Introduction
DNA double-strand breaks (DSB) are the primary
lesion induced by ionizing radiation and are most commonly
associated with cell death (1). The
cell has developed two major pathways of response to DSBs:
Non-homologous end-joining (NHEJ) and homologous recombination (HR)
(1). NHEJ is active throughout the
cell cycle and is the repair pathway that deals with the majority
of radiation-induced DSBs. NHEJ relies on a large protein complex,
DNA-dependent protein kinase (DNA-PK), to bind to the ends of
broken DNA and bring them together for direct ligation. DNA-PK is
composed of the Ku70/80 end-binding proteins and the DNA-PK
catalytic subunit (DNA-PKcs). The kinase activity of DNA-PK is
directly dependent on the functional Ku subunit; however, the
kinase activity is also affected by the loss of DNA-PKcs (2,3).
It appears that the phosphorylation activity of the
DNA-PKcs contributes to the DSB repair capability of DNA-PK.
DNA-PKcs not only have the ability to phosphorylate various NHEJ
proteins, it is also able to autophosphorylate itself (4–6).
DNA-PKcs has various phosphorylation sites throughout its protein
structure, with the most critical sites located in the T2609 and
the S2056 clusters (5–9). Site-directed mutants involving
phosphorylation sites in the T2609 and S2056 clusters result in
cell lines with various levels of radiosensitivity, ranging from a
DNA-PKcs and Ku null phenotype to very mild sensitivity. This
varying sensitivity was previously demonstrated in a synchronized
G1 population of cells exposed to low linear energy transfer (LET)
radiation Cs137 γ radiation (10).
High LET radiation is more effective at killing
cells than low LET radiation. This increase in cell death can be
observed as an increased relative biological effectiveness (RBE)
when comparing the D10 value of cells exposed to 250 KeV
X-rays or γ irradiation to the D10 value of cells
exposed to high LET radiation. High LET radiation creates various
types of complex DNA damage in small clusters within the DNA strand
(11–14), including DBS, single-stranded breaks
(SSBs) and base damage. Due to its complexity, the cell takes
considerably longer to repair this high LET radiation-induced DNA
damage (15,16). In contrast to the types of DNA
damage caused by high and low LET radiation, cisplatin induces
inter- and intrastrand cross-linking and DNA adducts (17–19).
Cisplatin-induced damage is often resolved by the nucleotide
excision repair pathway (20);
however, occasionally, cisplatin can result in DSBs in dividing
cells, and these DSBs require the NHEJ and HR pathways for complete
repair (21).
The aim of the present study was to determine the
relative sensitivity to high LET radiation of site-directed mutant
cells, containing phosphorylatable residues in the T2609 cluster,
S2056 cluster (19,20) and carboxyl-terminus phosphoinositide
3 kinase (PI3K) domain of DNA-PKcs. Furthermore, the current study
expands upon a previous study conducted by Chen et al
(8), which focused only on the
sensitivity of these DNA-PKcs mutants to low LET radiation.
Materials and methods
Cell lines and cell culture
The present study utilized a wild-type Chinese
hamster ovary (CHO) cell line (CHO10B2) provided by Dr. Joel
Beford, Department of Evrionmental & Radiological Health
Sciences, Colorado State University (Fort Collins, CO, USA);
NHEJ-deficient xrs-5 (Ku80 mutated) and V3 cells; HR-deficient 51D1
(Rad51D mutated) cells provided by Dr. Larry Thompson, Biosciences
and Biotechnology Division, Lawrence Livermore National Laboratory
(Livermore, CA, USA); and 14 cell lines derived from DNA-PKcs null
V3 cells with complementary human DNA-PKcs containing amino acid
substitutions at specific positions (shown in Table I). Cells were cultured in minimal
essential medium-α (Gibco Life Technologies, Indianapolis, IN, USA)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St.
Louis, MO, USA), and 1% penicillin, streptomycin and amphotericin B
(Gibco Life Technologies, Carlsbad, CA, USA), and maintained at
37°C in a humidified atmosphere of 5% CO2 in air.
 | Table ICell lines derived from DNA-PKcs null
V3 cells with human DNA-PKcs complementary DNA, containing amino
acid substitutions at various positions in the DNA-PKcs
constructs. |
Table I
Cell lines derived from DNA-PKcs null
V3 cells with human DNA-PKcs complementary DNA, containing amino
acid substitutions at various positions in the DNA-PKcs
constructs.
| |
Substituted
domain |
|---|
| |
|
|---|
| | S2056 cluster | T2609 cluster | PI3K |
|---|
| |
|
|
|
|---|
| Cell line | Altered DNA-PKcs
mutant | S2023 | S2029 | S2041 | S2051 | S2056 | T2609 | S2612 | T2620 | S2624 | T2638 | T2647 | Y3715 | L3750 | K3752 | D3921 |
|---|
| L-1 | Wild-type | | | | | | | | | | | | | | | |
| L-2 | V3-7A | | | | | A | A | A | A | A | A | A | | | | |
| L-3 | V3-6A | | | | | | A | A | A | A | A | A | | | | |
| L-4 | V3-2A | | | | | A | A | | | | | | | | | |
| L-5 | V3-S2056A | | | | | A | | | | | | | | | | |
| L-6 | V3-T2609A | | | | | | A | | | | | | | | | |
| L-8 | V3-KA4 | | | | | | | | | | | | | | R | |
| L-9 | V3-KB20 | | | | | | | | | | | | | R | R | |
| L-10 | V3-KC23a | | | | | | | | | | | | Δ | | | |
| L-11 | V3-KD51 | | | | | | | | | | | | | | | N |
| L-12 | V3-5A | A | A | A | A | A | | | | | | | | | | |
| L-14 | V3-3A | | | | | | A | | | | A | A | | | | |
Irradiation and cell treatment
Logarithmic phase cells were irradiated aerobically
at room temperature. The radiation source was a JL Shepherd and
Associates (San Fernando, CA, USA) irradiator that emitted
Cs137 γ-rays at a rate of 2.5 Gy/min, and the cells were
irradiated using accelerated Fe ions, C ions and protons at the
National Institute of Radiation Sciences (Chiba, Japan). The LET of
the radiation used were as followed: 500 MeV/nucleon initial energy
and LET 200 keV/μm monoenergetic Fe ions; 290 MeV/nucleon initial
energy and average LET 50 keV/μm spread-out Bragg peak (SOBP) C
ions; 70 MeV/nucleon initial energy and LET 1 keV/μm monoenergetic
protons; and, 0.663 MeV initial energy and LET 0.3 keV/μm
Cs137 γ radiation. Additionally, the cells were exposed
to various concentrations of cisplatin (3.3–33 μM) for 1 h prior to
plating for the survival experiments. Survival curves were obtained
by measuring the colony-forming ability of the irradiated cell
populations. Briefly, post-irradiation, the cells were plated onto
60-mm plastic petri dishes and incubated for 7–10 days for colony
formation. The dishes were then fixed with 100% ethanol and stained
with 0.1% crystal violet solution. A colony with >50 cells was
scored as a survivor.
RBE
Prism 5™ software (GraphPad Software, Inc., La
Jolla, CA, USA) was used to draw survival curves from the survival
fraction obtained from the survival assay. This software was also
used to obtain D10 values, the dose required to kill 90%
of cells, and RBE values, by dividing the D10 values of
the γ-ray exposure by the D10 values obtained various
radiation exposures.
Statistical analysis
Statistical comparison of the mean values was
performed using a two tailed t-test. P<0.05 was considered to
indicate a statistically significant difference. Error bars
indicate the standard error of the means and confidence interval
values were calculated using Prism 5™ software (GraphPad Software,
Inc.). Additionally, variation amongst the cell lines was
calculated using the D10 and mean values obtained from
Prism 5™ software.
Results
Effect of site-specific mutations on
sensitivity to low LET charged particle radiation (γ-rays and
protons)
To investigate the role of DNA-PKcs in cellular
sensitivity to low LET charged particles, the D10 values
of the various DNA-PKcs mutants exposed to proton radiation were
compared to the D10 values of the same cell lines
exposed to γ-rays. Asychronized cells were exposed to 70
MeV/nucleon initial energy and LET 1 keV/μm protons or 0.663 MeV
initial energy and LET 0.3 keV/μm of Cs137 γ-ray
radiation, and were immediately sub-cultured and plated for colony
formation assays. As shown in Table
II, the D10 values obtained from the Prism5™
software indicated marginal variation between the DNA-PKcs mutants
when exposed to proton radiation, similar to the values observed
when the cells were exposed to γ irradiation. Furthermore, the
xrs-5 cells demonstrated the highest sensitivity and the control
cells demonstrated the highest resistance in these two groups. The
majority of cell lines exhibited sensitivities similar to or more
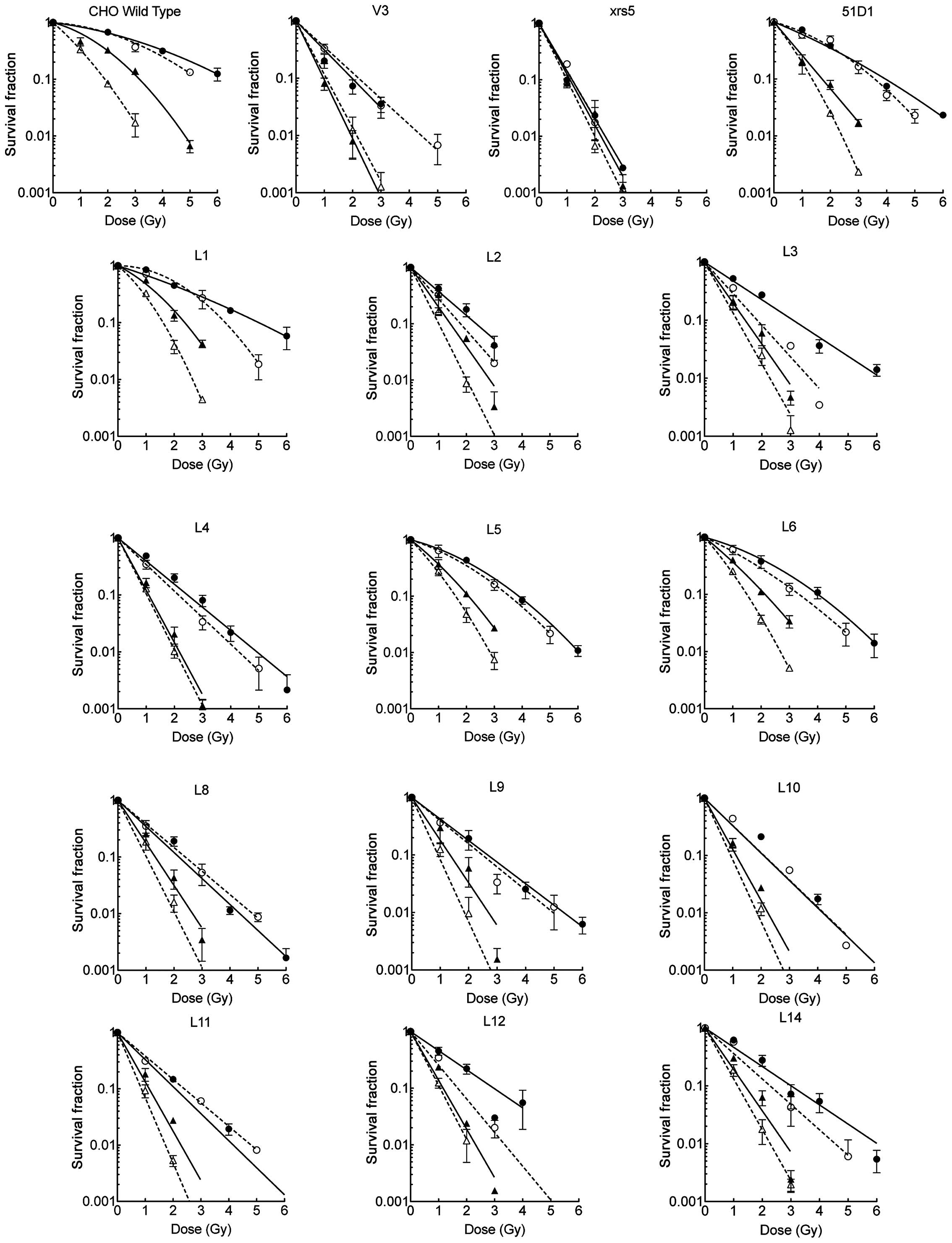
resistant than V3. The complete survival curves shown in Fig. 1 highlight the differing
sensitivities between the DNA-PKcs mutant cell lines.
 | Table IID10 values of control and
mutant cell lines to ionizing radiation and cisplatin. |
Table II
D10 values of control and
mutant cell lines to ionizing radiation and cisplatin.
| D10 (95%
confidence interval)a |
|---|
|
|
|---|
| Cell line | γ-rays, Gy | Proton, Gy | C ion, Gy | Fe ion, Gy | Cisplatin, μM |
|---|
| CHO10B2 | 6.37
(5.87–6.87) | 5.31
(4.86–5.77) | 3.16
(2.94–3.32) | 1.89
(1.52–2.1) | 29.29
(23.15–35.12) |
| XRS5 | 1.18
(1.05–1.31) | 1.19
(0.90–1.46) | 1.11
(0.94–1.28) | 1.00
(0.95–1.05) | 9.79
(8.85–10.70) |
| V3 | 1.98
(1.76–2.19) | 2.21
(1.98–2.44) | 0.97
(0.89–1.06) | 1.07
(0.93–1.21) | 11.86
(10.96–12.75) |
| 51D1 | 3.95
(3.69–4.16) | 3.57
(3.24–3.79) | 1.71
(1.53–1.87) | 1.35
(1.31–1.40) | 6.15
(5.40–6.88) |
| L-1 | 5.01
(4.52–5.46) | 3.82
(3.52–4.24) | 2.38
(2.17–2.53) | 1.61
(1.46–1.72) | 19.41
(14.21–24.30) |
| L-2 | 2.35
(2.00–2.69) | 1.79
(1.24–2.30) | 1.42
(1.25–1.60) | 1.01
(0.93–1.09) | 15.45
(14.31–16.57) |
| L-3 | 3.09
(2.87–3.30) | 1.84
(1.63–2.04) | 1.42
(1.25–1.58) | 1.14
(1.01–1.26) | 12.31
(9.27–15.18) |
| L-4 | 2.47
(2.29–2.64) | 2.15
(1.91–2.37) | 1.09
(1.00–1.18) | 1.02
(0.98–1.06) | 11.81
(10.55–13.02) |
| L-5 | 3.88
(3.62–4.07) | 3.54
(2.98–3.85) | 2.08
(1.93–2.19) | 1.61
(1.32–1.79) | 14.09
(12.03–16.08) |
| L-6 | 4.01
(3.35–4.37) | 3.35
(2.58–3.73) | 2.14
(1.93–2.29) | 1.50
(1.39–1.59) | 11.67
(10.77–12.56) |
| L-8 | 2.18
(2.00–2.35) | 2.40
(2.21–2.59) | 1.33
(1.13–1.53) | 1.02
(0.90–1.13) | 13.27
(11.19–15.26) |
| L-9 | 2.67
(2.43–2.91) | 2.48
(2.09–2.86) | 1.35
(1.05–1.63) | 0.93
(0.80–1.05) | 17.76
(12.15–23.03) |
| L-10 | 2.09
(1.86–2.31) | 2.10
(1.70–2.49) | 1.12
(1.02–1.22) | 0.93
(0.82–1.04) | 11.30
(8.85–13.63) |
| L-11 | 2.08
(1.90–2.26) | 2.40
(2.24–2.56) | 1.14
(1.01–1.27) | 0.86
(0.81–0.92) | 14.37
(11.01–17.55) |
| L-12 | 2.97
(2.45–3.46) | 1.68
(1.51–1.85) | 1.16
(0.92–1.40) | 1.05
(0.90–1.20) | 11.71
(8.98–14.29) |
| L-14 | 3.01
(2.67–3.34) | 2.27
(1.88–2.65) | 1.40
(1.19–1.60) | 1.14
(1.05–1.23) | 16.17
(13.52–18.71) |
Effect of site-specific mutations on
sensitivity to high LET radiation (C and Fe ions)
Considering that the results for low LET charged
particles were similar to those for low LET γ-rays, the effect of
site specific mutations on the cells’ sensitivity to high LET
radiation (Fe and C ions only) was investigated. Asychronized cells
were exposed to 500 MeV/nucleon initial energy and 200 keV/μm
monoenergetic Fe ions or 290 MeV/nucleon initial energy and average
50 keV/μm SOBP C ions, and were immediately subcultured and plated
for colony formation assays. As Table
II indicates, all DNA-PKcs mutants exhibited D10
values similar to the V3 cells, with the exception of L-5 and L-6.
L-5 and L-6 demonstrated similar D10 values when
compared with the L-1 cell line.
Effect of site-specific mutations on
RBE
Following calculation of the D10 values
for each DNA-PKcs mutant and the control CHO10B2 cells at each
exposure, the values were compared. As shown in Table III, the RBE values for all
DNA-PKcs mutants are similar, demonstrating that the single point
mutations do not increase the effectiveness of high LET
radiation.
 | Table IIIRelative biological
effectivenessa. |
Table III
Relative biological
effectivenessa.
| Relative biological
effectiveness |
|---|
|
|
|---|
| Cell line | γ-rays | Protons | C ions | Fe ions |
|---|
| CHO10B2 | 1 | 1.20 | 2.02 | 3.37 |
| XRS5 | 1 | 1.00 | 1.06 | 1.18 |
| V3 | 1 | 0.90 | 2.04 | 1.85 |
| 51D1 | 1 | 1.11 | 2.31 | 2.93 |
| L-1 | 1 | 1.31 | 2.11 | 3.11 |
| L-2 | 1 | 1.31 | 1.65 | 2.32 |
| L-3 | 1 | 1.68 | 2.18 | 2.71 |
| L-4 | 1 | 1.15 | 2.27 | 2.42 |
| L-5 | 1 | 1.10 | 1.87 | 2.41 |
| L-6 | 1 | 1.20 | 1.87 | 2.67 |
| L-8 | 1 | 0.91 | 1.64 | 2.14 |
| L-9 | 1 | 1.08 | 1.98 | 2.87 |
| L-10 | 1 | 1.00 | 1.87 | 2.25 |
| L-11 | 1 | 0.87 | 1.82 | 2.42 |
| L-12 | 1 | 1.77 | 2.56 | 2.83 |
| L-14 | 1 | 1.33 | 2.15 | 2.64 |
Effect of site-specific mutations on the
sensitivity of mutants to cisplatin
Cisplatin-induced DNA damage, unlike
radiation-induced DNA damage, rarely causes DSBs. The various types
of CHO cell were exposed to cisplatin, subcultured and plated for a
survival assay. As shown in Table
II, the DNA-PKcs mutants exhibit varying sensitivities to
cisplatin. All the DNA-PKcs mutants investigated in the present
study were more sensitive than the control CHO10B2 cells, however,
no statistically significant difference between the sensitivity of
the CHO10B2 and V3 cell lines was identified.
Comparison between radiation and
cisplatin sensitivity
To better understand the role of DNA-PKcs, the
sensitivity of cisplatin exposure was compared with each of the low
and high LET ionizing radiations used. The γ-ray D10
values were plotted on the X-axis, the Fe ion D10 values
were plotted on the Y-axis and the D10 values of
cisplatin were plotted in a bubble chart style, allowing the
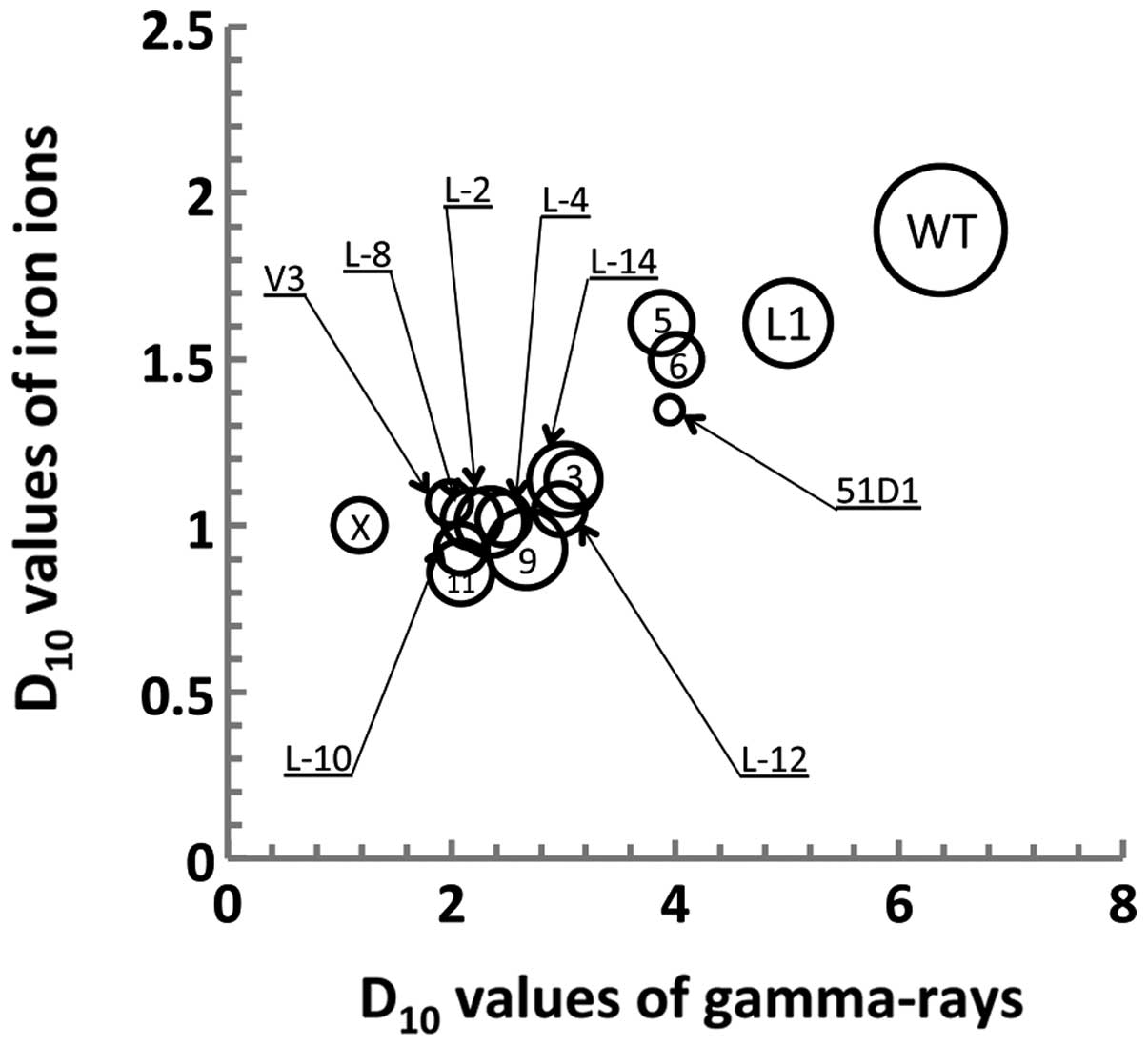
variance among each exposure group to be determined (Fig. 2).
As demonstrated in Fig.
2, radiation sensitivities between γ-ray and Fe ion radiation
were correlated with the wild-type and DNA repair-deficient cell
lines. However, the bubble chart indicated a lack of correlation
between cisplatin and radiation sensitivity. L-11, one of the most
sensitive mutants to γ-rays was also the most sensitive mutant to
Fe ions. By contrast, L-6, which is one of the mutants most
sensitive to cisplatin, is one of the mutants that is most
resistant to γ irradiation.
Discussion
The present study expands on a previous study, which
demonstrated that low LET radiation damage to the DNA appears to
only require a portion of the phosphorylation sites on the DNA-PKcs
protein (10). Considering that
high LET radiation produces complex damage to the DNA, this type of
damage involves DSBs as well as adducts, cross-links and SSBs, all
within a small region of DNA (11,12).
In the current study, two types of low LET radiation, γ-rays and
protons, and two types of high LET radiation, C and Fe ions, were
utilized. C, Fe and proton radiation are all charged particles. As
shown in Table II, L-5 and L-6
were the most resistant DNA-PKcs mutant cells to γ-ray and Fe ion
radiation, however, L-5 and L-6 were also less resistant than the
control and corrected cell lines (L-1) after γ-ray exposure. L-5
and L-6 each contain a single mutation in the S2056 and T2609
cluster, respectively. The L-4 cell line also contains these two
point mutations, however, and was observed to exhibit increased
sensitivity to γ-ray and Fe ion radiation. Thus, the L-4, L-3 and
L-12 D10 data indicates that the V3 phenotype requires a
mutation in the S2056 cluster and the T2609 cluster, or a complete
loss of one of these clusters. Furthermore, the results obtained
from the L-8, -9, -10 and -11 cell lines illustrate that the PI3K
cluster is as important for repair of high LET-induced damage as it
is for the repair of low LET-induced damage (10).
In addition to evaluating the role of specific
phosphorylation DNA-PKcs sites in the repair of high LET
radiation-induced DNA damage, the role of these phosphorylation
sites in the repair of cisplatin-induced DNA damage was also
investigated. As indicated in Fig.
2 and Table II, it appears
that a single point mutation in the S2056, T2609 or PI3K cluster
results in cisplatin sensitivity similar to that of the V3 null
mutant. In contrast to the sensitivity to low and high LET-induced
DNA damage, complete loss of a cluster or mutations in two clusters
does not increase the sensitivity of the cell lines to
cisplatin-induced DNA damage.
Finally, the correlation between the sensitivities
of all the cell lines to low and high LET radiation-induced versus
cisplatin-induced DNA damage was evaluated. A lack of correlation
was identified, indicating that the contribution of cross-linking
DNA damage, as a result of ionizing radiation, to cell death is
minor. High LET radiation creates complex DNA damage consisting of
DSB, SSBs, cross-linking and various other types of single
nucleotide damage (11,13,14).
Based on the D10 values of the L-5 and L-6 cell lines,
single point mutations in the S2056 or T2609 clusters exhibited
partial sensitivity to low LET radiation but appear to be
insufficient for creating a V3 phenotype upon exposure to low LET
radiation; however, L-5 and L-6 demonstrated a V3-like phenotype
when exposed to cisplatin. This is in contrast to low LET-induced
damage or cisplatin-induced damage, which requires a single
mutation among three clusters to induce a V3 phenotype.
In conclusion, the present study demonstrated that
the entire DNA-PKcs protein is required for repair of low LET
radiation and cisplatin-induced DNA damage. However, a single
mutation in the PI3K domain, multiple mutations within the S2056 or
T2609 clusters, or two mutations in the S2056 and T2609 clusters,
are required for the repair of high LET radiation-induced DNA
damage. These results indicate that the interaction of two clusters
may synergistically contribute to the repair of high LET
radiation-induced DNA damage. However, further studies are required
to investigate high LET-induced DNA damage and the associated
molecular repair mechanisms.
Acknowledgements
The authors would like to thank Dr Akiko M. Ueno
(Ueno Radiation Biology Research Fund), Dr John H. Benable (Venable
Memorial Scholarship), the Technology Fee Stipend Student
Experimental Learning Fund of the College of Veterinary Medicine
and Biosciences (Colorado State University, Fort Collins, CO, USA),
the Cyclotron, Heavy Ion Medical Accelerator in Chiba (Chiba,
Japan) and the International Open Laboratory of the National
Institute of Radiological Sciences (Chiba, Japan) for supporting
the present study.
References
|
1
|
Rothkamm K, Krüger I, Thompson LH and
Löbrich M: Pathways of DNA double-strand break repair during the
mammalian cell cycle. Mol Cell Biol. 23:5706–5715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finnie NJ, Gottlieb TM, Blunt T, et al:
DNA-dependent protein kinase activity is absent in xrs-6 cells:
implications for site-specific recombination and DNA double-strand
break repair. Proc Natl Acad Sci USA. 92:320–324. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peterson SR, Kurimasa A, Oshimura M, et
al: Loss of the catalytic subunit of the DNA-dependent protein
kinase in DNA double-strand-break-repair mutant mammalian cells.
Proc Natl Acad Sci USA. 92:3171–3174. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurimasa A, Kumano S, Boubnov NV, et al:
Requirement for the kinase activity of human DNA-dependent protein
kinase catalytic subunit in DNA strand break rejoining. Mol Cell
Biol. 19:3877–3884. 1999.PubMed/NCBI
|
|
5
|
Chan DW, Chen BP, Prithivirajsingh S, et
al: Autophosphorylation of the DNA-dependent protein kinase
catalytic subunit is required for rejoining of DNA double-strand
breaks. Genes Dev. 16:2333–2338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding Q, Reddy YV, Wang W, et al:
Autophosphorylation of the catalytic subunit of the DNA-dependent
protein kinase is required for efficient end processing during DNA
double-strand break repair. Mol Cell Biol. 23:5836–5848. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen BP, Uematsu N, Kobayashi J, et al:
Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs
phosphorylations at the Thr-2609 cluster upon DNA double strand
break. J Biol Chem. 282:6582–6587. 2007. View Article : Google Scholar
|
|
8
|
Chen BP, Chan DW, Kobayashi J, et al: Cell
cycle dependence of DNA-dependent protein kinase phosphorylation in
response to DNA double strand breaks. J Biol Chem. 280:14709–14715.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui X, Yu Y, Gupta S, Cho YM, Lees-Miller
SP and Meek K: Autophosphorylation of DNA-dependent protein kinase
regulates DNA end processing and may also alter double-strand break
repair pathway choice. Mol Cell Biol. 25:10842–10852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagasawa H, Little JB, Lin YF, et al:
Differential role of DNA-PKcs phosphorylations and kinase activity
in radiosensitivity and chromosomal instability. Radiat Res.
175:83–89. 2011. View
Article : Google Scholar :
|
|
11
|
Hada M and Georgakilas AG: Formation of
clustered DNA damage after high-LET irradiation: a review. J Radiat
Res. 49:203–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goodhead DT: Initial events in the
cellular effects of ionizing radiations: clustered damage in DNA.
Int J Radiat Biol. 65:7–17. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sutherland BM, Bennett PV, Schenk H, et
al: Clustered DNA damages induced by high and low LET radiation,
including heavy ions. Phys Med. 17(Suppl 1): 202–204. 2001.
|
|
14
|
Sutherland BM, Bennett PV, Weinert E,
Sidorkina O and Laval J: Frequencies and relative levels of
clustered damages in DNA exposed to gamma rays in radioquenching
vs. nonradioquenching conditions. Environ Mol Mutagen. 38:159–165.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leatherbarrow EL, Harper JV, Cucinotta FA
and O’Neill P: Induction and quantification of gamma-H2AX foci
following low and high LET-irradiation. Int J Radiat Biol.
82:111–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmid TE, Dollinger G, Beisker W, et al:
Differences in the kinetics of gamma-H2AX fluorescence decay after
exposure to low and high LET radiation. Int J Radiat Biol.
86:682–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roos WP and Kaina B: DNA damage-induced
cell death by apoptosis. Trends Mol Med. 12:440–450. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung Y and Lippard SJ: Direct cellular
responses to platinum-induced DNA damage. Chem Rev. 107:1387–1407.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez VM, Fuertes MA, Alonso C and
Perez JM: Is cisplatin-induced cell death always produced by
apoptosis? Mol Pharmacol. 59:657–663. 2001.PubMed/NCBI
|
|
20
|
Basu A and Krishnamurthy S: Cellular
responses to cisplatin-induced DNA damage. J Nucleic Acids.
2010:2010. View Article : Google Scholar
|
|
21
|
Vilenchik MM and Knudson AG: Endogenous
DNA double-strand breaks: production, fidelity of repair, and
induction of cancer. Proc Natl Acad Sci USA. 100:12871–12876. 2003.
View Article : Google Scholar : PubMed/NCBI
|