Introduction
Esophageal cancer is the eighth most common type of
cancer and ranks as the sixth leading cause of cancer-related
mortality worldwide (1,2). In particular, China contains areas of
the highest incidences of esophageal cancer in the world, with
esophageal cancer classified as the sixth most frequently diagnosed
type of cancer (3) and the fourth
most common cause of cancer mortality in China (4). In the previous two decades,
comprehensive therapeutic regimes aimed at improving survival have
been widely applied, however, the overall five-year relative
survival rate is just 16.9% (5) due
to the aggressive nature of this type of malignancy. Currently, the
traditional tumor-node-metastasis (TNM) classification systems of
the International Union Against Cancer (UICC) and American Joint
Committee on Cancer are the most important tools used for
predicting the prognosis of esophageal cancer patients and the only
method used on a routine basis (6).
However, esophageal cancer is highly heterogeneous, and tumors with
the same TNM stage demonstrate differences in clinical course and
treatment response. Thus, the identification of factors to predict
malignant potential and prognosis is of great importance.
Telomeres are unique structures composed of
double-stranded tandem repeats of TTAGGG at the end of a chromosome
(7). Telomerase, a
ribonucleoprotein enzyme, consists of a catalytic protein unit,
human telomerase reverse transcriptase (hTERT), and human
telomerase RNA. Together, the two units synthesize telomeric
sequences that allow tumor cells to escape from cellular senescence
when reactivated or are upregulated by tumor cells to allow
indefinite proliferation (8). The
expression of hTERT closely correlates with telomerase activity and
serves as an indicator of telomerase activation (9,10).
Although previous studies have demonstrated that high hTERT
expression is a predictive and prognostic biomarker of a poor
outcome in a range of malignancies, including Ewing’s sarcoma, and
colorectal, gastric and breast carcinoma (11–14),
studies regarding the role of hTERT expression in esophageal cancer
are sparse and thus warrant further investigation.
The ubiquitin proteasome pathway (UPP) and the
lysosome degradation system are two distinctive proteolytic
mechanisms required for intracellular protein degradation (15). The UPP participates in protein
quality control via the degradation of misfolded proteins (16) in order to regulate basic cellular
activities, such as cell cycle control, the DNA damage response,
apoptosis and tumorigenesis (17).
The functioning of the UPP is achieved by the coordinated action of
a cascade of three enzyme species: Ubiquitin activating enzymes
(E1), ubiquitin-conjugating enzymes (E2) and ubiquitin ligases (E3)
(16,18). Ubiquitin-conjugating enzyme E2D 3
(UBE2D3), also known as UBCH5C, is a member of the E2 family. In
our previous study, a yeast two-hybrid screen was performed to
identify that a mutual association exists between hTERT and the
hTERT-interacting protein UBE2D3; downregulation of UBE2D3 resulted
in the accumulation of hTERT, indicating that UBE2D3 potentially
has a role in the hTERT signaling pathway (19). Thus, the aim of the present study
was to elucidate the clinical significance of hTERT and UBE2D3
expression in esophageal cancer.
Patients and methods
Patients and follow-up
The esophageal cancer tissue specimens were obtained
from patients who had undergone curative surgery (esophagectomy
with lymph node dissection) at the Zhongnan Hospital of Wuhan
University (Wuhan, Hubei, China) between January 2006 and December
2012. No patients had previously received palliative resection,
pre-operative chemotherapy or radiotherapy, and no patients
exhibited synchronous or metachronous cancer in other organs or
succumbed in the month following surgery. All post-operative
specimens were diagnosed with esophageal cancer by two pathologists
and the TNM stage was determined according to the seventh edition
of the UICC TNM system (20).
According to the aforementioned criteria, 150
patients were included in the present study. Follow-up data were
available for all patients on a one to three month basis, the most
recent follow-up date was June 10, 2014, and the median follow-up
time was 37 months (range, 14–90 months). In addition, a total of
91 (60.7%) patients succumbed during the follow-up period. Any
recurrence in the anastomotic mediastinum, esophagus or regional
lymph nodes was defined as local-regional recurrence, and
recurrence identified via blood flow was defined as distant
metastasis, such as liver, lung and bone metastasis. Overall
survival (OS) time was defined as the period between the date of
the surgical procedure and the date of mortality. Furthermore, the
present study was conducted in accordance with the Declaration of
Helsinki of the World Medical Association and the protocols were
approved by the Ethics Committee of Zhongnan Hospital of Wuhan
University, with consent obtained from all patients.
Immunohistochemical analysis and
evaluation
Immunohistochemical staining was performed using the
streptavidin-biotin method to detect hTERT and UBE2D3 protein
expression levels. Firstly, the 4 μm-wide sections of esophageal
cancer and adjacent tissues were incubated at room temperature for
60 min, followed by exposure to dimethylbenzene for 10 min. The
sections were then deparaffinized in 100, 95 and 75% ethyl alcohol
for 5 min, respectively. Next, the tissue samples were microwaved
in citrate buffer (0.1 mol/l, pH 6.0) for 10 min at 1,000 W.
Endogenous peroxidase was blocked by incubating the sections in 3%
hydrogen peroxide for 5 min at room temperature. Subsequent to
washing with distilled water and incubating with goat serum (Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China) for 1 h,
the sections were incubated with primary monoclonal hTERT (cat no.,
ab32020; dilution, 1:100; Abcam, Cambridge, MA, USA) or polyclonal
UBE2D3 (cat no., 11677–1-AP; dilution, 1:50; Proteintech Group,
Inc., Chicago, IL, USA) antibodies at room temperature for 2 h.
Biotinylated secondary goat anti-rabbit antibodies and
peroxidase-conjugated streptavidin obtained from the DAKO Universal
LSAB™ kit (Dako, Glostrup, Denmark) were applied for 20 min each.
Finally, the sections were incubated in 3′3′-diaminobenzidine for 5
min, followed by hematoxylin counterstaining. Two negative controls
were performed by replacing the primary antibody with non-immune
serum and omitting the application of the secondary antibody.
The intensity of the immunostaining was evaluated
using light microscopy. Two independent investigators, who were
blinded to the clinicopathological data, evaluated the levels of
protein expression in each section, and the staining intensity was
scored as follows: No staining, 0; weak staining, 1; moderate
staining, 2; and strong staining, 3. Additionally, the extent of
staining was scored according to the percentage of
positively-stained cells in the entire carcinoma-involved area, as
follows: Absent, 0; sporadic, 1–10%; local, 11–25%; occasional,
26–50%; majority, 51–75%; and large majority, 76–100%. For hTERT
expression, an intensity score of ≥2 and ≥11% of cells with
positive hTERT staining was considered to indicate high expression,
and an intensity score of <2 or <11% of cells with hTERT
positive staining was considered to indicate low expression
(21). By contrast, UBE2D3 protein
expression levels were classified as high when UBE2D3 staining was
present in >50% of cells (22).
Expression in the cytoplasm and nucleus was regarded as an
indication of positive expression for the two proteins (22,23).
Using this method, the expression of hTERT and UBE2D3 was detected
in 150 esophageal cancer and 30 adjacent tissue samples.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The Pearson
χ2 test or Fisher’s exact test were used to compare
qualitative variables. Kaplan-Meier analysis was performed for
univariate analysis and significance among patient subgroups was
calculated using the log-rank test. Furthermore, the Cox
proportional hazard model was used to conduct multivariate analysis
and Spearman correlation coefficients were applied for analyzing
the association between the expression of the two proteins.
Two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Major clinicopathological features and
immunohistochemical findings
Included in the present study were 145 samples of
squamous cell cancer and five other pathological types. The age
range of the patients was 39–85 years (mean ± standard deviation,
60.0±9.42 years). Additional clinical data for the current patients
are presented in Table I.
 | Table IPatient demographics and
clinicopathological characteristics. |
Table I
Patient demographics and
clinicopathological characteristics.
| Characteristic | n (%) |
|---|
| Age, years |
| <60 | 70 (46.7) |
| ≥60 | 80 (53.3) |
| Gender |
| Male | 124 (82.7) |
| Female | 26 (17.3) |
| Location |
| Upper thoracic | 21 (14.0) |
| Middle/lower
thoracic | 129 (86.0) |
| Tumor size, cm |
| <5 | 81 (54.0) |
| ≥5 | 69 (46.0) |
| Histological
gradea |
| G1/2 | 119 (79.3) |
| G3/G4 | 31 (20.7) |
| T stage |
| T1–2 | 65 (43.3) |
| T3–4 | 85 (56.7) |
| N stage |
| N0 | 76 (50.7) |
| N1–3 | 74 (49.3) |
| TNM stage |
| Early (I–II) | 88 (58.7) |
| Advanced
(III–IV) | 62 (41.3) |
| Adjuvant
radiotherapy |
| Yes | 40 (26.7) |
| No | 110 (73.3) |
| Adjuvant
chemotherapy |
| Yes | 70 (46.7) |
| No | 80 (53.3) |
| Recurrence |
| No | 66 (44.0) |
| Yes | 84 (56.0) |
| Recurrence
location |
|
Local-regional | 50 (59.5) |
| Distant | 34 (40.5) |
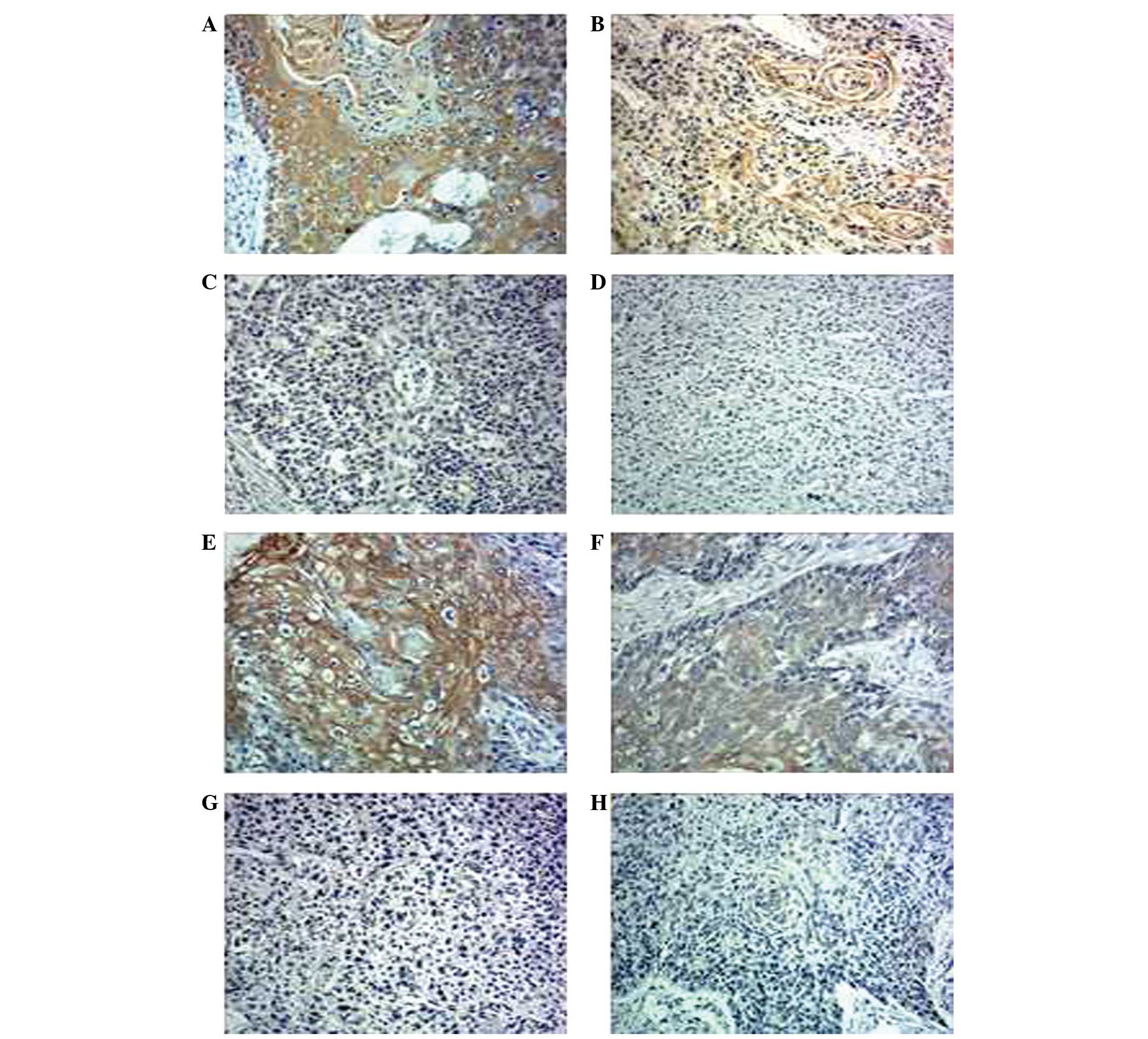
Immunohistochemical analysis of all esophageal
cancer and healthy adjacent tissues was performed. According to the
aforementioned classification system, 11 healthy adjacent tissue
samples with high hTERT expression and 21 with high UBE2D3
expression were identified, compared with 91 esophageal cancer
tissue samples with high hTERT expression and 55 with high UBE2D3
expression. Thus, the expression levels of these two proteins
demonstrated a significant difference between the cancerous and
adjacent tissues (P=0.015 and P=0.001, respectively). Fig. 1A–D shows representative examples of
hTERT expression in esophageal cancer samples and Fig. 1E–H shows representative examples of
UBE2D3 expression. According to the aforementioned staining
intensity and extent scores, Fig. 1A,
B, E and F demonstrated high expression levels, however,
Fig. 1C, D, G and H demonstrated
low UBE2D3 expression levels.
Association between expression levels of
hTERT and UBE2D3, and various clinicopathological features
As summarized in Table
II, hTERT expression was significantly higher in larger-sized,
poorly-differentiated, and advanced T, N and TNM stage tumors
(P=0.039, P=0.032, P=0.030, P=0.018 and P=0.012, respectively).
Furthermore, UBE2D3 expression levels were significantly lower in
poorly-differentiated, advanced N stage and recurrent cases of
esophageal cancer (P=0.007, P=0.016 and P=0.008, respectively).
 | Table IIAssociation between hTERT and UBE2D3
expression levels, and various clinicopathological
characteristics. |
Table II
Association between hTERT and UBE2D3
expression levels, and various clinicopathological
characteristics.
| hTERT
expression | UBE2D3
expression |
|---|
|
|
|
|---|
| Characteristic | Low, n (%)
(n=59) | High, n (%)
(n=91) | P-value | Low, n (%)
(n=95) | High, n (%)
(n=55) | P-value |
|---|
| Age, years | | | 0.067 | | | 0.651 |
| <60 | 33 (47.1) | 37 (52.9) | | 43 (61.4) | 27 (38.6) | |
| ≥60 | 26 (32.5) | 54 (67.5) | | 52 (65.0) | 28 (35.0) | |
| Gender | | | 0.154 | | | 0.811 |
| Male | 52 (41.9) | 72 (58.1) | | 78 (62.9) | 46 (37.1) | |
| Female | 7 (26.9) | 19 (73.1) | | 17 (65.4) | 9 (34.6) | |
| Location | | | 0.900 | | | 0.261 |
| Upper
thoracic | 8 (38.1) | 13 (61.9) | | 11 (52.4) | 10 (47.6) | |
| Middle/lower
thoracic | 51 (39.5) | 78 (60.5) | | 84 (65.1) | 45 (34.9) | |
| Tumor size, cm | | | 0.039 | | | 0.262 |
| <5 | 38 (46.9) | 43 (53.1) | | 48 (59.3) | 33 (40.7) | |
| ≥5 | 21 (30.4) | 48 (69.6) | | 47 (68.1) | 22 (31.9) | |
| Histological
gradea | | | 0.032 | | | 0.007 |
| G1/2 | 52 (43.7) | 67 (56.3) | | 64 (57.1) | 48 (42.9) | |
| G3/4 | 7 (22.6) | 24 (77.4) | | 31 (81.6) | 7 (18.4) | |
| T stage | | | 0.030 | | | 0.154 |
| T1–2 | 32 (49.2) | 33 (50.8) | | 37 (56.9) | 28 (43.1) | |
| T3–4 | 27 (45.8) | 58 (63.7) | | 58 (68.2) | 27 (31.8) | |
| N stage | | | 0.018 | | | 0.016 |
| N0 | 37 (48.7) | 39 (51.3) | | 41 (53.9) | 35 (46.1) | |
| N1–3 | 22 (29.7) | 52 (70.3) | | 54 (73.0) | 20 (27.0) | |
| TNM stage | | | 0.012 | | | 0.347 |
| Early (I–II) | 42 (47.7) | 46 (52.3) | | 53 (60.2) | 35 (39.8) | |
| Advanced
(III–IV) | 17 (27.4) | 45 (72.6) | | 42 (67.7) | 20 (32.3) | |
| Recurrence | | | 0.09 | | | 0.008 |
| No | 31 (47.0) | 35 (53.0) | | 34 (51.5) | 32 (48.5) | |
| Yes | 28 (33.3) | 56 (66.7) | | 61 (72.6) | 23 (27.4) | |
| Recurrence
locationb | | | 0.677 | | | 0.731 |
|
Local-regional | 16 (32.0) | 34 (68.0) | | 37 (74.0) | 13 (26.0) | |
| Distant | 12 (35.3) | 22 (64.7) | | 24 (70.6) | 10 (29.4) | |
Survival analysis
In the 150 cases, the median OS time was 20.0 months
(range, 5–84 months), and the one-, three- and five-year survival
rates were 72.3, 40.7 and 25.0%, respectively. As indicated in
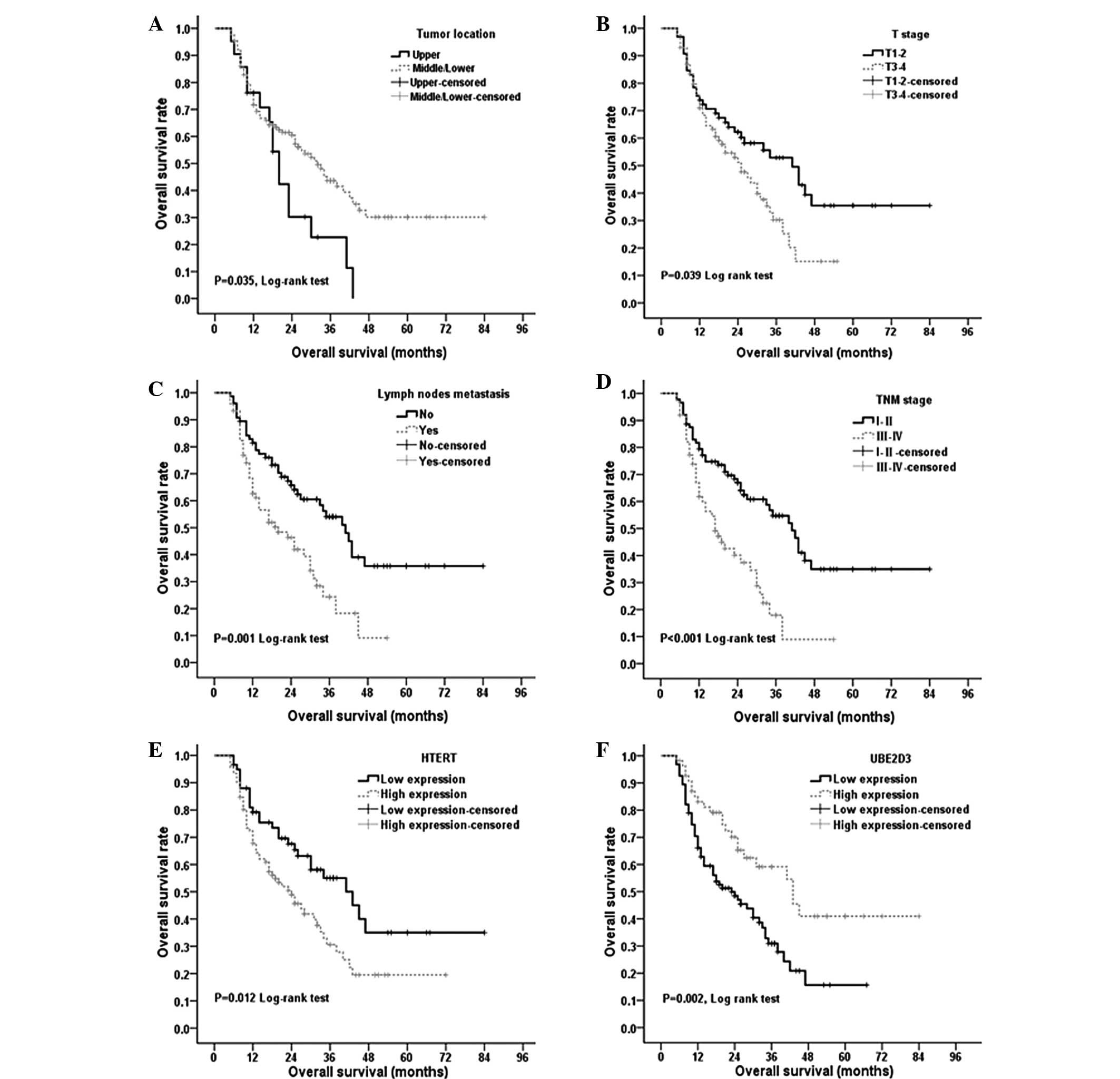
Fig. 2, a number of traditional
factors were associated with the OS time of the esophageal cancer
patients, including tumor location, fibrous membrane invasion,
lymph node status and TNM stage (P<0.05 for all; Fig. 2A–D). In addition, low expression
levels of hTERT and high expression levels of UBE2D3 were
associated with improved OS time (P=0.012 and P=0.002,
respectively; Fig. 2E and F).
Multivariate analysis
Univariate analysis (Table III) indicated that tumor location,
T, N and TNM stage, and hTERT and UBE2D3 expression may predict
esophageal cancer prognosis, therefore, these factors were
integrated into multivariate analysis using Cox proportional
hazards analysis. It was subsequently identified that tumor
location, lymph node status and UBE2D3 expression level were
independent prognostic factors in esophageal cancer (Table IV).
 | Table IIIUnivariate analysis of factors
regarding overall survival. |
Table III
Univariate analysis of factors
regarding overall survival.
| Characteristic | n (%) | Five-year survival
rate, % |
χ2a | P-value |
|---|
| Age, years | | | 0.459 | 0.498 |
| <60 | 70 (46.7) | 29.0 | | |
| ≥60 | 80 (53.3) | 22.0 | | |
| Gender | | | 0.068 | 0.405 |
| Male | 124 (82.7) | 28.0 | | |
| Female | 26 (17.3) | 17.0 | | |
| Location | | | 4.455 | 0.035 |
| Upper
thoracic | 21 (14.0) | 11.0 | | |
| Middle/lower
thoracic | 129 (86.0) | 30.0 | | |
| Tumor size, cm | | | 3.812 | 0.051 |
| <5 | 81 (54.0) | 29.8 | | |
| ≥5 | 69 (46.0) | 20.2 | | |
| Histological
gradeb | | | 0.068 | 0.795 |
| G1/2 | 119 (79.3) | 27.0 | | |
| G3/4 | 31 (20.7) | 19.0 | | |
| T stage | | | 4.278 | 0.039 |
| T1–T2 | 65 (43.3) | 35.5 | | |
| T3–T4 | 85 (56.7) | 15.0 | | |
| N stage | | | 11.290 | 0.001 |
| N0 | 76 (50.7) | 35.8 | | |
| N1–3 | 74 (49.3) | 9.1 | | |
| TNM stage | | | 16.000 | <0.001 |
| Early (I–II) | 88 (58.7) | 34.9 | | |
| Advanced
(III–IV) | 62 (41.3) | 17.0 | | |
| Adjuvant
radiotherapy | | | 0.293 | 0.588 |
| Yes | 40 (26.7) | 26.0 | | |
| No | 110 (73.3) | 23.0 | | |
| Adjuvant
chemotherapy | | | 0.004 | 0.951 |
| Yes | 70 (46.7) | 26.1 | | |
| No | 80 (53.3) | 24.8 | | |
| hTERT
expression | | | 6.353 | 0.012 |
| Low | 59 (39.3) | 35.0 | | |
| High | 91 (60.7) | 19.5 | | |
| UBE2D3
expression | | | 9.145 | 0.002 |
| Low | 95 (63.3) | 15.7 | | |
| High | 55 (36.7) | 40.9 | | |
 | Table IVMultivariate Cox proportional hazards
analysis of overall survival. |
Table IV
Multivariate Cox proportional hazards
analysis of overall survival.
| Overall
survival |
|---|
|
|
|---|
| Factora | HR | 95% CI | P-value |
|---|
| Tumor location | 0.476 | 0.270–0.841 | 0.011 |
| T stage | 1.086 | 0.674–1.754 | 0.734 |
| N stage | 1.694 | 1.055–2.721 | 0.029 |
| hTERT
expression | 1.589 | 0.990–2.551 | 0.055 |
| UBE2D3
expression | 0.487 | 0.293–0.807 | 0.005 |
Correlation between hTERT and UBE2D3
expression levels
The Spearman correlation coefficient for hTERT and
UBE2D3 expression was r=-0.18 (P=0.027), indicating that hTERT and
UBE2D3 expression are negatively correlated with each other.
Discussion
The present study investigated 150 esophageal cancer
and 30 adjacent tissues to evaluate the prognostic value of a
number of conventional clinicopathological and cellular molecular
factors. In accordance with previously conducted studies (24–26),
univariate analysis demonstrated that traditional clinical
parameters, such as T, N and overall TNM stage, were important
factors for predicting the survival rate, whereas tumor histology
and adjuvant therapy were not (Table
II). However, whether tumor location affects the prognosis of
esophageal cancer is a controversial topic. Yang et al
(27) demonstrated that the tumor
location did not impact the survival rate of esophageal cancer;
however, in the seventh edition of the UICC TNM system (20), tumor location (upper and middle
thoracic versus lower thoracic) was important for grouping T2-3N0M0
squamous cell cancers. The present univariate and multivariate
analysis indicated that tumor location (upper versus middle and
lower thoracic) was associated with survival rate and may be an
independent prognostic factor in esophageal cancer. In addition,
the present study identified that lymph node involvement may be an
independent prognostic factor for esophageal cancer, which was
consistent with the results of previous studies (28,29).
hTERT confers limitless replicative potential to
cancer cells (30), and previous
studies have established immortalized human esophageal epithelial
cell models by the introduction of hTERT (31). Furthermore, hTERT is able to promote
the development of invasive esophageal squamous cell cancer by
interacting with epidermal growth factor receptor and p53 (32). Telomerase activity has been
extensively studied in various types of malignant tumor for
clinical, diagnostic and/or prognostic purposes (12,13,33),
and it has been proposed for use as a marker of poor prognosis in
such tumors. The present study determined that hTERT was more
frequently elevated in the esophageal cancer tissues compared with
the adjacent healthy tissues. In the cancer tissues, the expression
of hTERT was also elevated in tumors with large size, poor
differentiation, deep tumor invasion, lymph node metastasis and
advanced TNM stage. Furthermore, strong expression of hTERT was
correlated with OS time, indicating that hTERT participates in the
progress of esophageal cancer and may be a poor prognostic
biomarker of esophageal cancer tumors. However, in multivariate
analysis, hTERT expression was not an independent prognostic
factor, therefore, a combination test of telomerase activity with
other prognostic factors may be necessary.
UBE2D3 is a member of E2 family and is a crucial
component of the ubiquitination cascade, acting as a key mediator
of the interaction between E1 and E3 (34,35).
The whole ubiquitination process is responsible for 80% of
proteasomal cellular protein degradation. Upregulation of UBE2D3 in
acute promyelocytic leukemia cells leads to the ubiquitination of
cyclin D1 and its degradation in the proteasome (36). However, in the absence of UBE2D3,
cyclin D1 is not degraded and tumor cells continue to cycle
(37). Mittal et al
(38) reported that knocking down
UBE2D3 in human breast cancer cells resulted in elevated cyclin D1
levels, and that a low level of UBE2D3 expression was a determinant
factor in the progression of metastatic breast cancer. These two
studies indicated that UBE2D3 expression is involved in cell cycle
regulation via the degradation of cyclin D1; in consideration of
this biological behavior, the present study proposes that UBE2D2
expression levels may promote tumor development. Furthermore, the
current study identified that the expression of UBE2D3 was
significantly lower in the esophageal cancer tissues compared with
the adjacent healthy tissues, as well as significantly lower in the
cancer tissues with lymph node involvement and poor
differentiation. In addition, UBE2D3 appeared to be an independent
prognostic factor for esophageal cancer. Thus, it is proposed that
UBE2D3 expression may be involved in the progression of esophageal
cancer.
Most notably, Spearman correlation coefficient
analysis revealed a negative correlation between UBE2D3 and hTERT
protein expression levels, which was consistent with a previous
study (19). These results indicate
that hTERT and UBE2D3 may interact with each other, validating the
proposal that UBE2D3 potentially has a role in the hTERT signaling
pathway. However, the mechanism by which the ubiquitination process
of UBE2D3 is involved in the interaction with the hTERT pathway,
and whether UBE2D3 expression exists as a universal phenomenon in
all types of tumor, requires additional studies to be conducted in
the future.
In conclusion, the present study demonstrated that
hTERT and UBE2D3 expression are negatively correlated, and that the
two proteins demonstrate a strong association with the prognosis in
esophageal cancer. Furthermore, the expression of UBE2D3, lymph
node involvement and tumor location were independent predictive
prognostic factors; thus, UBE2D3 expression may be a promising
prognostic biomarker in esophageal cancer. However, the current
study was based on retrospective analysis and semi-quantitative
research, therefore, prospective randomized clinical trials and
basic quantitative research are required to evaluate the clinical
utility of the present study results.
Acknowledgements
The present study was sponsored by the National
Natural Science foundation of china (grant no. 81071825) and the
Fundamental Research Funds for the Central Universities (grant no.
2042014kf0114).
References
|
1
|
Yang H and Chen YX: Improvement analysis
of article quality in World Journal of Gastroenterology during
2008–2012. World J Gastroenterol. 19:7830–7835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao WM, Zheng WH and Ling ZQ:
Epidemiologic risk factors for esophageal cancer development. Asian
Pac J Cancer Prev. 12:2461–2466. 2011.
|
|
3
|
Wang X, Fan JC, Wang AR, et al:
Epidemiology of esophageal cancer in Yanting - regional report of a
national screening programme in China. Asian Pac J Cancer Prev.
14:2429–2432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin Y, Totsuka Y, He Y, et al:
Epidemiology of esophageal cancer in Japan and China. J Epidemiol.
23:233–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gertler R, Stein HJ, Langer R, et al:
Long-term outcome of 2920 patients with cancers of the esophagus
and esophagogastric junction: evaluation of the New Union
Internationale Contre le Cancer/American Joint Cancer Committee
staging system. Ann Surg. 253:689–698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu W, Zhang Y, Liu D, et al: Telomeres -
structure, function, and regulation. Exp Cell Res. 319:133–141.
2013. View Article : Google Scholar
|
|
8
|
Philippi C, Loretz B, Schaefer UF and Lehr
CM: Telomerase as an emerging target to fight cancer -
opportunities and challenges for nanomedicine. J Control Release.
146:228–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Counter CM, Meyerson M, Eaton EN, et al:
Telomerase activity is restored in human cells by ectopic
expression of hTERT (hEST2), the catalytic subunit of telomerase.
Oncogene. 16:1217–1222. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ulaner GA, Hu JF, Vu TH, et al: Telomerase
activity in human development is regulated by human telomerase
reverse transcriptase (hTERT) transcription and by alternate
splicing of hTERT transcripts. Cancer Res. 58:4168–4172.
1998.PubMed/NCBI
|
|
11
|
Proctor A, Brownhill SC and Burchill SA:
The promise of telomere length, telomerase activity and its
regulation in the translocation-dependent cancer ESFT; clinical
challenges and utility. Biochim Biophys Acta. 1792:260–274. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simsek BC, Pehlivan S and Karaoglu A:
Human telomerase reverse transcriptase expression in colorectal
tumors: correlations with immunohistochemical expression and
clinicopathologic features. Ann Diagn Pathol. 14:413–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang G, Wang W, Zhou J and Yang X:
Correlation between telomerase activity and matrix
metalloproteinases 2 expression in gastric cancer. Cancer Biomark.
13:21–28. 2013.PubMed/NCBI
|
|
14
|
Poremba C, Heine B, Diallo R, et al:
Telomerase as a prognostic marker in breast cancer: high-throughput
tissue microarray analysis of hTERT and hTR. J Pathol. 198:181–189.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu Y, Chen C, Pan J, et al: The Ubiquitin
Proteasome Pathway (UPP) in the regulation of cell cycle control
and DNA damage repair and its implication in tumorigenesis. Int J
Clin Exp Pathol. 5:726–738. 2012.PubMed/NCBI
|
|
16
|
Amm I, Sommer T and Wolf DH: Protein
quality control and elimination of protein waste: the role of the
ubiquitin-proteasome system. Biochim Biophys Acta. 1843:182–196.
2014. View Article : Google Scholar
|
|
17
|
Clague MJ and Urbé S: Ubiquitin: same
molecule, different degradation pathways. Cell. 143:682–685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleiger G and Mayor T: Perilous journey: a
tour of the ubiquitin-proteasome system. Trends Cell Biol.
24:352–359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Yang L, Hu L, et al: Inhibition of
UBE2D3 expression attenuates radiosensitivity of MCF-7 human breast
cancer cells by increasing hTERT expression and activity. PLoS One.
8:e646602013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; sWest Sussex: 2009
|
|
21
|
Yang CH, Hung WC, Wang SL, et al:
Immunoexpression and prognostic role of hTERT and cyclin D1 in
urothelial carcinoma. APMIS. 116:309–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujita T, Ikeda H, Kawasaki K, et al:
Clinicopathological relevance of UbcH10 in breast cancer. Cancer
Sci. 100:238–248. 2009. View Article : Google Scholar
|
|
23
|
Kumaki F, Kawai T, Hiroi S, et al:
Telomerase activity and expression of human telomerase RNA
component and human telomerase reverse transcriptase in lung
carcinomas. Hum Pathol. 32:188–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yam PC, Tong D and Law S: Comparisons of
sixth and seventh edition of the American Joint Cancer Committee
staging systems for esophageal cancer. Ann Surg Oncol. 21:583–588.
2014. View Article : Google Scholar
|
|
25
|
Hsu PK, Wu YC, Chou TY, et al: Comparison
of the 6th and 7th editions of the American Joint Committee on
Cancer tumor-node-metastasis staging system in patients with
resected esophageal carcinoma. Ann Thorac Surg. 89:1024–1031. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong JC, Murphy JD, Wang SJ, et al:
Chemoradiotherapy before and after surgery for locally advanced
esophageal cancer: a SEER-Medicare analysis. Ann Surg Oncol.
20:3999–4007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang HX, Hou X, Liu QW, et al: Tumor
location does not impact long-term survival in patients with
operable thoracic esophageal squamous cell carcinoma in China. Ann
Thorac Surg. 93:1861–1866. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marjanovic G, Schricker M, Walch A, et al:
Detection of lymph node involvement by cytokeratin
immunohistochemistry is an independent prognostic factor after
curative resection of esophageal cancer. J Gastrointest Surg.
15:29–37. 2011. View Article : Google Scholar
|
|
29
|
Dhupar R, Correa AM, Ajani J, et al:
Concordance of studies for nodal staging is prognostic for worse
survival in esophageal cancer. Dis Esophagus. 27:770–776. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vallböhmer D, Brabender J, Metzger R and
Hölscher AH: Genetics in the pathogenesis of esophageal cancer:
possible predictive and prognostic factors. J Gastrointest Surg.
14(Suppl 1): S75–S80. 2010. View Article : Google Scholar
|
|
31
|
Cheung PY, Deng W, Man C, et al: Genetic
alterations in a telomerase-immortalized human esophageal
epithelial cell line: Implications for carcinogenesis. Cancer Lett.
293:41–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okawa T, Michaylira CZ, Kalabis J, et al:
The functional interplay between EGFR overexpression, hTERT
activation, and p53 mutation in esophageal epithelial cells with
activation of stromal fibroblasts induces tumor development,
invasion, and differentiation. Genes Dev. 21:2788–2803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakabe R, Murakami Y, Uemura K, et al:
Prognostic significance of telomerase activity and human telomerase
reverse transcriptase expression in ampullary carcinoma. Ann Surg
Oncol. 19:3072–3080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Page RC, Pruneda JN, Amick J, et al:
Structural insights into the conformation and oligomerization of
E2~ubiquitin conjugates. Biochemistry. 51:4175–4187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye Y and Rape M: Building ubiquitin
chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 10:755–764.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams J: The proteasome: structure,
function, and role in the cell. Cancer Treat Rev. 29(Suppl 1): 3–9.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khanna-Gupta A and Berliner N: ATRA:
Finding targeted APL therapy targets. Blood. 110:476–477. 2007.
View Article : Google Scholar
|
|
38
|
Mittal MK, Singh K, Misra S and Chaudhuri
G: SLUG-induced elevation of D1 cyclin in breast cancer cells
through the inhibition of its ubiquitination. J Biol Chem.
286:469–479. 2011. View Article : Google Scholar :
|