Introduction
Intracranial tumors are relatively common in infants
and young children; the number of cases is second only to leukemia
(1) and the tumors are the most
common type of solid tumor among the pediatric malignancies and
have the highest mortality rates (2). Furthermore, intracranial tumors
significantly affect quality of life and are one of the major
causes of infant mortality (3,4). The
incidence of brain tumors in infants has reportedly been gradually
increasing within recent years (5).
The clinical manifestations, location and
histological features of brain tumors in infants and young children
less than two years old reportedly differ by age (6). Supratentorial tumors occur more
frequently than infratentorial tumors in the youngest patients.
Tumor type also varies by age. In the neonatal period, germ cell
tumors, particularly teratoma, are the most common tumor type
(7), however, in slightly older
infants, astrocytoma, a neuroepithelial tumor, predominates
(8,9). The majority of intracranial tumors are
low-grade tumors (5), followed by
ependymomas and medulloblastomas (10).
Furthermore, the clinical manifestations of
intracranial tumors are different in this age group. Increased head
circumference, headaches and vomiting are typical, resulting from
increased intracranial pressure or obstructive hydrocephalus
(11). Non-specific clinical
symptoms may include irritability, drowsiness, seizures,
developmental delay, difficulty swallowing and hoarseness. As the
infant skull is not fully developed, its compensatory ability is
greater than that of older children. Consequently, symptoms often
appear relatively late (8),
delaying the diagnosis and affecting the overall prognosis.
Although the typical initial symptoms of intracranial hypertension,
such as vomiting, are common, the inability of young patients to
articulate their symptoms increases the difficulties involved in
making an early and accurate diagnosis, and often contributes to
misdiagnosis (12).
The rapid growth of the immature central nervous
system during the first two years of life makes it acutely
sensitive to the detrimental effects of radiotherapy and
chemotherapy, thus, producing relatively serious side-effects in
this age group compared with older children. Treatment-associated
neurological effects include leukoencephalopathy, neurological and
intellectual deficits, growth retardation, and endocrinological
abnormalities (13). Therefore,
radiography and chemotherapy are rarely prescribed in this age
group, adding to the difficulty of treating brain tumors in the
very young (14,15).
The development and refinement of novel
neurosurgical techniques, as well as advanced diagnostic
instruments has increased the importance of surgery as a treatment
strategy for brain tumors (16).
However, the small surgical field and the specific anesthetic
requirements in children under two years old increases the
difficulty of performing such surgery (17). Furthermore, the limited blood volume
and relatively immature physiological functioning of infants only
increases the surgical challenge (18). Despite these constraints, surgical
tumor resection can significantly improve the overall prognosis of
infants and children with brain tumors who are less than two years
old.
The purpose of the present study was to evaluate the
distinct features of pediatric neurologic neoplasms, including
disease onset, clinical manifestations, histopathological features,
characteristics and treatment outcomes, in infants and young
children aged two years and under who presented with brain
tumors.
Patients and methods
Patients
The present retrospective study included 32 infants
aged two years and under with primary brain tumors, who were
accurately diagnosed, treated and followed up between January 2003
and March 2013 in the Department of Neurosurgery at Fudan
University Children’s Hospital (Shanghai, China). The protocol of
the current study was approved by the Institutional Review Board of
Fudan University Children’s Hospital.
Diagnosis and classification
Diagnosis was based on a combination of three
modalities: Pathological examination, clinical symptoms and
diagnostic imaging. The results were obtained by performing
ultrasound imaging diagnosis in combination with examination of
detailed computed tomography (CT) and magnetic resonance imaging
(MRI) findings. In addition, histological classification was
conducted according to the 2007 World Health Organization (WHO)
classification of tumors of the central nervous system (19). For the purpose of evaluation, the
patients were divided into conservative treatment (n=12) and
surgical intervention (n=20) groups, and data regarding disease
onset, clinical manifestations, histopathological features,
characteristics and treatment outcomes were compared.
Statistical analysis
Data were analyzed using SPSS statistical software
(version 18.0; SPSS, Inc., Chicago, IL, USA). Patient gender and
age, tumor location and histological type, and clinical
manifestations were evaluated. The associations between the
treatment strategy received and the prognosis were also determined.
Due to the small sample size, continuous variables are presented as
the median and interquartile range (IQR). Categorical variables are
presented by count and percentage. Differences between the
conservative treatment and surgical intervention arms were compared
using the non-parametric Mann-Whitney U test for the continuous
variables and Fisher’s exact test with Yate’s correction for the
categorical variables. In addition, Kaplan-Meier curves were used
to calculate the overall survival and follow-up times, and the
differences between the survival curves were determined by
performing the log-rank test. Overall survival time was defined as
the date of hospitalization to the date of mortality from the brain
tumor. Patients who were alive at the time of the analysis were
evaluated at the date of last contact on December 31, 2013. All
statistical assessments were two-sided and P≤0.05 was considered to
indicate a statistically significant difference.
Results
Patient demographics
The baseline characteristics of the current series
of 32 pediatric patients with brain tumors are summarized in
Table I. No significant differences
were noted in the demographic characteristics between the two
groups (P>0.05). The median age was 16.0 months (IQR, 8.3–23.3)
for the conservative treatment arm and 17.5 months (IQR, 10.0–23.0)
for the surgical arm. Furthermore, the median follow-up time was
18.0 months (IQR, 1.0–40.8) for the conservative treatment arm and
24.0 months (IQR, 9.3–40.5) for the surgical intervention arm. The
conservative treatment arm included six (50%) males compared with
11 (55%) males in the surgical intervention arm. Five patients
(41.7%) in the conservative treatment arm were infants under one
year old, and seven (58.3%) were children one to two years old. By
contrast, nine (45%) patients in the surgical arm were under one
year old, and 11 (55%) were one to two years old. In the
conservative treatment arm, six patients (50%) presented with
supratentorial brain tumors and six (50%) with infratentorial brain
tumors; however, in the surgical arm, eight patients (40%)
presented with supratentorial brain tumors and 12 (60%) with
infratentorial brain tumors. Additionally, the most common
histological types in the conservative treatment group were
astrocytoma, ependymoma and medulloblastoma (three cases each; 25%
each); compared with astrocytoma (seven cases; 35%) and ependymoma
(six cases; 30%) in the surgical arm.
 | Table IPatient demographic characteristics at
baseline. |
Table I
Patient demographic characteristics at
baseline.
| Characteristic | Conservative
treatment group (n=12) | Surgical intervention
group (n=20) | Overall patients
(n=32) | P-value |
|---|
| Age,
monthsa | 16.0 (8.3–23.3) | 17.5 (10.0–23.0) | 16.5 (9.3–23.0) | 0.744 |
|
0–12b | 5 (41.7) | 9 (45.0) | 14 (43.8) | 0.854 |
|
13–24b | 7 (58.3) | 11 (55.0) | 18 (56.3) | |
| Follow-up period,
monthsa | 18.0 (1.0–40.8) | 24.0 (9.3–40.5) | 21.5 (6.0–40.8) | 0.326 |
| Male
genderb | 6 (50.0) | 11 (55.0) | 17 (53.1) | 0.784 |
|
Locationb | | | |
0.370c |
| Supratentorial | 6 (50.0) | 8 (40.0) | 14 (43.8) | |
| Saddle zone | 3 (25.0) | 0 (0.0) | 3 (9.4) | |
| Hemisphere | 3 (25.0) | 7 (35.0) | 10 (31.3) | |
| Lateral
ventricles | 0 (0.0) | 1 (5.0) | 1 (3.1) | |
|
Infratentorial | 6 (50.0) | 12 (60.0) | 18 (56.3) | |
| Cerebellar
vermis | 2 (16.7) | 5 (25.0) | 7 (21.9) | |
| Cerebellar
hemisphere | 2 (16.7) | 3 (15.0) | 5 (15.6) | |
| Fourth
ventricle | 1 (8.3) | 1 (5.0) | 2 (6.3) | |
| Posterior
fossa | 1 (8.3) | 3 (15.0) | 4 (12.5) | |
| Histological
typeb | | | | 0.650 |
| Astrocytoma | 3 (25.0) | 7 (35.0) | 10 (31.3) | |
| Ependymoma | 3 (25.0) | 6 (30.0) | 9 (28.1) | |
|
Medulloblastoma | 3 (25.0) | 3 (15.0) | 6 (18.8) | |
|
Craniopharyngioma | 1 (8.3) | 0 (0.0) | 1 (3.1) | |
| Hemangioma | 1 (8.3) | 1 (5.0) | 2 (6.3) | |
| Ganglion nerve
glioma | 0 (0.0) | 1 (5.0) | 1 (3.1) | |
| Teratoma | 1 (8.3) | 0 (0.0) | 1 (3.1) | |
| Atypical
teratoma/rhabdoid tumor | 0 (0.0) | 1 (5.0) | 1 (3.1) | |
|
Rhabdomyosarcoma | 0 (0.0) | 1 (5.0) | 1 (3.1) | |
Overall survival
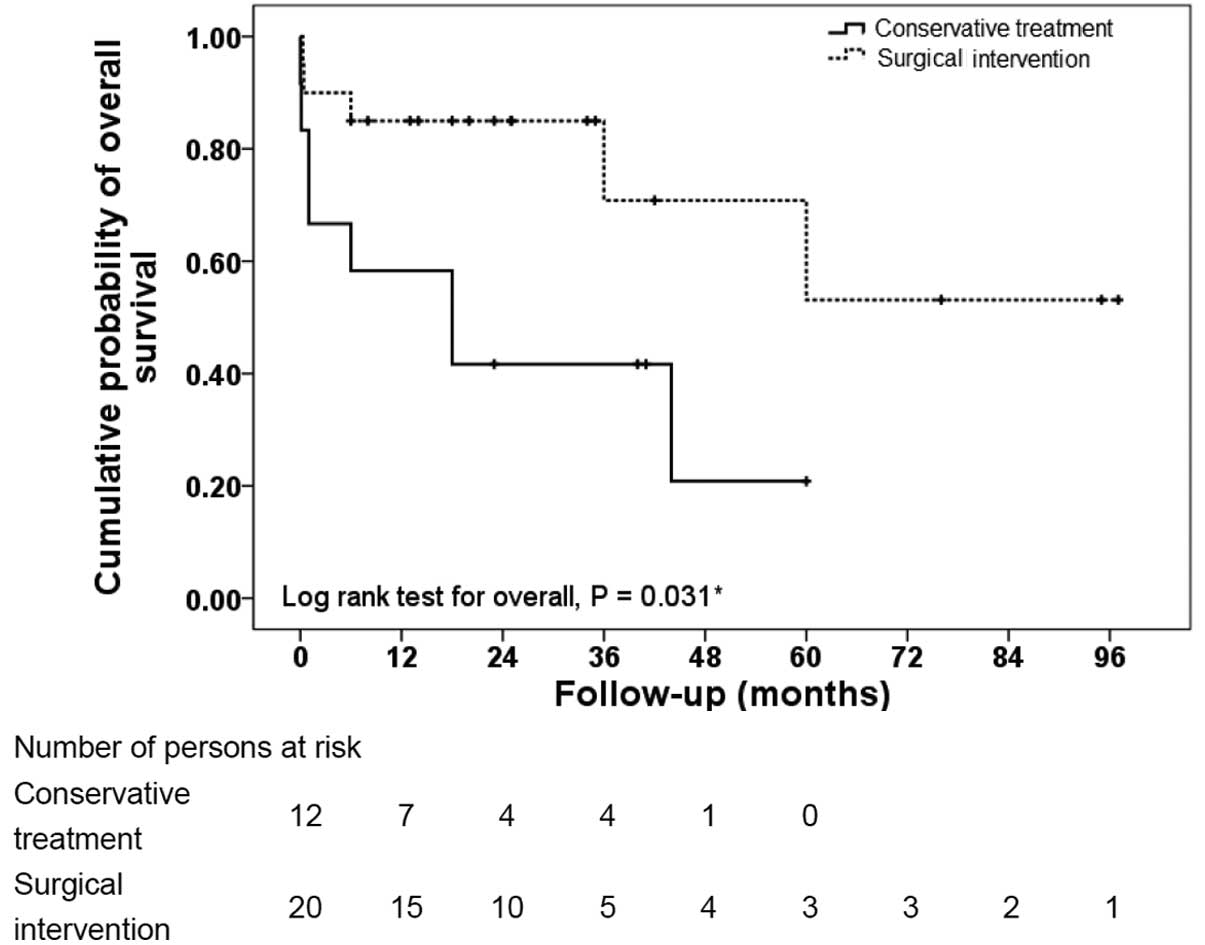
Fig. 1 represents a
comparison between the Kaplan-Meier curves of overall survival for
the conservative treatment and surgical intervention arms. The
survival rate was significantly higher in the surgical intervention
arm (P=0.030, log-rank test). Fig.
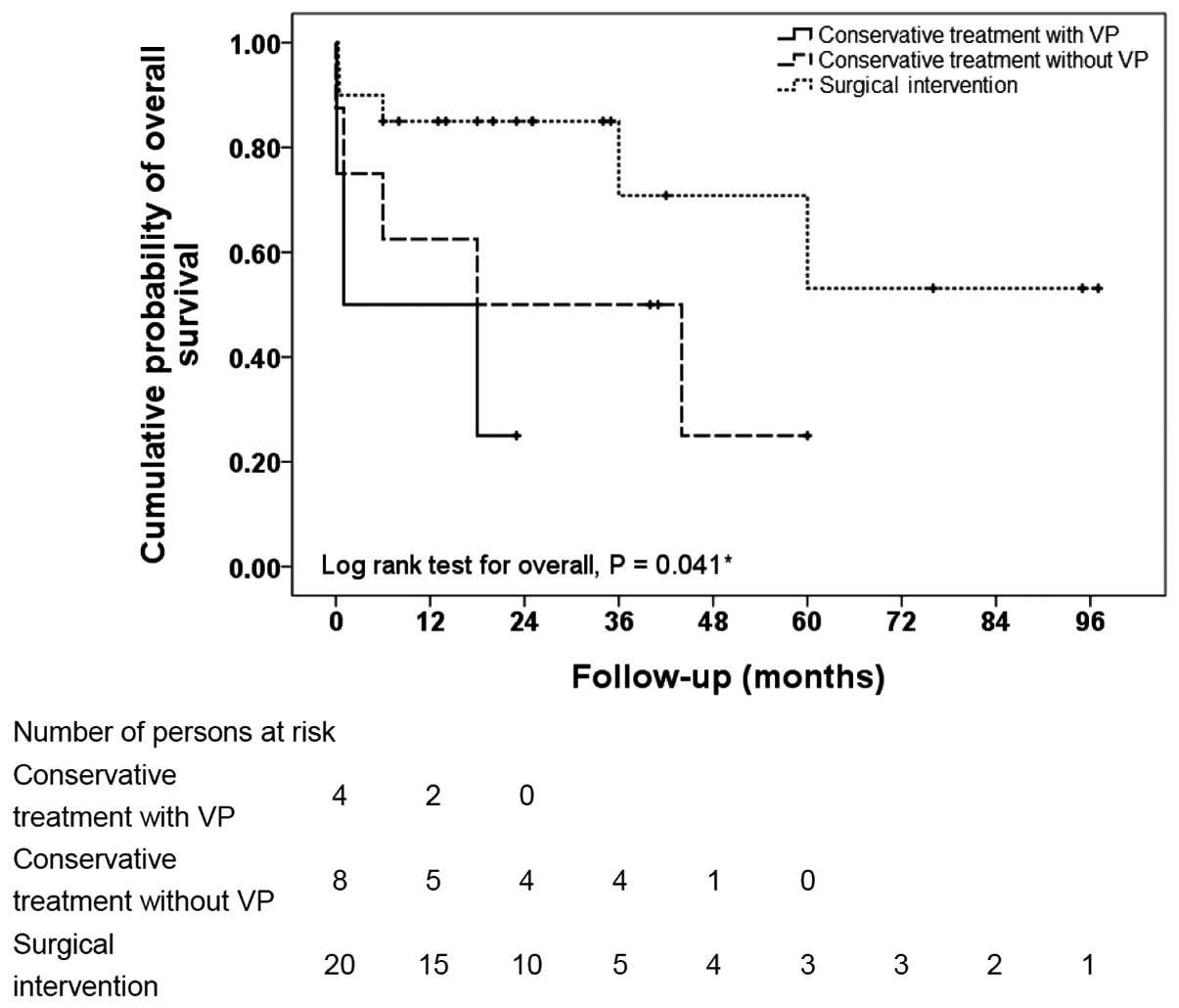
2 indicates the Kaplan-Meier curves of overall survival for the
following three treatment arms: Conservative treatment with
ventriculoperitoneal shunting, conservative treatment without
shunting and surgical intervention. The overall survival rate was
significantly different between the three treatment groups
(P=0.041, log-rank test). Patients who received surgery survived
for the longest period of time, followed in order by those treated
with conservative therapy alone and those treated conservatively
with ventriculoperitoneal shunting.
Diagnosis
The majority of patients were admitted to the
hospital between 15 days and 4 months after disease onset, defined
as the presence of symptoms based on descriptions by the patients
or parents. The mean disease course was 52 days (range, 3 h to five
months). The most common primary symptoms were caused by
intracranial hypertension, and included headaches, nausea and
vomiting (11 cases; 34.4%). Limb weakness or limited activity was
observed in 10 cases (31.3%). In four cases (12.5%), the tumor was
discovered during the preliminary examination. Furthermore, three
patients (9.4%) experienced convulsive seizures, four (12.5%)
exhibited abnormal vision and one (3.1%) exhibited precocious
sexual behavior. Additionally, two patients had been transferred to
Fudan University Children’s Hospital from other hospitals after
being misdiagnosed with a gastrointestinal disorder.
Treatment strategies
In total, 20 children (62.5%) underwent surgical
tumor resection. Of these, 13 exhibited middle- and low-grade
tumors, while seven presented with high-grade tumors; thus, the
ratio between low- and high-grade tumors was ~2:1. A total of 14
patients were discharged following improvement in their symptoms or
upon being considered cured, accounting for 70% of the children who
underwent surgery or 43.8% of all infants and children involved in
the current study. Tumors recurred in two patients (6.3%), each
within the first post-operative year, and one infant (3.1%)
succumbed due to post-operative sputum aspiration. Additionally,
the mean survival time of the infants and children who underwent
surgical tumor resection was 67.6 months [95% confidence interval
(CI), 46.6–88.6 months].
Of the 12 infants and children who received
conservation treatment alone, eight presented with low-grade tumors
and four with high-grade tumors; thus, the ratio between low- and
high-grade tumors was 2:1. The mean survival time in these patients
was 25.3 months (95% CI, 11.4–39.3 months). By contrast, the mean
survival time for the four patients who received conservative
therapy with ventriculoperitoneal shunting was 10.5 months (95% CI,
0.6–20.5 months).
Discussion
The clinical features, diagnosis and treatment
strategies associated with brain tumors in infants and children
under two years of age are unique. In the present 10-year
epidemiological study of such tumors in a single institution, it
was identified that a marginally greater number of males were
affected than females, that more tumors were infratentorial than
suptratentorial (56.2 vs. 43.8%, respectively), that intracranial
hypertension was the most common onset symptom and that astrocytoma
was the most common tumor type. Furthermore, prognosis differed
according to the type of treatment received: Surgical tumor
resection was associated with a mean post-operative survival time
of 67.6 months; conservative treatment with medications and
observation was associated with a mean survival time of 25.3
months; and the four patients who received conservative therapy
combined with ventriculoperitoneal shunting exhibited a mean
survival time of 10.5 months.
The incidence of brain tumors in children is
reported to be gradually increasing (1). Although the exact reasons behind this
increasing trend are unknown, we propose that it is due to a
combination of factors. For example, advanced imaging techniques
have improved the overall ability to diagnose brain tumors in this
age group, therefore, it is possible that the actual incidence has
not increased, but that tumors are being diagnosed earlier.
Additionally, factors such as global environmental effects and
exposure to various carcinogens may be contributing to a real
increase in the incidence of brain tumors (1). However, in the current study, which
included all infants and young children admitted to Fudan
University Children’s Hospital in a 10-year period between 2003 and
2013, no increase occurred in the number of brain tumors in
children aged two years and under. Furthermore, the types of tumor
observed in the present study were generally common types; only one
atypical teratoid rhabdoid tumor was identified, however, no
embryonal tumors or other infant tumors that may be expected in a
series of this size were diagnosed.
The occurrence of brain tumors in children who are
less than two years old is associated with distinct patterns. For
example, males are marginally more likely than females to develop
brain tumors (6), as supported by
the cases analyzed in the present study. Additionally,
supratentorial tumors are reported to occur at a marginally higher
rate than other types of tumors (8)
Furthermore, incidence increases with increasing age, therefore,
patients who are closer to two years of age exhibit a marginally
higher incidence of brain tumor compared with patients who are less
than one year old. In addition, patients less than one year old are
reported to develop more supratentorial than infratentorial tumors.
This complicates the diagnosis, as the wide supratentorial space in
younger children delays the appearance of clinical symptoms
compared with older children (20,21).
However, contradictory results were observed in the current series,
with the occurrence of a marginally higher incidence (56.3%) of
infratentorial brain tumors. One possible cause for this
discrepancy is that the current study only represents cases from a
single center and included a relatively small number of patients.
Additionally, geographical and cultural factors may have
contributed to the difference.
Astrocytoma, which is a neuroepithelial tumor, is
reported to be the most common histological tumor type in infants
under two years old (10,22). This is compatible with the findings
of the current study, which identified astrocytoma as the most
frequently diagnosed tumor type, occurring in 9/32 patients
(28.1%). Tumors in this age group are typically located in the
supratentorial and infratentorial cerebellar hemispheres, and
low-grade tumors are most prevalent (23,24).
The majority of infratentorial tumors in the present series were
medulloblastoma and pilocytic astrocytoma, and the number of
low-grade tumors was similar to the number of high-grade tumors.
Furthermore, the tumors were predominantly distributed around the
midline. The majority of supratentorial tumors were located in the
sellar region, and the majority of the infratentorial tumors were
located in the cerebellar vermis, possibly as they are embryonal in
nature (10). Similar to the
results reported in a previous study (10), the three most common histological
tumor types observed in the current study were astrocytoma,
ependymoma and medulloblastoma.
Two cases in the current study were misdiagnosed as
gastrointestinal diseases in other hospitals due to unexplained
vomiting. The tumors were diagnosed only after the patients were
transferred to Fudan University Children’s Hospital for additional
treatment, which delayed an early diagnosis and the commencement of
appropriate treatment. These two cases underscore the fact that the
diagnosis is often delayed in this age group. This is partially due
to infants being unable to articulate their symptoms. Parents
typically notice that their child is irritable most of the time and
cries more often than usual, however, they attribute the symptoms
to another cause (25). Physicians
may also attribute the symptoms to more common causes.
Headaches are typically a feature of functional
diseases in older children, whereas in infants, headaches are often
the result of organic disease (26,27).
Therefore, it is important to take note of headaches in infants. In
the current series, a relatively high proportion of patients (10
patients; 31.3%) were admitted to the hospital exhibiting specific
neurological signs, such as epilepsy or limb dysfunction..
Ultrasound imaging is an important diagnostic
modality for the early detection of intracranial tumors in this age
group (28). Four patients were
admitted to Fudan University Children’s Hospital after ultrasound
detected intracranial lesions during initial examinations, allowing
for the early initiation of treatment. Additionally, enhanced CT
and MRI are important for localizing and providing a qualitative
diagnosis of tumors in this age group (29). The pre-operative images obtained
using these techniques correlated well with the pathological
findings for those infants who underwent surgical tumor resection.
Thus, ultrasound imaging combined with the use of enhanced CT and
MRI may facilitate a correct diagnosis of the intracranial location
of the majority of common brain tumors, greatly improving the
accuracy of the diagnosis (30,31).
Surgery is considered to be a direct and effective
means of treating brain tumors in infants and young children, and
surgeons advise that tumors should be removed as completely as
possible, as the extent of resection correlates closely with
overall prognosis (32,33). In a previous study, increasing the
extent of resection was associated with improved survival,
regardless of age, degree of disability or WHO grade, in children
with malignant astrocytomas (32).
However, the majority of tumors in infants grow near the midline or
occur in important functional areas of the brain, therefore, it is
challenging to avoid damaging these structures during surgery.
Intraoperative nerve navigation facilitates the definition of the
extent of tumor resection and protects important neural structures
when resecting tumors maximally (34), which is important in improving the
post-operative prognosis and quality of life (35). Thus, Fudan University Children’s
Hospital have recently commenced the use of intraoperative nerve
navigation for brain tumor resections, with the aim of maximizing
patient outcomes.
The 20 patients who underwent surgical tumor
resection in the current series exhibited relatively satisfactory
prognoses. A total of 14 patients were discharged after their
symptoms improved or when they were considered cured, and the mean
survival time of 67.6 months was a significant improvement on the
25.3-month mean survival time of the patients who received
conservative treatment (P=0.030). However, surgery may not be an
option for critically ill patients who would not survive the
procedure or who would not derive any benefits from surgical tumor
excision. Therefore, high surgical risk is a prognostic factor for
pediatric patients. Infants who are not candidates for surgery are
typically prescribed medication, which may be combined with
ventriculoperitoneal shunting to relieve clinical symptoms
(36).
In the present study, single ventriculoperitoneal
shunting was performed in four infants with severe obstructive
hydrocephalus secondary to tumor compression who were not
candidates for surgery. Although the shunts relieved the high
intracranial pressure and clinical symptoms caused by the
obstructed cerebrospinal fluid, the mean survival time of 10.5
months was significantly less than the mean survival time of the
infants who were treated using surgery or conservative therapy
alone (P=0.041). One possible explanation for this significant
difference is that these infants were more critically ill than
those who were treated with conservative therapy alone. Two of the
four patients presented with high-grade tumors and two with
low-grade tumors; this ratio of high-grade tumors in the
ventriculoperitoneal shunting treatment group is marginally higher
compared with the non-ventriculoperitoneal shunting and
conservative therapy group. Additionally, it is possible that
single ventriculoperitoneal shunting may accelerate tumor spread in
the peritoneal cavity or otherwise aggravate the disease (37).
In the present study, two patients experienced tumor
recurrence within the first post-operative year, at an incidence
rate of 10% of the total surgical cohort. One patient presented
with a low-grade tumor and the other with a high-grade tumor.
According to the WHO classification system, the ratio between low-
and high-grade tumors is similar in infants who undergo surgical
treatment and those who receive conservative treatment (19).
In the present study, post-operative recurrence and
mortality predominantly occurred in patients whose tumors were
located near vital centers, such as the brain stem or ventricle.
These areas are of relatively high surgical risk, and it is
difficult to completely remove the affected tissue (38). One such patient in the present study
succumbed post-operatively due to aspirating sputum.
The present study was a retrospective chart review
and, thus, had certain inherent limitations, which included limited
patient histories and a small sample size. In addition, the current
study included a relatively limited series of 32 infants who were
treated for brain tumors at Fudan University Children’s Hospital
and were available for follow-up. Patients lost to follow-up were
excluded, however, a comparison of the histopathological diagnoses
of the two treatment groups indicated no significant difference in
the tumor classification or grade. Therefore, standard deviation
could be controlled for when the statistical analysis was
conducted.
Pathological examination was not possible for those
infants who had received conservative treatment. However, among all
32 pediatric cases of common brain tumors, ultrasound imaging
diagnoses were combined with the details of enhanced CT and MRI
examination, allowing the observation of relatively unique clinical
manifestations of brain tumors and positioning signs in the infants
and young children, which ensured the greatest degree of diagnostic
accuracy.. However, errors in diagnosis are inevitable and may
affect the results. Despite possible discrepancies, the ratio
between the low- and high-grade tumors in the surgical cohort was
similar to the ratio in the patients who received conservative
therapy, demonstrating, to an extent, the reliability of the
results of the present study.
In conclusion, surgical tumor resection remains a
direct and effective method for treating infant brain tumors. The
current study indicates that surgical tumor resection may improve
the overall prognosis of infants aged two years and under who
presented with brain tumors. Ventriculoperitoneal shunts may pre-
and post-operatively facilitate the improvement of clinical
symptoms by relieving intracranial pressure from accumulated
cerebrospinal fluid; however, they do not increase long-term
survival. In addition, high surgical risk is a prognostic factor in
this pediatric patient population.
Acknowledgements
The authors would like to thank Mr. Zheng Shan, the
Vice President of Fudan University Children’s Hospital, for
supporting the present study.
References
|
1
|
Baldwin RT and Preston-Martin S:
Epidemiology of brain tumors in childhood - a review. Toxicol Appl
Pharmacol. 199:118–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magnani C, Aareleid T, Viscomi S, Pastore
G and Berrino F; EUROCARE Working Group. Variation in survival of
children with central nervous system (CNS) malignancies diagnosed
in Europe between 1978 and 1992: the EUROCARE study. Eur J Cancer.
37:711–721. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macedoni-Luksic M, Jereb B and Todorovski
L: Long-term sequelae in children treated for brain tumors:
impairments, disability, and handicap. Pediatr Hematol Oncol.
20:89–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aarsen FK, Paquier PF, Reddingius RE,
Streng IC, Arts WF, Evera-Preesman M and Catsman-Berrevoets CE:
Functional outcome after low-grade astrocytoma treatment in
childhood. Cancer. 106:396–402. 2006. View Article : Google Scholar
|
|
5
|
Raaschou-Nielsen O, Sørensen M, Carstensen
H, Jensen T, Bernhardtsen T, Gjerris F and Schmiegelow K:
Increasing incidence of childhood tumours of the central nervous
system in Denmark, 1980–1996. Br J Cancer. 95:416–422. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sala F, Colarusso E, Mazza C, Talacchi A
and Bricolo A: Brain tumors in children under 3 years of age.
Recent experience (1987–1997) in 39 patients. Pediatr Neurosurg.
31:16–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isaacs H Jr: I. Perinatal brain tumors: a
review of 250 cases. Pediatr Neurol. 27:249–261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bishop AJ, McDonald MW, Chang AL and
Esiashvili N: Infant brain tumors: incidence, survival, and the
role of radiation based on Surveillance, Epidemiology, and End
Results (SEER) Data. Int J Radiat Oncol Biol Phys. 82:341–347.
2012. View Article : Google Scholar
|
|
9
|
Johnson MW, Eberhart CG, Perry A, Tihan T,
Cohen KJ, Rosenblum MK, Rais-Bahrami S, Goldthwaite P and Burger
PC: Spectrum of pilomyxoid astrocytomas: intermediate pilomyxoid
tumors. Am J Surg Pathol. 34:1783–1791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furuta T, Tabuchi A, Adachi Y, Mizumatsu
S, Tamesa N, Ichikawa T, Tamiya T, Matsumoto K and Ohmoto T:
Primary brain tumors in children under age 3 years. Brain Tumor
Pathol. 15:7–12. 1998. View Article : Google Scholar
|
|
11
|
Hanieh S, Hanieh A, Bourne AJ and Byard
RW: Brain tumours in infancy: a clinicopathological study. J Clin
Neurosci. 4:181–185. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larouche V, Huang A, Bartels U and Bouffet
E: Tumors of the central nervous system in the first year of life.
Pediatr Blood Cancer. 49(Suppl): 1074–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DiRosso C, Ianelli A and Papacci F:
Infantile brain tumors. Tumors of the Pediatric Central Nervous
System. 2. Keating RF, Goodrich JT and Packer R: Thieme; New York:
pp. 3942013
|
|
14
|
Magdum SA: Neonatal brain tumours - a
review. Early Hum Dev. 86:627–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thorp N: Basic principles of paediatric
radiotherapy. Clin Oncol (R Coll Radiol). 25:3–10. 2013. View Article : Google Scholar
|
|
16
|
Kim YH, Song SW, Lee JY, et al: Surgically
treated brain tumors: a retrospective case series of 10,009 cases
at a single institution. World Neurosurg. 76:555–563. 2011.
View Article : Google Scholar
|
|
17
|
Soriano SG, Eldredge EA and Rockoff MA:
Pediatric neuroanesthesia. Anesthesiol Clin North America.
20:389–404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gaggero R, Consales A, Fazzini F, et al:
Epilepsy associated with supratentorial brain tumors under 3 years
of life. Epilepsy Res. 87:184–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dunham C, Pillai S and Steinbok P: Infant
brain tumors: a neuropathologic population-based institutional
reappraisal. Hum Pathol. 43:1668–1676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramanan M and Chaseling R: Paediatric
brain tumours treated at a single, tertiary paediatric
neurosurgical referral centre from 1999 to 2010 in Australia. J
Clin Neurosci. 19:1387–1391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ibrahim K and Appleton R: Seizures as the
presenting symptom of brain tumours in children. Seizure.
13:108–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balestrini MR, Micheli R, et al: Brain
tumors with symptomatic onset in the first two years of life.
Childs Nerv Syst. 10:104–110. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rickert CH, Probst-Cousin S and Gullotta
F: Primary intracranial neoplasms of infancy and early childhood.
Childs Nerv Syst. 13:507–513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rangwala LM and Liu GT: Pediatric
idiopathic intracranial hypertension. Surv Ophthalmol. 52:597–617.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohen BH: Headaches as a symptom of
neurological disease. Semin Pediatr Neurol. 2:144–150. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fleming AJ and Chi SN: Brain tumors in
children. Curr Probl Pediatr Adolesc Health Care. 42:80–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Orbach D, Sarnacki S, Brisse HJ, et al:
Neonatal cancer. Lancet Oncol. 14:e609–e620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan SN and Sepahdari AR: Orbital masses:
CT and MRI of common vascular lesions, benign tumors, and
malignancies. Saudi J Ophthalmol. 26:373–383. 2012. View Article : Google Scholar
|
|
30
|
Parmar HA, Pruthi S, Ibrahim M and Gandhi
D: Imaging of congenital brain tumors. Semin Ultrasound CT MR.
32:578–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koob M and Girard N: Cerebral tumors:
specific features in children. Diagn Interv Imaging. 95:965–983.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McGirt MJ, Chaichana KL, Gathinji M, et
al: Independent association of extent of resection with survival in
patients with malignant brain astrocytoma. J Neurosurg.
110:156–162. 2009. View Article : Google Scholar
|
|
33
|
Khalil EM: Treatment results of adults and
children with medulloblastoma NCI, Cairo University experience. J
Egypt Natl Canc Inst. 20:175–186. 2008.PubMed/NCBI
|
|
34
|
Vabulas M, Kumar VA, Hamilton JD, Martinez
JJ, Rao G, Sawaya R and Prabhu SS: Real-time atlas-based
stereotactic neuronavigation. Neurosurgery. 74:128–134. 2014.
View Article : Google Scholar
|
|
35
|
Steinbok P, Mangat JS, Kerr JM, Sargent M,
Suryaningtyas W, Singhal A and Cochrane D: Neurological morbidity
of surgical resection of pediatric cerebellar astrocytomas. Childs
Nerv Syst. 29:1269–1275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roujeau T, Di Rocco F, Dufour C, et al:
Shall we treat hydrocephalus associated to brain stem glioma in
children? Childs Nerv Syst. 27:1735–1739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han YP, Zhao Y, He XG and Ma J: Peritoneal
metastasis of third ventricular atypical teratoid/rhabdoid tumor
after VP shunt implantation for unexplained hydrocephalus. World J
Pediatr. 8:367–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garzón M, García-Fructuoso G, Guillén A,
Suñol M, Mora J and Cruz O: Brain stem tumors in children and
adolescents: single institutional experience. Childs Nerv Syst.
29:1321–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|