border in the vascular phase upon
Sonazoid contrast-enhanced ultrasonography. However, no enhancement
was observed in the center of the lesion, while a defect in the
entire tumor was observed in the post-vascular phase (Fig. 3).
 | Table IBlood laboratory findings on
admission. |
Table I
Blood laboratory findings on
admission.
| Diagnostic blood
tests | Result |
|---|
| Biochemistry |
| CRP | 0.2 mg/dl |
| Na | 133 mEq/l |
| K | 4,1 mEq/l |
| Cl | 93 mEq/l |
| TP | 7.3 g/dl |
| Alb | 4.2 g/dl |
| T-Bil | 0.5 mg/dl |
| AST | 30 IU/l |
| ALT | 38 IU/l |
| LDH | 139 IU/l |
| ALP | 243 IU/l |
| GGT | 50 IU/l |
| LDL-C | 78 mg/dl |
| HDL-C | 56 mg/dl |
| TG | 70 mg/dl |
| BUN | 8 mg/dl |
| Cr | 0.73 mg/dl |
| PT | 88% |
| PT-INR | 1.07 |
| Hematology |
| WBC | 6000/μl |
| RBC |
421×104/μl |
| Hgb | 14.4 g/dl |
| Hct | 41.3% |
| PLT |
13.0×104/μl |
| Serology |
| HCV-Ab | 0.1 COI |
| HBs-Ag | 0.02 IU/l |
| HBc-Ab | - |
| ANA | 40 |
| AMAM2 | <0.5 |
| Tumor markers |
| CEA | 1.5 ng/ml |
| CA19-9 | 2 U/ml |
| AFP | 1.5 ng/ml |
| PIVKA II | 427 mAU/ml |
Nutrition therapy was commenced immediately
following admission by combining a dysphagia diet with tube
feeding. Although a temporary inflammatory reaction and
acceleration of the fibrinolytic system were identified on blood
testing performed on post-admission day 8, with a D-dimer level of
1.9 mg/dl and a C-reactive protein (CRP) level of 8.1 mg/dl, these
improved naturally. As a result of a subsequent in-depth
inspection, the cause of dysphagia was identified as progressive
bulbar paralysis. However, the bulbar paralysis was not considered
to be associated with the carcinoma.
The AFP level increased slightly to 1.8 ng/ml and
the PIVKA-II decreased by 41.0 mAU/ml, as observed on blood testing
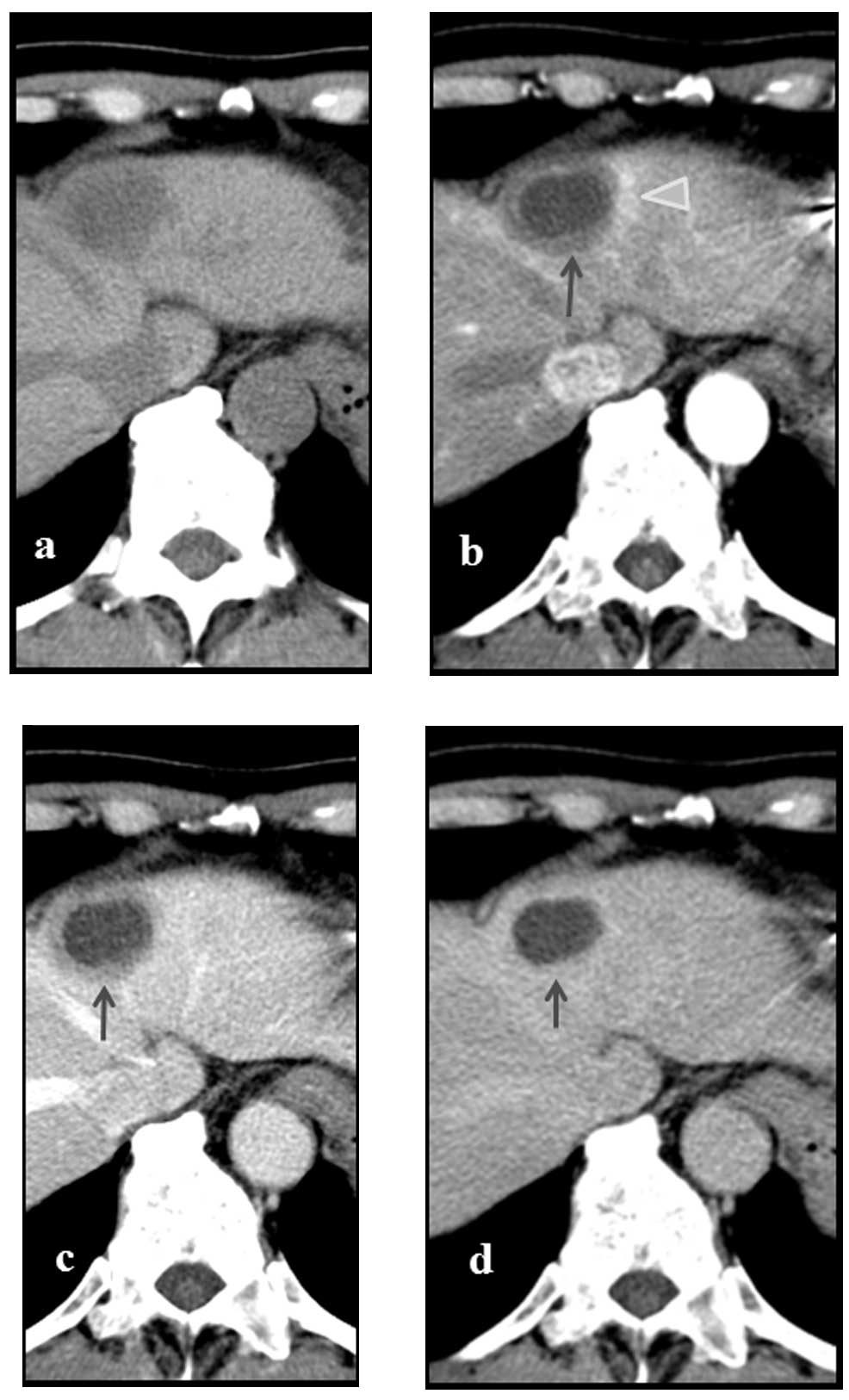
on post-admission day 23. An additional abdominal contrast-enhanced
CT was performed on post-admission day 29, revealing no change in
the tumor size, which remained at ~30 mm. However, the contrast
enhancement of the tumor observed at the time of admission was no
longer present, and a faint contrast enhancement that was 5 mm in
width was instead observed surrounding the tumor. Additionally, a
5-mm wide deeply-stained region, hypothesized to be an
arterio-portal (A-P) shunt was observed in the vicinity of the
tumor (Fig. 4). The vascular and
post-vascular phases of Sonazoid contrast-enhanced ultrasonography
(Sonazoid perfluorobutane; GE Healthcare, Oslo, Norway), performed
on post-admission day 30, revealed no enhancement in the tumor, but
a defect ~30 mm in size was observed (Fig. 5). Necrosis of the tumor was
suspected. However, as viable persistence of the malignant tumor
could not be excluded, a hepatic left lobe excision was performed
on post-admission day 43.
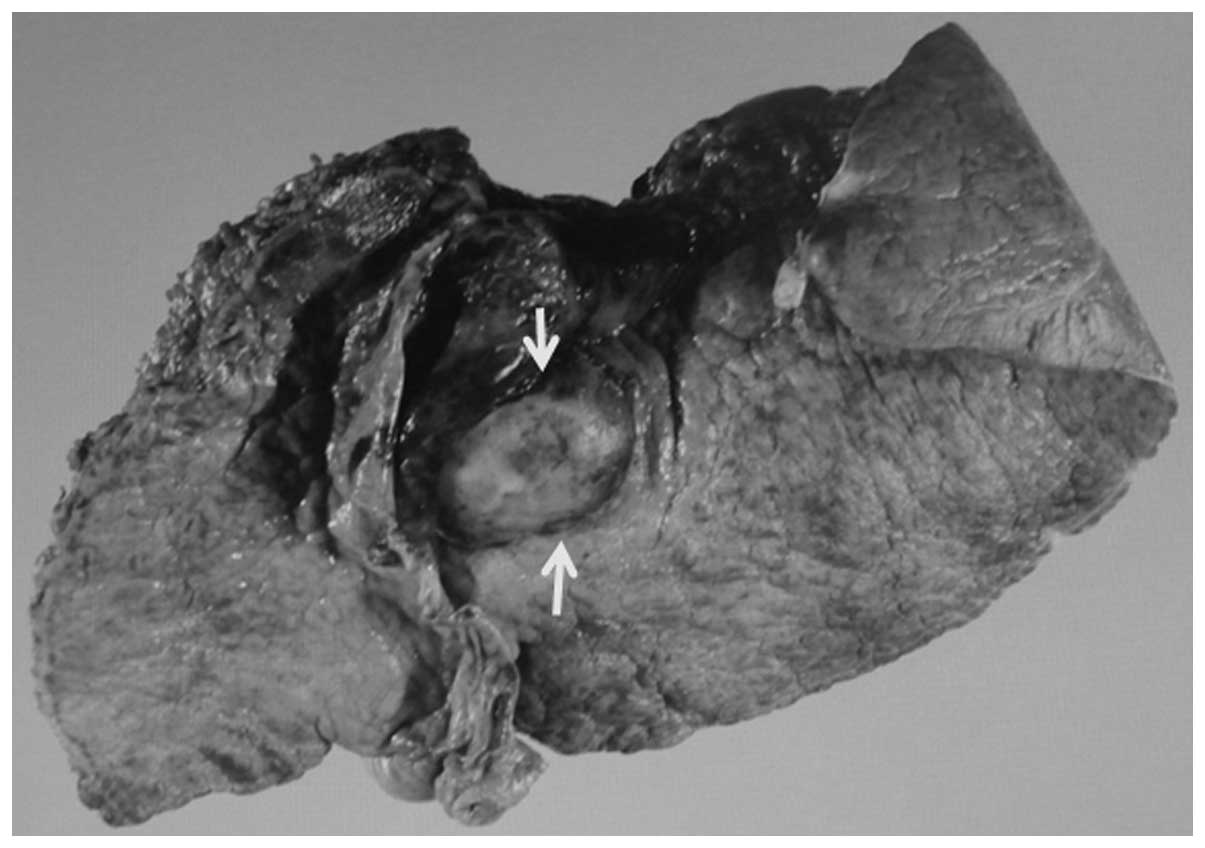
A visual inspection of the excised specimen revealed
a 25×18 mm well-defined white-colored circular tumor in the S2
liver segment (Fig. 6).
Pathological imaging revealed the formation of a
fibrous capsule ~1 mm in size surrounding the tumor, and the entire
region within the tumor was necrotized. As cellular necrosis is
considered to be a valvate-arrangement of oval cells, which was
observed in a region of the present tumor, the resulting diagnosis
was HCC in which necrosis had commenced. Outside the fibrous
capsule, ~3 mm of fibrous tissue was observed. Occlusion due to
thrombus was observed within the blood vessels passing through the
fibrous capsule. The background liver disease was chronic hepatitis
(Fig. 7).
Eight months subsequent to admission, no re-increase
of the tumor marker was observed and no recurrence of HCC was
identified in the follow-up CT.
Discussion
Although a pre-operative histological diagnosis was
not performed for the present patient, HCC was suspected due to the
presence of alcoholic liver disease, which was the causative factor
of HCC, an increased PIVKA-II value and the tumor exhibiting a high
density in the arterial phase and a low density in the portal and
delayed phases of abdominal contrast-enhanced CT, which was
performed at the time of admission. The possibility of a metastatic
hepatic tumor and cholangiocarcinoma was considered. However,
findings that indicated the presence of a malignancy other than a
hepatic tumor were not observed upon close systematic inspection,
and as no expansion of the intrahepatic bile duct was observed in
the vicinity of the tumor, the possibility of a different
malignancy was considered to be low. The contrast enhancement at
the tumor border observed upon contrast-enhanced CT at admission
was not observed at all on the CT performed on the post-admission
day 29 and necrosis within the tumor was suspected. However, the
persistence of cancer could not be ruled out. As a result, and due
to the cellular necrosis being considered to be a
valvate-arrangement of oval cells, which was observed within the
fibrous capsule, a diagnosis of HCC in which necrosis was
histopathologically caused was made. In addition, the entire tumor
was necrotized.
The possible causes of spontaneous necrosis of HCC
include hepatic circulation impairment due to massive bleeding or
shock (6,19), damage to the lining fibrous capsule
of the artery supplying the tumor due to catheterization (20), a sudden enlargement of the tumor
(7), a reduced blood supply to the
cancer nodule due to the formation of a fibrous capsule (7), damage to the cancer nodule due to
inflammatory cells, such as lymphocytes, and cytokines, such as
TNF-α (7,9,21),
abstinence from drinking (18), and
the use of herbal medicines (18).
It has been reported that HCCs are generally observed with various
levels of necrosis depending on the post-operative histopathology
(5).
HCC, in which complete necrosis is
histopathologically diagnosed is rare. A search of the literature
on PubMed (National Center for Biotechnology Information, US
National Library of Medicine, Bethesda, MD, USA) was performed
using the keywords ‘spontaneous complete necrosis of hepatocellular
carcinoma AND regression’, and 12 cases published between 1978 and
2012 were found, in addition to the present case (Table II). Not all studies are included in
the table, as the results contained cases diagnosed by only
diagnostic imaging. Upon investigating the reported cases, an
increased number of male patients was found compared with female
patients, namely 11 cases, and the mean age was 67 years old. The
smallest tumor was 30 mm in size and the largest was 130 mm in
size, with a mean of 67 mm. The cause of hepatic disease was HBV in
four cases, HCV in three cases and alcohol in two cases, including
overlapping cases without an apparent tendency, and the background
hepatic disorder was chronic hepatitis in five cases and liver
cirrhosis in four cases, exhibiting no apparent bias. The cause of
the spontaneous necrosis of the tumor was, including overlapping
cases, reported to be the formation of a fibrous capsule in 10
cases, immunological reaction in eight cases, artery occlusion in
five cases, portal vein embolization in one case, massive bleeding
in one case and herbal medicine in one case, with no apparent
tendency, although many cases exhibited overlapping symptoms.
 | Table IIAnalysis of the reported patients with
hepatocellular carcinoma undergoing spontaneous complete
necrosis. |
Table II
Analysis of the reported patients with
hepatocellular carcinoma undergoing spontaneous complete
necrosis.
| Study | Author, Year
(ref) | Patient age | Gender | HBV or HCV | Alcoholic liver
disease | Tumor size, mm | Capsule
formation | Liver disease | Factors involved in
necrosis |
|---|
| 1 | Our case, 2012 | 68 | M | No | Positive | 30 | Positive | CH | A-P shunt,
thrombi |
| 2 | Yokoyama et
al, 2012 (11) | 80 | M | No | ND | 68 | Positive | ND | Imune, thrombi |
| 3 | Maejima et al,
2011 (10) | 68 | M | HCV | Positive | 100 | Positive | CH | Imune |
| 4 | Arakawa et al,
2008 (12) | 78 | F | HBV | No | 30 | Positive | ND | Imune |
| 5 | Ohta et al,
2005 (1) | 74 | M | No | ND | 60 | Positive | ND | Imune, thrombi |
| 6 | Li et al,
2003 (13) | 53 | M | HBV | No | 30 | ND | LC | Imune |
| 7 | Iiai et al,
2003 (14) | 69 | M | HCV | ND | 40 | Positive | CH | Portal vein
thrombi |
| 8 | Morimoto et
al, 2002 (15) | 73 | M | No | Positive | 100 | Positive | CH | Thrombi |
| 9 | Izuishi et
al, 2000 (7) | 50 | M | HCV | ND | 40 | Positive | CH | Imune |
| 10 | Ozeki et al,
1996 (16) | 69 | F | ND | No | 30 | Positive | LC | Imune |
| 11 | Markovic et
al, 1996 (9) | 62 | M | HBV | No | 130 | Positive | LC | Imune |
| 12 | Gaffy et al,
1990 (6) | 63 | M | No | No | 100 | ND | LC | Herbs,
bleeding |
| 13 | Andreola et
al, 1987 (8) | 75 | M | HBV | ND | 120 | Positive | ND | Thrombi |
In the present study, occlusion of the artery
supplying the carcinoma, due to the formation of thrombus, and the
formation of a fibrous capsule were observed, causing declined
blood flow to the tumor. This resulted in rapid complete
spontaneous necrosis of the tumor. Although the cause of the
thrombus formation is unknown, the present patient exhibited a
dehydrating tendency due to dysphagia resulting from progressive
bulbar paralysis, and was in a state prone to the formation of
thrombus due to undergoing pacemaker implantation. Overall, these
factors were surmised to be the causes of the hepatic tumor. A
temporary inflammatory reaction was observed on post-admission day
8, indicated by a CRP level of 8.1 mg/dl, and it was hypothesized
that necrosis of the tumor progressed over the same period.
Although no uniform conclusion has been obtained
regarding the treatment of cases with complete spontaneous necrosis
of HCC, relapse of spontaneously necrotized HCC has been reported
(22) and viable persistence of the
tumor cells has also been often observed, despite advanced necrosis
being diagnosed in imaging examinations (5). It is hypothesized that the same
treatment should be administered to patients with advanced necrosis
as is administered to those with general HCC, while taking into
consideration the possibility of persisting tumor cells.
In the present study, a patient with HCC that
developed spontaneous complete necrosis was admitted to the Tokyo
Rosai Hospital, with the diagnosis being confirmed by surgical
resection and pathological evaluation. The cause of the complete
spontaneous necrosis was surmised to be occlusion of the blood
vessel supplying the tumor, due to the formation of thrombus, along
with the formation of a thick fibrous capsule and large ischemia
generated in the tumor. This phenomenon provides a valuable insight
into spontaneous complete necrosis of HCC.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
CT
|
computed tomography
|
|
PIVKA- II
|
Protein induced by vitamin K absence
or antagonists II
|
|
A-P
|
arterio-portal
|
References
|
1
|
Ohta H, Sakamoto Y, Ojima H, et al:
Spontaneous regression of hepatocellular carcinoma with complete
necrosis: case report. Abdom Imaging. 30:734–737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Llovet JM: Major achievements
in hepatocellular carcinoma. Lancet. 373:614–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kojiro M and Roskams T: Early
hepatocellular carcinoma and dysplastic nodule. Semin Liver Dis.
25:133–142. 2005. View Article : Google Scholar
|
|
4
|
Cole WH: Efforts to explain spontaneous
regression of cancer. J Surg Oncol. 17:201–209. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto M, Takasaki K, Watayo T, et al:
Spontaneous necrosis due to a hepatocellular carcinoma. Gan No
Rinsho. 37:491–496. 1991.(In Japanese).
|
|
6
|
Gaffy MJ, Joyce JP, Carlson GS and Esteban
JM: Spontaneous regression of hepatocellular carcinoma. Cancer.
65:2779–2783. 1990. View Article : Google Scholar
|
|
7
|
Izuishi K, Ryu M, Hasebe T, et al:
Spontaneous total necrosis of hepatocellular carcinoma: report of a
case. Hepatogastroenterology. 47:1122–1124. 2000.PubMed/NCBI
|
|
8
|
Andreola S, Audisio RA, Mazzaferro V, et
al: Spontaneous massive necrosis of a hepatocellular carcinoma.
Tumori. 73:203–207. 1987.PubMed/NCBI
|
|
9
|
Markovic S, Ferlan-Marolt V and Hlebanja
Z: Spontaneous regression of hepatocellular carcinoma. Am J
Gastroenterol. 91:392–393. 1996.PubMed/NCBI
|
|
10
|
Maejima R, Nakayama H, Horii T, et al: A
case of spontaneous complete necrosis of hepatocellular carcinoma
sized 10 cm in diameter demonstrated by the resected specimen.
Nihon Shokakibyo Gakkai Zasshi. 108:1902–1909. 2011.(In Japanese).
PubMed/NCBI
|
|
11
|
Yokoyama T, Yoshida H, Hirakata A, et al:
Spontaneous complete necrosis of advanced hepatocellular carcinoma.
J Nippon Med School. 79:213–217. 2012. View Article : Google Scholar
|
|
12
|
Arakawa Y, Mori H, Ikegami T, et al:
Hepatocellular carcinoma with spontaneous regression: report of the
rare case. Hepatogastroenterology. 55:1770–1772. 2008.PubMed/NCBI
|
|
13
|
Li AJ, Wu MC, Cong WM, et al: Spontaneous
complete necrosis of hepatocellular carcinoma: a case report.
Heptobilitary Pancreat Dis Int. 2:152–154. 2003.
|
|
14
|
Iiai T, Sato Y, Nabatame N, et al:
Spontaneous complete regression of hepatocellular carcinoma with
portal vein tumor thrombus. Hepatogastroenterology. 50:1628–1630.
2003.PubMed/NCBI
|
|
15
|
Morimoto Y, Tanaka Y, Itoh T, et al:
Spontaneous necrosis of hepatocellular carcinoma: a case report.
Dig Surg. 19:413–418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozeki Y, Matsubara N, Tateyama K, et al:
Spontaneous complete necrosis of hepatocellular carcinoma. Am J
Gastroenterol. 91:391–392. 1996.PubMed/NCBI
|
|
17
|
Tocci G, Conte A, Guarascio P and Visco G:
Spontaneous remission of hepatocellular carcinoma after massive
gastrointestinal hemorrhage. BMJ. 300:641–642. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takayasu K, Marumatsu Y, Shima Y, et al:
Necrosis of hepatocellular carcinoma as a result of subintimal
injury incurred by hepatic angiography: report of two cases. Am J
Gastroenterol. 81:979–983. 1986.PubMed/NCBI
|
|
19
|
Ohba K, Omagari K, Nakamura T, et al:
Abscopal regression of hepatocellular carcinoma after radiotherapy
for bone metastasis. Gut. 43:575–577. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Storey RE, Huerta AL, Khan A and Laber DA:
Spontaneous complete regression of hepatocellular carcinoma. Med
Oncol. 28:948–950. 2011. View Article : Google Scholar
|
|
21
|
Cheng HM and Tsai MC: Regression of
hepatocellular carcinoma spontaneous or herbal medicine related? Am
J Med. 32:579–585. 2004.
|
|
22
|
Lee HS, Lee JS, Woo GW, et al: Recurrent
hepatocellular carcinoma after spontaneous regression. J
Gastroenterol. 35:552–556. 2000. View Article : Google Scholar : PubMed/NCBI
|