Introduction
Primary hepatic lymphoma (PHL) is a rare malignancy
with nonspecific clinical features. PHL is defined by liver
involvement at presentation; however, it does not affect the
spleen, lymph nodes, peripheral blood, bone marrow or other tissues
for at least six months following diagnosis (1). Main syptoms that are often exhibited
include hepatic abnormalities in asymptomatic patients leading to
the onset of fulminant hepatic failure with the rapid progression
of encephalopathy leading to a comatose state and death.
Hepatomegaly is also very common, and symptoms of jaundice may be
found upon physical examination. Symptoms are usually nonspecific,
and may include right upper quadrant and epigastric pain, fatigue,
weight loss, fever, anorexia and nausea (2). PHL may occur at any age, the median
age of PHL occurrence is 50 years, with a male to female ratio of
2–3:1 (3–5). Previous studies have indicated that
the prevalence of PHL was 0.4% among extranodal NHL cases and
0.016% among all NHL cases (6,7). The
etiology of PHL remains unclear, however, certain viruses,
including hepatitis C virus (HCV), human immunodeficiency virus
(HIV) and Epstein-Barr virus (EBV) may be involved. HCV infection
is detected in 40–60% of patients with PHL (8–10). The
pathogenesis of PHL remains unclear and may be associated with
hepatitis C virus, human immunodeficiency virus, epstein-barr
virus, or human T-lymphotropic virus infections, liver cirrhosis,
systemic lupus erythematosus, and immunosuppressive therapy
(8). To date, no optimal treatment
method exists for PHL due to the rarity of this malignancy.
However, surgical resectioning, radiotherapy and chemotherapy are
currently available as treatment modalities. In the present study,
the characteristics of PHL and a potential treatment strategy were
evaluated in a patient presenting PHL. Written informed consent was
obtained from the patient.
Case report
In April 2006, an abdominal computed tomography (CT)
scan revealed multiple solid hypodense lesions with a size of 18×18
mm in a 56-year-old male undergoing a routine physical examination
at The Affiliated Cancer Hospital of Zhengzhou University
(Zhengzhou, China). After four months, further CT scans detected an
irregular nodular mass with a size of at least 125×100 mm. The
patient did not present any symptoms, such as fever, respiratory
problems, weakness, anorexia or weight loss. The patient had a
20-year history of hepatitis B virus infection and had been
receiving treatment with lamivudine.
Clinical examination detected hepatomegaly (45 mm)
below the right costal margin, while no other abnormalities were
observed. The liver was nontender, with an irregular surface on the
right side. Viral markers were found to be positive for hepatitis B
and negative for hepatitis C. Fluorescence in situ
hybridization revealed EBV-DNA levels of 5×106
copies/ml. The results of further examinations, including a
baseline electrocardiogram, chest CT scan, complete blood count,
liver function test and kidney function test, were found to be
normal. In addition, the levels of lactase dehydrogenase, uric
acid, calcium, α-fetoprotein, β-2-microglobulin and carcinoma
embryonic antigen were normal. An abdominal and pelvic CT scan
revealed an irregular nodular mass in the right lobe of the liver
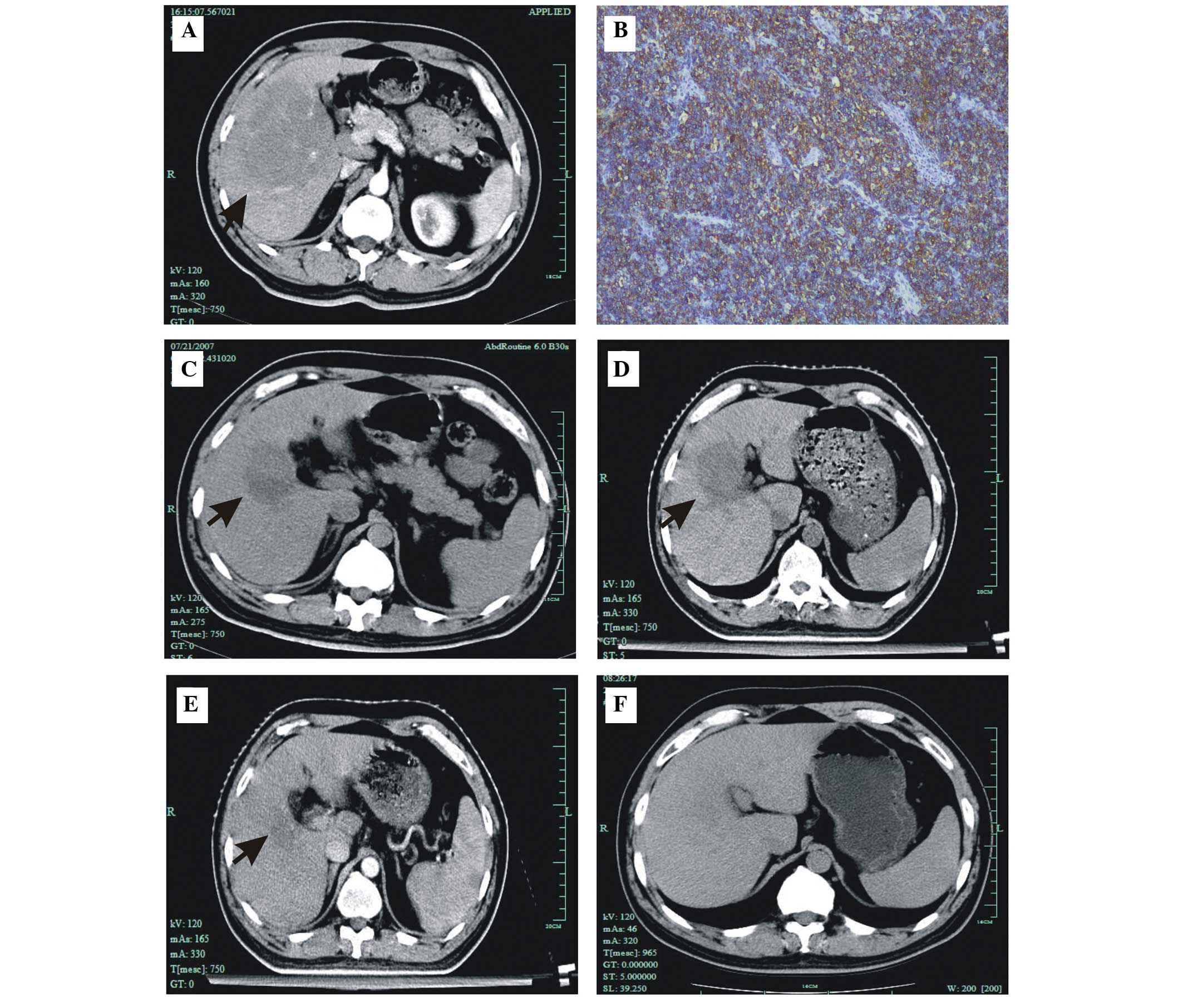
with a size of at least 125×100 mm (Fig. 1A). No other abnormal findings were
detected at the abdomen and pelvis. Furthermore, a chest and neck
CT scan demonstrated normal lung fields and no mediastinal or
paratracheal lymphadenopathy. Bone marrow biopsy identified no
evidence of tumor involvement. Ultimately, a diagnostic liver
biopsy was performed, which revealed sheets and nests of round
cells, surrounded by mature lymphoid cells at the periphery. These
cells were positive for the CD20 and leukocyte common antigen
markers (Fig. 1B). The findings
were consistent with non-Hodgkin’s lymphoma (NHL) of diffuse large
B-cell type. Therefore, the final diagnosis of the patient in the
present study was PHL of diffuse large B-cell type at stage IE,
according to the Ann Arbor staging system, since a single
extranodal site was involved (11).
The patient received R-CHOP chemotherapy, which
involved administration of the following: 375 mg/m2
rituximab [intravenously (IV)] over 6 h on day –1; 750
mg/m2 cyclophosphamide over 3 h, 2 mg vincristine and 50
mg/m2 doxorubicin (IV) on day 1; and 40 mg/m2
prednisolone (orally) on days 1–5, every 21-day cycle. An abdominal
CT scan was performed following two cycles of chemotherapy, which
revealed a marked regression of the lesion to 58×58 mm in the right
lobe of the liver (Fig. 1C).
Following two further cycles of R-CHOP, an abdominal CT scan
detected that the mass was slightly enlarged, having a size of
62×59 mm (Fig. 1D).
Subsequently, the right liver of the patient was
treated with radiotherapy at a dose of 40 Gy over 28 days (2 Gy
fractions, days 1–5). CT scans revealed further regression of the
liver lesion to 26×32 mm following radiotherapy (Fig. 1E). In addition, the patient received
treatment with the R-Hyper-CVAD/R-HD MTX-ara-C regimen, following
radiotherapy. The regimen consisted of two cycles of dose-intensive
therapy courses with rituximab and Hyper-CVAD (R-Hyper-CVAD)
therapy alternating with rituximab and high-dose methotrexate (MTX)
and cytosine arabinoside (ara-C) therapy (R-HD MTX-ara-C). The
R-Hyper-CVAD regimen involved administration of the following: 375
mg/m2 rituximab (IV) on day –1; 300 mg/m2
cyclophosphamide (IV) over 3 h every 12 h for six doses on days
1–3; 300 mg/m2 mesna by continuous infusion initiated
along with cyclophosphamide administration and ending 6 h after the
last dose of cyclophosphamide; 2 mg vincristine (IV) on days 4 and
11; 50 mg/m2 doxorubicin (IV) on day 4; and 40 mg
dexamethasone daily on days 1–4 and 11–14. The R-HD MTX-ara-C
regimen involved administration of the following: 375
mg/m2 rituximab on day 1; 200 mg/m2 MTX (IV)
over 2 h, followed by 800 mg/m2 IV over 24 h on day 1;
15 mg citrovorum factor rescue every 6 h, initiated 24 h after
completion of MTX infusion and increased to 50 mg every 6 h when
MTX levels were >20 mmol/l at the end of the infusion, >1
mmol/l at 24 h after infusion or >0.1 mmol/l at 48 h after
infusion, until MTX levels were <0.1 mM; 3 g/m2 ara-C
over 2 h every 12 h on days 2 and 3; and 50 mg methylprednisolone
(IV) twice daily on days 1 through 3. After three cycles of the
R-Hyper-CVAD/R-HD MTX-ara-C regimen, abdominal CT scans revealed
that the liver lesion disappeared and the patient achieved complete
remission (Fig. 1F). Following
complete remission, the patient was subjected to maintenance
therapy by administration of 375 mg/m2 rituximab on day
1 and every three months thereafter for a total of seven doses. The
patient remained in a stable and healthy condition, with regular
follow-ups for >3 years.
Discussion
Non-Hodgkin’s lymphoma (NHL) is a common malignant
disease, which presents with liver involvement in 10% of patients
at an advanced stage of the disease (12). Primary hepatic lymphoma (PHL) refers
to an extranodal lymphoma of the liver without involvement of any
other organ, such as the lymph nodes or spleen (1). PHL is an unusual form of NHL that
usually presents with constitutional symptoms, hepatomegaly and
symptoms of cholestatic jaundice. Previous studies indicated that
the prevalence of PHL was 0.4% among extranodal NHL cases and
0.016% among all the NHL cases (6,7). The
etiology of PHL remains unknown; however, HCV and human
immunodeficiency virus have been found to be implicated (7,8,13). HCV
infection is detected in 40–60% of patients with PHL (8–10). The
frequent association with HCV indicates that this virus may be
involved in the pathogenesis of PHL (14,15).
Furthermore, PHL is detected in immunocompromised patients;
however, to the best of our knowledge, the association between PHL
occurrence and immune deficiency has not been reported thus far. In
the current study, the patient did not present HCV infection or
immunodeficiency symptoms. The present study demonstrated that HBV
or Epstein-Barr virus infection, which are important etiological
factors for tumorigenesis, were a minor complication of the primary
hepatic B-cell lymphoma case (8).
Therefore, PHL may occur in patients without any prior liver
disease.
To date, no consensus has been reached on the
optimal treatment for PHL. Previous studies have reported the use
of surgical resection, radiotherapy and chemotherapy as treatment
modalities for PHL, alone or in combination (16–18).
For low-volume localized PHL, surgical resection may be an
effective treatment option, alone or in combination with
chemotherapy (1,19,20).
Since relapse following surgery is common and PHL is
chemosensitive, chemotherapy may be employed in all the PHL cases.
A study using transplantation combined chemotherapy conducted at
the MD Anderson Cancer Center between 1974 and 1995 reported an
overall complete remission rate of 83% and a five-year survival
rate of 17% (21). Another study
demonstrated that the five-year survival rate of primary hepatic
diffuse large B-cell lymphoma (DLBCL) cases was 43%, while patients
receiving rituximab along with cytotoxic treatment were
successfully treated (19). In
addition, a study from a group in France demonstrated that 24
HCV-seropositive systemic DLBCL cases exhibited a significantly
lower two-year survival rate (56%) compared with 72 HCV-negative
DLBCL cases (80%; P<0.02) (22).
However, a good prognosis was observed in primary hepatic DLBCL
cases following cytotoxic treatment with or without rituximab
(23,24). In addition, a previous study
revealed that antiviral treatment following cytotoxic treatment
contributed towards a significantly increased disease-free survival
period in 69 HCV-seropositive B-cell lymphoma cases (25). The use of the R-CHOP regimen has
been found to increase the complete remission rate and prolong the
survival significantly (17,24).
These data strongly indicate that rituximab and cytotoxic
treatment, followed by antiviral treatment, may result in an
improved clinical outcome in primary hepatic and systemic
HCV-seropositive DLBCL cases. The case reported in the present
study was found to be sensitive to the R-CHOP regimen. However,
resistance to the treatment was developed and the R-Hyper-CVAD/R-HD
MTX-ara-C regimen was used in combination with radiotherapy.
Complete remission was achieved in the patient. In conclusion, PHL
is a rare disease and thus, at present no standard treatment has
been identified. In the present case, the patient was treated with
R-CHOP, radiotherapy and R-Hyper-CVAD/R-HD MTX-ara-C, and complete
remission was achieved. Follow-up has shown that the patient
remains in a stable condition. Thus, this case indicates that
comprehensive treatment may be beneficial for patients with
PHL.
References
|
1
|
Avlonitis VS and Linos D: Primary hepatic
lymphoma: a review. Eur J Surg. 165:725–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masood A, Kairouz S, Hudhud KH, et al:
Primary non-Hodgkin lymphoma of liver. Curr Oncol. 16:74–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Resende V, Oliveira TS, Gomes RT, et al:
Primary hepatic lymphoma: A case report. Int J Surg Case Rep.
4:1165–1168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raimondo L, Ferrara I, Stefano AD, et al:
Primary hepatic lymphoma in a patient with previous rectal
adenocarcinoma: a case report and discussion of etiopathogenesis
and diagnostic tools. Int J Hematol. 95:320–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lei KI: Primary non-Hodgkin’s lymphoma of
the liver. Leuk Lymphoma. 29:293–299. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freeman C, Berg JW and Cutler SJ:
Occurrence and prognosis of extranodal lymphomas. Cancer.
29:252–260. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trněný M, Sálková J, Dlouhá J and
Stříteský J: Hepatic involvement in patients with non-Hodgkins
lymphoma. Vnitr Lek. 59:606–611. 2013.(In Czech).
|
|
8
|
Kikuma K, Watanabe J, Oshiro Y, et al:
Etiological factors in primary hepatic B-cell lymphoma. Virchows
Arch. 460:379–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Page RD, Romaguera JE, Osborne B, et al:
Primary hepatic lymphoma: favorable outcome after combination
chemotherapy. Cancer. 92:2023–2029. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohamed M and Fernando R: Diagnostic and
therapeutic quandaries in a patient with primary hepatic lymphoma
and concurrent hepatitis C infection. Indian J Hematol Blood
Transfus. 30(Suppl 1): S394–S397. 2014. View Article : Google Scholar
|
|
11
|
Rosenberg SA: Validity of the Ann Arbor
staging classification for the non-Hodgkin’s lymphomas. Cancer
Treat Rep. 61:1023–1027. 1977.PubMed/NCBI
|
|
12
|
Ma YJ, Chen EQ, Chen XB, et al: Primary
hepatic diffuse large B cell lymphoma: A case report: Primary
hepatic diffuse large B cell lymphoma. Hepat Mon. 11:203–205.
2011.PubMed/NCBI
|
|
13
|
Willenbrock K, Kriener S, Oeschger S and
Hansmann ML: Nodular lymphoid lesion of the liver with simultaneous
focal nodular hyperplasia and hemangioma: discrimination from
primary hepatic MALT-type non-Hodgkin’s lymphoma. Virchows Arch.
448:223–227. 2006. View Article : Google Scholar
|
|
14
|
Agmon-Levin N, Berger I, Shtalrid M,
Schlanger H and Sthoeger ZM: Primary hepatic lymphoma: a case
report and review of the literature. Age Ageing. 33:637–640. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salmon JS, Thompson MA, Arildsen RC and
Greer JP: Non-Hodgkin’s lymphoma involving the liver: clinical and
therapeutic considerations. Clin Lymphoma Myeloma. 6:273–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouliaris K, Christodoulidis G, Koukoulis
G, et al: A primary hepatic lymphoma treated with liver resection
and chemotherapy. Case Rep Surg. 2014:7495092014.PubMed/NCBI
|
|
17
|
Serrano-Navarro I, Rodríguez-López JF,
Navas-Espejo R, et al: Primary hepatic lymphoma-favorable outcome
with chemotherapy plus rituximab. Rev Esp Enferm Dig. 100:724–728.
2008.(In Spanish).
|
|
18
|
Nasr Ben Ammar C, Chaari N, Kochbati L, et
al: Primary non-Hodgkin lymphoma of the liver: case report and
review of the literature. Cancer Radiother. 10:595–601. 2006.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noronha V, Shafi NQ, Obando JA and Kummar
S: Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol
Hematol. 53:199–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lei KI: Primary non-Hodgkin’s lymphoma of
the liver. Leuk Lymphoma. 29:293–299. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avolio AW, Agnes S, Barbarino R, et al:
Post-transplant lymphoproliferative disorders after liver
transplantation: analysis of early and end late cases in a 255
patient series. Transplant Proc. 39:1956–1960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Besson C, Canioni D, Lepage E, et al;
Groupe d’Etude des Lymphomes de l’Adulte Programs. Characteristics
and outcome of diffuse large B-cell lymphoma in hepatitis C
virus-positive patients in LNH 93 and LNH 98 Groupe d’Etude des
Lymphomes de l’Adulte Programs. J Clin Oncol. 24:953–960. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Renzo A, Perna F, Persico M, et al:
Excellent prognosis and prevalence of HCV infection of primary
hepatic and splenic non-Hodgkin’s lymphoma. Eur J Haematol.
81:51–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zafar MS, Aggarwal S and Bhalla S:
Complete response to chemotherapy in primary hepatic lymphoma. J
Cancer Res Ther. 8:114–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
La Mura V, De Renzo A, Perna F, et al:
Antiviral therapy after complete response to chemotherapy could be
efficacious in HCV-positive non-Hodgkin’s lymphoma. J Hepatol.
49:557–563. 2008. View Article : Google Scholar : PubMed/NCBI
|