Introduction
Endometrial cancer (EC) is one of the most common
gynecological malignancies that affect the health of women
worldwide (1). An increase in the
incidence and morbidity of EC in young women, in whom the
preservation of fertility is of particular importance, indicates
the importance of identifying treatment options for this disease,
as the current standard treatment for EC is total hysterectomy
(2). Therefore, the pathogenetic
mechanism of EC has been the subject of extensive research efforts
in previous decades. The tumorigenesis of EC is a complicated
process that involves multiple factors, stages and gene mutations.
Numerous factors or processes involved in the pathogenesis of EC
remain unidentified, although several factors closely associated
with the occurrence of EC are known, including oncogenes,
anti-oncogenes, estrogen, progestin, estrogen and progestin
receptors, DNA mismatch repair genes and satellite instability
(3,4).
Numb is an evolutionarily conserved developmental
protein that plays a critical role in cell-fate determination and
differentiation. Numb was first identified as a mediator of cell
division in neural progenitors in Drosophila, in which asymmetric
segregation of Numb results in the two daughter cells acquiring
different fates (5). Previous
research has revealed an association between Numb expression and
cancer development (6–11) that involves several important
cellular processes, including cell polarity, cell division and
epithelial to mesenchymal transition (EMT), as well as multiple
signaling pathways, such as Notch, Hedgehog and p53 (8). In addition, numerous proteins have
been identified as binding partners for Numb, including the
Par3-Par6-aPKC complex, E-cadherin, integrin, ligand of
Numb-protein X and the dualoxidase activator/Numb interacting
protein (12–15). As these pathways and proteins are
involved in the onset and metastasis of several malignancies, it is
reasonable to assume that Numb plays a role in cancer
development.
The role of Numb as a tumor suppressor in breast
cancer has been proposed by several studies that reported that the
removal of Numb resulted in reduced TP53 levels and impaired TP53,
apoptosis and DNA-damage checkpoint activation. In addition, the
tumor suppressor role of Numb was demonstrated to be associated
with the formation of the Numb/TP53/MDM2 complex (16). Reduced Numb expression was
correlated with decreased disease-free survival and a higher risk
of developing distant metastases from breast cancer (17). However, the role of Numb in
tumorigenesis differs in other types of tumors. In experimental
glioma, Numb overexpression does not exert a tumor suppressor
function and does not impair cell proliferation in vitro or
induce differentiation of neural or glial cells (18). Additionally, two newly-identified
isoforms of Numb, Numb5 and Numb6, have been revealed to act as
oncogenes (9).
Regardless of its exact function, the Numb protein
has been increasingly associated with tumorigenesis. However, there
are no reports on the role of Numb in EC. In the present study, the
potential association between Numb and EC was investigated and, to
the best of our knowledge, the present study demonstrated for the
first time that Numb plays a role in the development of EC.
Materials and methods
Cell culture and clinical samples
Human embryonic kidney 293T cells were obtained from
the Developmental and Stem Cell Institute of West China Second
University Hospital (Chengdu, Sichuan, China). Human endometrial
HEC-1B cancer cells were obtained from the Gynecological Oncology
Laboratory of West China Second University Hospital. The cell lines
were cultured in Dulbecco’s modified Eagle’s medium-high glucose
(DMEM-HG; Gibco Life Technologies, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (Hyclone, Logan, UT, USA), 40,000 mU/ml
penicillin and 40 μg/ml streptomycin (both Corning Life Sciences -
Mediatech Inc., Manassas, VA, USA), at 37°C in a humidified
incubator with a 5% CO2 atmosphere. Upon reaching 90%
confluence, the cells were dissociated using 0.25% trypsin and
subcultured.
Between August 2008 and March 2009, eight patients
with EC, aged between 37 and 81 years, who had been treated at the
Department of Gynecology and Obstetrics (West China Second
University Hospital), were enrolled in the present study.
Hysterectomy, bilateral salpingo-oophorectomy, lymphadenectomy and
cytological examination of the peritoneal fluid were performed.
Patients who were not found to possess macroscopic lesions during
the procedure and who received a non-EC post-operative pathological
diagnosis, such as cervical cancer, were excluded. For each
patient, normal endometrial and EC tissues were collected and
promptly stored in 4% paraformaldehyde (PFA) subsequent to being
obtained. Patients lacking a normal endometrium were excluded. The
present study was approved by the Medicine Ethics Committee of West
China Second Hospital of Sichuan University. Consent was obtained
from the patients prior to collecting the patient-derived
tissues.
Immunohistochemistry
The tissue was fixed in 4% PFA and then embedded in
paraffin. A standard immunohistochemical staining procedure was
performed. Briefly, a deparaffinization series was performed using
xylene and ethanol. Antigen retrieval was achieved by boiling
tissue slides with 0.01 mol/l citric buffer. Hydrogen peroxide was
used to quench the endogenous peroxidase activity. Subsequent to
blocking, the sections were incubated with a polyclonal rabbit
anti-human Numb72 antibody (1:1,000; Developmental and Stem Cell
Institute of West China Second University Hospital) overnight at
4°C. The sections were incubated with the corresponding
biotinylated goat anti-rabbit immunoglobulin (Ig)G secondary
polyclonal antibodies (catalog number, 111-065-003; Jackson
Immunoresearch, West Grove, PA, USA) for 1 h at room temperature
followed by incubation with peroxidase-conjugated streptavidin
(catalog number, 016-030-084; Jackson Immunoresearch) for 30 min at
room temperature and then were stained with 3,3′-Diaminobenzidine.
The stained slides were counterstained with hematoxylin, dehydrated
using alcohol and xylene and mounted in resinous mounting media.
The tissue sections stained with isotype IgG were used as controls.
All the slides stained with the same antibody were processed
simultaneously. The stained tissue slides were analyzed under an
Olympus CKX41 microscope (Tokyo, Japan), and images were captured
by a digital camera DP70, Olympus) and recorded into a
microscope-linked PC computer. Average intensity quantification was
performed using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). Intensity was measured in
three equally divided regions. Average intensity per area was
determined by dividing the sum of all pixel intensities by the
measured area. All compared images were acquired under identical
parameters. The data were expressed as the mean ± standard error of
the mean.
Immunofluorescence (IF) analysis
Cells growing on coverslips were fixed with 4% PFA
for 15 min at room temperature and then washed three times with
PBS. Subsequent to blocking for 30 min, the coverslips were
incubated with a polyclonal rabbit anti-human Numb72 antibody
(Developmental and Stem Cell Institute of West China Second
University Hospital) and mouse anti-human nuclear pore complex
(NPC) proteins monoclonal antibody (1:1,000; catalog number,
MMS-120R; Covance, Inc., Princeton, NJ, USA) overnight at 4°C. The
cells were incubated with the corresponding polyclonal cy2
anti-rabbit (green fluorescence; 1:2,000; catalog number,
111-225-003) and polyclonal cy3 anti-mouse (red fluorescence;
1:2,000; catalog number, 111-165-003) secondary antibodies (Jackson
Immunoresearch) for 1 h at room temperature in the dark, and the
coverslips were mounted in antifade mounting media. The cells were
observed with a confocal laser microscope (LSM510; Zeiss,
Oberkochen, Germany). Images were captured using the LSM510
microscope, and fluorescence intensity quantification was performed
by Image-Pro Plus version 6.0 software. Fluorescence intensity was
measured in six equally divided regions. Average fluorescence
intensity per area was determined by dividing the sum of all pixel
intensities by the measured area. All compared images were acquired
under identical parameters. The data were expressed as the mean and
standard error of the mean.
Immunoblots
Lysates were extracted from five cell lines and
homogenized in lysis buffer [50 mM Tris-HCl, pH7.4; 1% NP-40; 0.25%
sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; and 1 mM PMSF,
supplemented with 1 μg/ml protein inhibitor cocktail (P2714,
Sigma-Aldrich, St. Louis, MO, USA) (prior to use)]. The protein
concentrations were determined by the bicinchoninic acid protein
assay. Equal amounts of protein were separated on a 10% SDS
polyacrylamide gel and transferred to PVDF membranes. Subsequent to
blocking for 1 h, the membranes were incubated with monoclonal
primary rabbit anti-human Numb (1:1,000; catalog number, 2756; Cell
Signaling Technology, Inc., Danvers, MA, USA) and monoclonal mouse
anti-human β-actin (1:2,000; catalog number, sc47778; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) antibodies overnight at 4°C
in blocking buffer, which consisted of Tris-buffered saline
containing 5% skim milk and 0.1% Tween-20 (TBS-T). The primary
antibody-bound membranes were washed three times in TBS-T and
incubated for 1 h at room temperature with horseradish
peroxidase-conjugated secondary polyclonal goat anti-rabbit IgG
antibody (1:3,000; catalog number, ZB2301; Zhongshan Golden Bridge,
Beijing, China) and polyclonal goat anti-mouse IgG antibody
(1:3,000; catalog number, ZB2305; Zhongshan Golden Bridge, Beijing,
China). Immunosignals were visualized using the Immun-Star WesternC
chemiluminescence kit (product number, 170–5070; Bio-Rad
Laboratories, Hercules, CA, USA). Each experiment was repeated
three times. Intensity determination was performed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistics
All statistical tests were performed using SPSS
Statistics, version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Numb expression in normal endometrial and
EC tissues
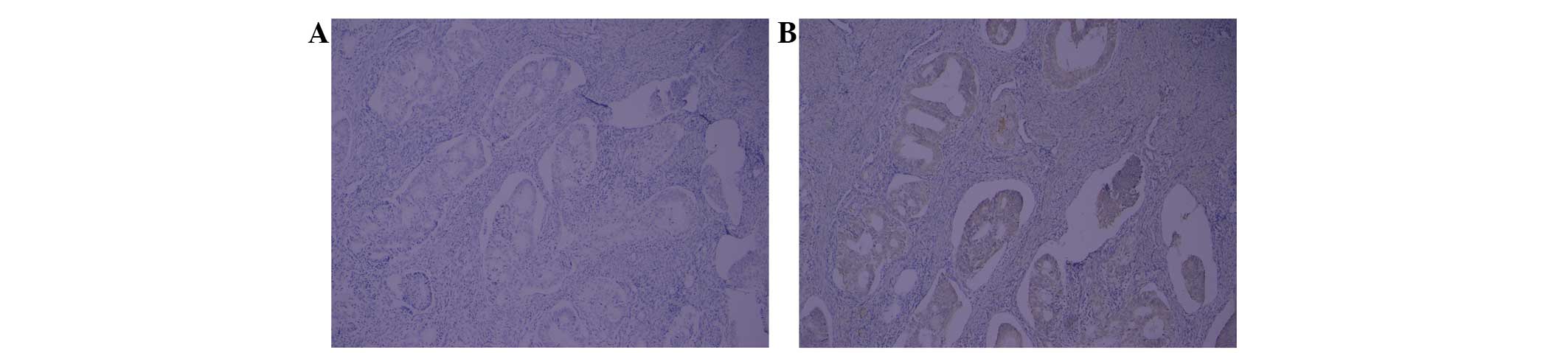
The expression of Numb72, one of four Numb isoforms,
was analyzed by immunohistochemistry (IHC) in the tissues of five
EC patients selected at random, and expression of the protein was
detected in all cases (Fig. 1).
The pathological characteristics of eight patients
with EC are described in Table I.
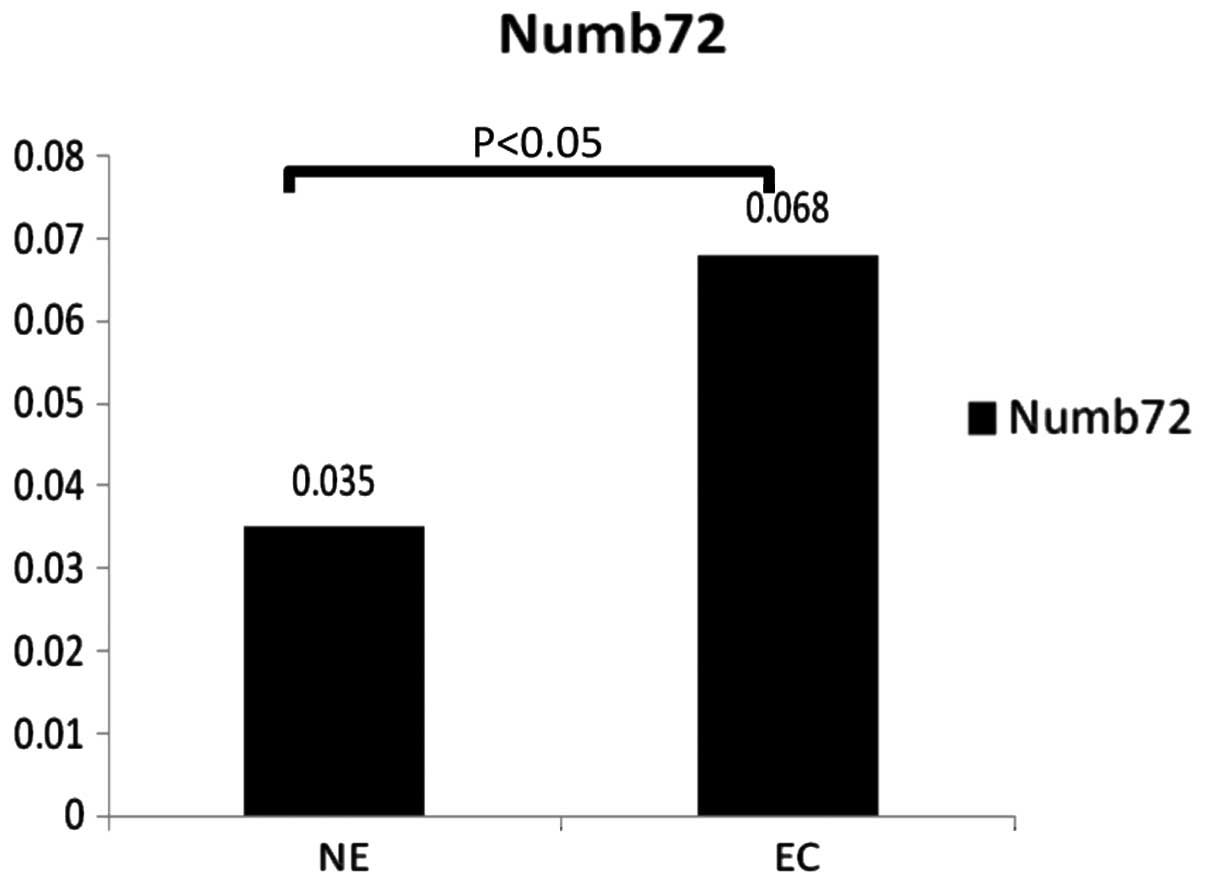
IHC analysis revealed that Numb72 was expressed at higher levels in
EC tissues compared with normal endometrial tissue (0.068±0.036 vs.
0.035±0.01, respectively; P<0.05; Figs. 2 and 3). In addition, the brown particles
demonstrating Numb72 expression were detected with a higher
frequency in the nucleus of EC tissues (Fig. 2).
 | Table IThe pathological characterises of
eight patients with endometrial cancer. |
Table I
The pathological characterises of
eight patients with endometrial cancer.
| Patient | Age, years | Histological
subtype | Stage | Depth of myometrium
invasion | Vascular
metastasis | Lymph node
invasion |
|---|
| 1 | 49 | Medium-low grade
endometroid EC | Ic | Whole | No | No |
| 2 | 81 | Low grade endometroid
EC | Ia | No | No | No |
| 3 | 50 | Medium grade
endometroid EC | Ib | <1/2 | No | No |
| 4 | 58 | Medium-low grade
endometroid EC | Ia | No | No | No |
| 5 | 51 | Low grade endometroid
EC | Ib | <1/2 | No | No |
| 6 | 59 | Low grade endometroid
EC | Ib | <1/2 | Yes | No |
| 7 | 37 | High-medium grade
endometroid EC | Ib | <1/2 | No | No |
| 8 | 56 | Medium-low grade
endometroid EC | Ib | <1/2 | No | No |
Numb72 expression in HEC-1B and 293T
cells
IF detection of Numb72
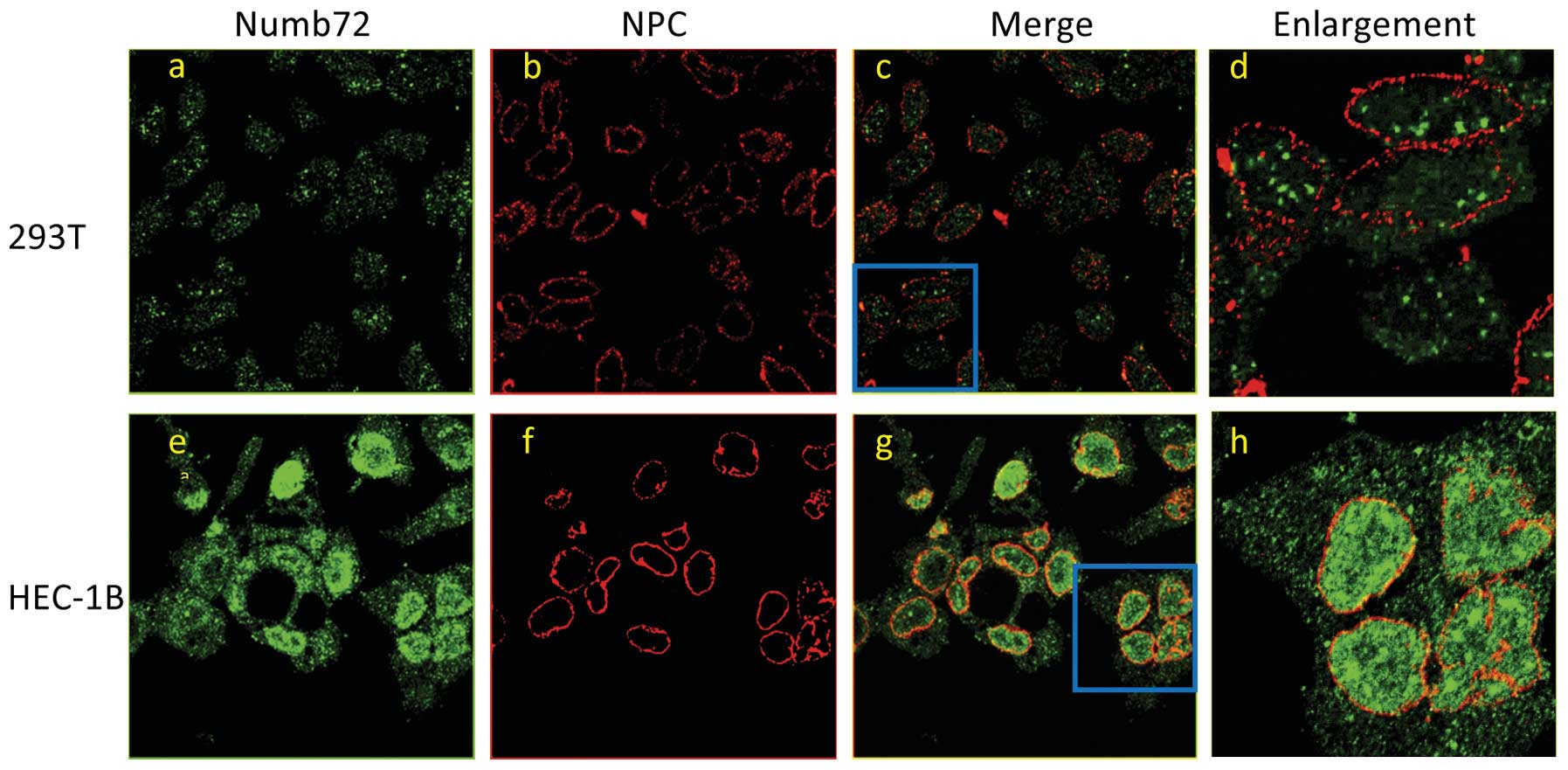
IF and laser scanning confocal microscopy (LSCM)
were used to study the intracellular localization and expression of
Numb in the 293T and HEC-1B cell lines. The results revealed that
Numb predominantly localized to the cytoplasm in 293T cells and the
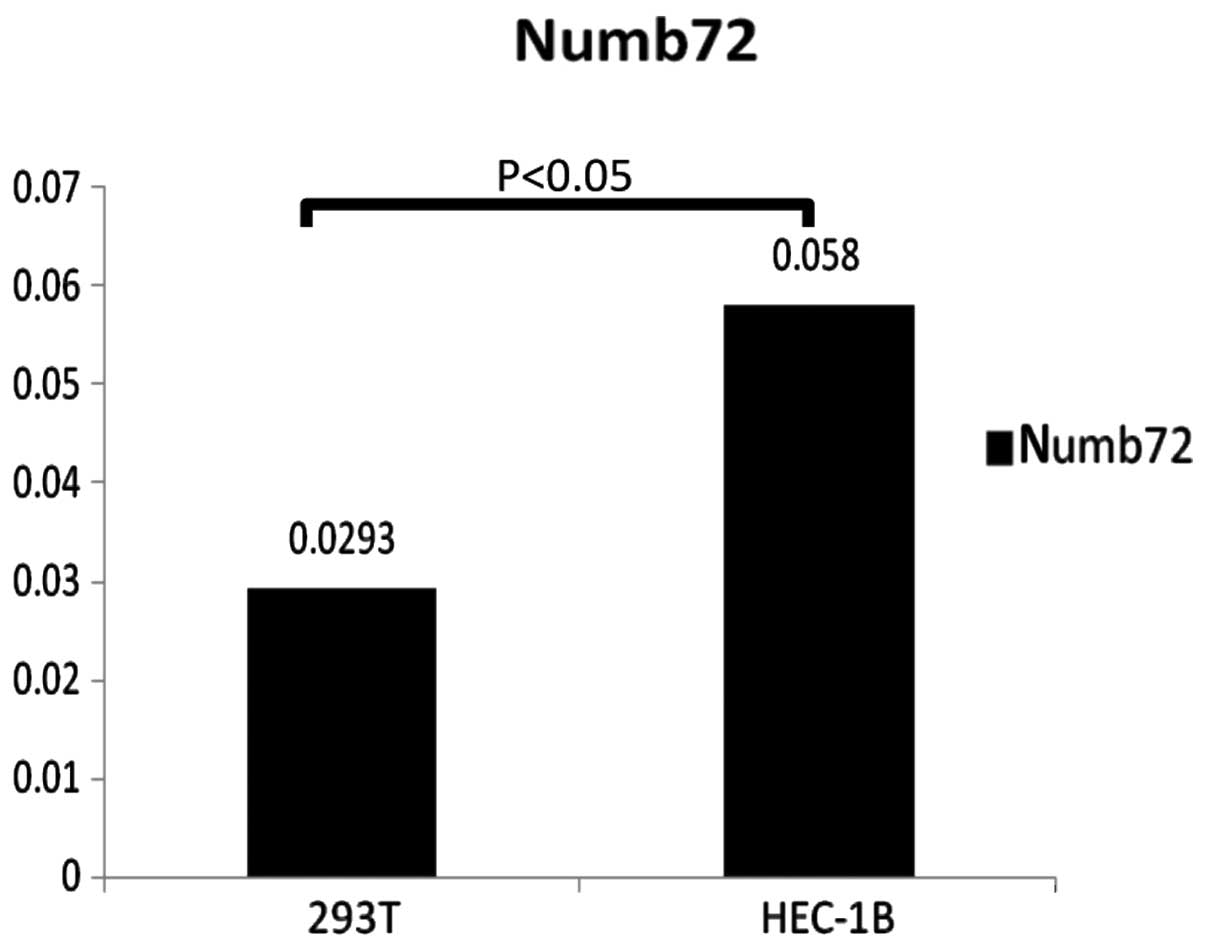
nucleus in HEC-1B cells, and that Numb72 expression levels were
higher in HEC-1B cells compared with 293T cells (0.058±0.004 vs.
0.0293±0.018; P<0.05; Figs. 4
and 5). The cells were probed with
an antibody against NPC proteins that specifically detects the
location of the nuclear membrane to identify the location of Numb,
which confirmed the aforementioned results.
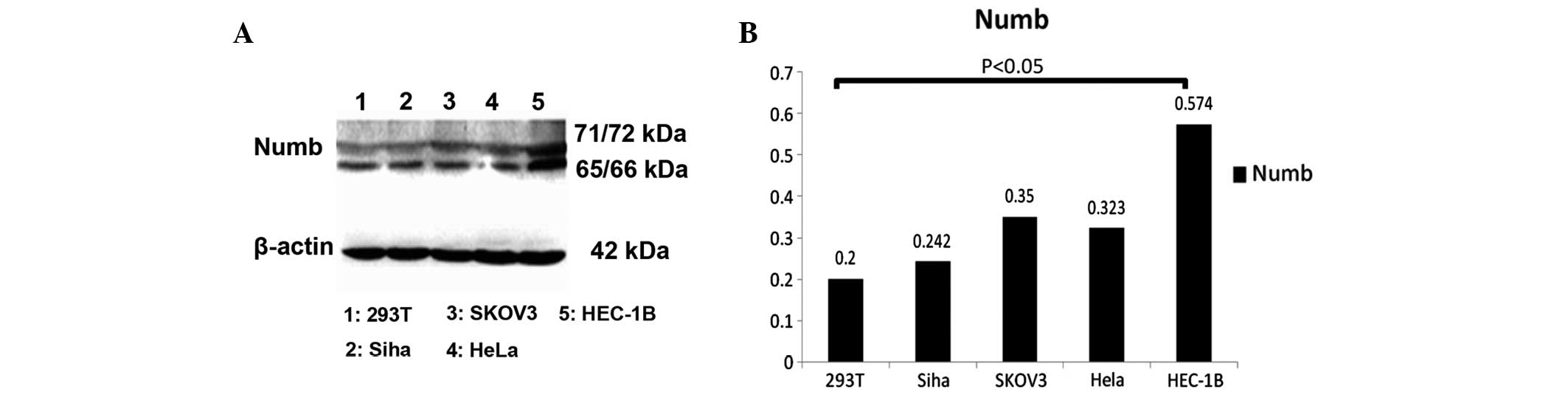
Western-blot analysis of Numb72
Numb72 expression was analyzed by western blotting
in the Siha, SKOV-3, HeLa, HEC-1B and 293T cell lines to determine
whether the levels of the Numb72 protein differ between normal
cells and cancer cells. The results revealed that Numb expression
is highest in HEC-1B cells, with a statistically significant
difference in the levels of the protein between HEC-1B and 293T
cells (0.574±0.17 and 0.198±0.08, respectively; P<0.05; Fig. 6).
Discussion
Mammalian Numb encodes four alternatively spliced
transcripts that generate four proteins ranging between 65 and 72
kDa in size. Numb contains an amino-terminal
phosphotyrosine-binding domain (PTB) and C-terminal proline-rich
region (PRR), comprising putative Src homology 3-binding sites, and
an Eps15 homology (EH) region [DPF; responsible for binding to
alpha-adaptin, and NPF; responsible for binding to the EH domain of
endocytic proteins). The various domains of Numb possess different
functions. The PTB domain is crucial for Numb function as PTB
domains are protein interaction domains, whereas the DPF and NPF
motifs are critical for the role of Numb as an endocytic adaptor
protein. A large 48-amino acid insert in the PRR region
distinguishes the Numb1 and Numb3 isoforms from Numb2 and Numb4,
which lack this sequence. A smaller 11-amino acid insert in the PTB
region also distinguishes Numb1 and Numb2 from Numb3 and Numb4
(19). In the present study, Numb72
was selected since it has been associated with the proliferation
and differentiation of cells, suggesting that Numb may be a
candidate factor involved in the association between Numb proteins
and cancer. Numb72 localizes to the plasma membrane due to the
insertion of 11 amino acids in the PTB region (19–21).
The present results revealed that Numb72 expression
was higher in EC compared with normal endometrial tissue, and
Numb72 localized to the plasma membrane and the nucleus in EC,
which was consistent with the results obtained from cervical cancer
cells (22). In the present study,
the asymmetric distribution of Numb in the apical membrane of cells
was not observed as has been reported in neurons, in which Numb
segregates to the apical daughter cell that remains as a
progenitor. Although the reasons for this discrepancy are not
clear, it may be due to differences in the experimental methods
used or the analysis of the protein at various cell cycle phases,
as Numb was demonstrated to localize to the plasma membrane during
the mitosis phase, while during interphase, Numb is phosphorylated
and distributed into the cytoplasm (23). In addition, the present results
revealed that Numb expression was gradually upregulated in
correlation with the differentiation grade of EC tissue. The
intracellular localization of Numb may be associated with
tumorigenesis and the degree of malignancy of tumors, although
further research with a larger number of clinical samples is
necessary to reach a statistically significant conclusion.
The expression of Numb in HEC-1B and 293T cells was
analyzed by LSCM, which not only confirmed the upregulation of
Numb72 in HEC-1B cells compared with 293T cells, but also revealed
the predominant nuclear localization in HEC-1B cells. The nuclear
localization of Numb72 in HEC-1B cells was confirmed by the
co-localization of Numb72 with NPC proteins in the nucleus. The
present immunohistochemical and IF results clearly indicate that
Numb72 expression is not restricted to the cytoplasm and plasma
membrane as previously reported, but that the protein is also
expressed in the nucleus of EC cells. This may indicate a
translocation of the overexpressed Numb72 protein from the
cytoplasm to the nucleus in EC cells. This result was confirmed in
cells overexpressing Numb72 following transfection with the
pD-RFP-numb72 plasmid (data not shown). The present data suggest
that increased expression of Numb72 may be associated with
tumorigenesis and its nuclear translocation may be a key underlying
mechanism.
Overall, the present results suggest that Numb may
be involved in the pathogenesis of EC. Furthermore, Numb does not
appear to play a protective role in EC, and its nuclear
translocation may represent a novel pathogenetic mechanism of EC
development. As Numb inhibits Notch signaling in the cytoplasm, the
translocation of Numb from the membrane or cytoplasm into the
nucleus may activate Notch signaling (24,25).
The role of Numb in the regulation of p53 activity is not clear.
Colaluca et al reported that Numb protects p53 from
MDM2-mediated degradation, and decreased levels of Numb result in
the downregulation of p53, leading to the occurrence of breast
cancer (16). This study also
reported that Numb forms a tricomplex with p53 and MDM2. However,
in the present study, increased Numb expression and nuclear
translocation in EC cells was correlated with increased p53
expression in the nucleus (data not shown), suggesting that Numb
may act in association with p53 and MDM2 in the nucleus of
endometrial cancer cells in a different manner than in breast
cancer cells. The role of Numb in tumorigenesis should be explored
in further detail in future studies.
Acknowledgements
This study received partial funding by the National
Natural Science Foundation of China (grant no. 81001149) and the
Shanghai Outstanding Youth Training Plan of China (grant no.
XYQ2011062).
References
|
1
|
Kandoth C, Schultz N, Cherniack AD, et al;
Cancer Genome Atlas Research Network. Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalogera E, Dowdy SC and Bakkum-Gamez JN:
Preserving fertility in young patients with endometrial cancer:
current perspectives. Int J Womens Health. 29:691–701. 2014.
|
|
3
|
SGO Clinical Practice Endometrial Cancer
Working Group. Burke WM, Orr J, et al: Endometrial cancer: a review
and current management strategies: part I. Gynecol Oncol.
134:385–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oda K, Stokoe D, Taketani Y, et al: High
frequency of coexistent mutations of PIK3CA and PTEN genes in
endometrial carcinoma. Cancer Res. 65:10669–10673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cayouette M and Raff M: Asymmetric
segregation of Numb: a mechanism for neural specification from
Drosophila to mammals. Nat Neurosci. 5:1265–1269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mine T, Matsueda S, Li Y, et al: Breast
cancer cells expressing stem cell markers CD44+ CD24lo
are eliminated by Numb-1 peptide-activated T cells. Cancer Immunol
Immunother. 58:1185–1194. 2009. View Article : Google Scholar :
|
|
7
|
Nishimoto Y and Okano H: New insight into
cancer therapeutics: induction of differentiation by regulating the
Musashi/Numb/Notch pathway. Cell Res. 20:1083–1085. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pece S, Confalonieri S, Romano PR and Di
Fiore PP: NUMB-ing down cancer by more than just a NOTCH. Biochim
Biophys Acta. 1815:26–43. 2011.
|
|
9
|
Zeng YL, Shao XM and Li HS: Numb
expression in colon cancer and its significance. Sichuan Da Xue Xue
Bao Yi Xue Ban. 43:6–8. 2012.(In Chinese).
|
|
10
|
Rennstam K, McMichael N, Berglund P, et
al: Numb protein expression correlates with a basal-like phenotype
and cancer stem cell markers in primary breast cancer. Breast
Cancer Res Treat. 122:315–324. 2010. View Article : Google Scholar
|
|
11
|
Karaczyn A, Bani-Yaghoub M, Tremblay R, et
al: Two novel human NUMB isoforms provide a potential link between
development and cancer. Neural Dev. 5:312010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng D, Sun Y, Gu S, et al: LNX (Ligand
of Numb-protein X) interacts with RhoC, both of which regulate
AP-1-mediated transcriptional activation. Mol Biol Rep.
37:2431–2437. 2010. View Article : Google Scholar
|
|
13
|
Sato K, Watanabe T, Wang S, et al: Numb
controls E-cadherin endocytosis through p120 catenin with aPKC. Mol
Biol Cell. 22:3103–3119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bogdanović O, Delfino-Machín M,
Nicolás-Pérez M, et al: Numb/Numbl-Opo antagonism controls retinal
epithelium morphogenesis by regulating integrin endocytosis. Dev
Cell. 23:782–795. 2012. View Article : Google Scholar
|
|
15
|
Nishimura T and Kaibuchi K: Numb controls
integrin endocytosis for directional cell migration with aPKC and
PAR-3. Dev Cell. 13:15–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colaluca IN, Tosoni D, Nuciforo P, et al:
NUMB controls p53 tumour suppressor activity. Nature. 451:76–80.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rennstam K, McMichael N, Berglund P, et
al: Numb protein expression correlates with a basal-like phenotype
and cancer stem cell markers in primary breast cancer. Breast
Cancer Res Treat. 122:315–324. 2010. View Article : Google Scholar
|
|
18
|
Euskirchen P, Skaftnesmo KO, Huszthy PC,
et al: NUMB does not impair growth and differentiation status of
experimental gliomas. Exp Cell Res. 317:2864–2873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dho SE, French MB, Woods SA and McGlade
CJ: Characterization of four mammalian numb protein isoforms.
Identification of cytoplasmic and membrane-associated variants of
the phosphotyrosine binding domain. J Biol Chem. 274:33097–33104.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dho SE, Trejo J, Siderovski DP and McGlade
CJ: Dynamic regulation of mammalian numb by G protein-coupled
receptors and protein kinase C activation: Structural determinants
of numb association with the cortical membrane. Mol Biol Cell.
17:4142–4155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verdi JM, Bashirullah A, Goldhawk DE, et
al: Distinct human NUMB isoforms regulate differentiation vs.
proliferation in the neuronal lineage. Proc Natl Acad Sci USA.
96:10472–10476. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Chen X, Ye F, Lu W and Xie X:
Symmetric division and expression of its regulatory gene Numb in
human cervical squamous carcinoma cells. Pathobiology. 76:149–154.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wirtz-Peitz F, Nishimura T and Knoblich
JA: Linking cell cycle to asymmetric division: Aurora-A
phosphorylates the Par complex to regulate Numb localization. Cell.
135:161–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McGill MA, Dho SE, Weinmaster G and
McGlade CJ: Numb regulates post-endocytic trafficking and
degradation of Notch1. J Biol Chem. 284:26427–26438. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gulino A, Di Marcotullio L and Screpanti
I: The multiple functions of Numb. Exp Cell Res. 316:900–906. 2010.
View Article : Google Scholar
|