Introduction
The clinical tumor size (cT), which is determined by
palpation during physical examination or by imaging, is important
in determining patient prognosis and treatment (1–4).
Clinical staging determines the necessity of pre-operative
chemotherapy and sentinel lymph node biopsy. However, the methods
used to determine tumor size can yield various results, which in
turn can affect treatment options and ultimately, patient outcomes.
For example, clinical staging based on physical examination is
subjective due to the examiner. This method is also not effective
for nonpalpable lesions (5). Breast
imaging methods, which include mammography, ultrasonography and
magnetic resonance imaging (MRI), are less subjective, but also
demonstrate certain limitations. The tumor boundary in mammography
is often unclear in dense breast tissues, particularly in Asian or
young women, and the tumor size may vary depending on the type of
mammography performed. Additionally, numerous studies indicate that
mammography underestimates the true tumor size compared to the
histopathological size (6–8). By contrast, breast ultrasonography
allows for easier measurement of the longest tumor dimension and is
strongly correlated with the histopathological size compared to
physical examination or mammography, although numerous studies
indicate that ultrasonography (USG) also underestimates the tumor
size compared with the histopathological size (6,7).
In contrast to cT, the pathological tumor size (pT)
is determined by microscopic measurement of surgical specimens, and
accurate pathological tumor staging (T staging) plays a decisive
role in determining whether to perform post-operative adjuvant
chemotherapy. Among biopsy methods, vacuum-assisted breast biopsy
(VABB), a more recently developed form of core needle biopsy, is
now widely used. This biopsy allows complete excision of target
lesions, with accuracy similar to that of excisional biopsy.
However, the fragmented specimens create certain challenges in
histopathological assessment (9).
Based on the importance of obtaining reliable information regarding
tumor size to accurately determine cancer stage, the purpose of the
present study was to investigate any differences between
pre-operative clinical T stage and histopathological T stage
subsequent to surgery in VABB-diagnosed breast cancer.
Materials and methods
The present retrospective study was conducted using
the medical records of 209 patients out of 294 potential
participants diagnosed with invasive breast cancer by VABB
(Mammotome®; Devicor Medical Products, Inc., Cincinnati,
OH, USA) at the Department of Surgery of Kangnam CHA Hospital, CHA
University College of Medicine (Seoul, Republic of Korea) between
January 2007 and December 2012. The remaining 85 patients were
excluded due to the cases reporting the use of neoadjuvant
chemotherapy, or the presence of ductal carcinoma in situ or
invasive breast cancer with extensive intraductal components. All
patients underwent surgical resection of breast cancer, comprising
modified radical mastectomy or breast-conserving surgery, with or
without sentinel lymph node biopsy. VABB was performed using an
eight-gauge needle on USG-assessed lesions classified as breast
imaging reporting and data system (BI-RADS) categories 3, 4a-c and
5. Complete excisional biopsy was performed for USG-assessed
lesions categorized as category 3 or 4a, but only incisional biopsy
using VABB was performed to obtain 3–5 core tissue samples in
lesions that were classified as category 4b and above. The
histological tumor size was measured in the long axis. The presence
of synchronous cancers and the extent of retroareolar involvement
were recorded using sonograms.
Breast ultrasonography was performed using duplex
sonography that uses B-mode and color Doppler with a probe of 7–12
MHz high frequency Linear Array (Logic 700; GE Healthcare
Bio-sciences, Pittsburgh, PA, USA; HDI 5000; Philips Ultrasound,
Bothell, WA, USA). The tumor size was determined based on the
longest dimension of the measured tumor, although the long
cytoplasmic processes of the tumor were excluded from measurement.
Sagittal and transverse views were obtained for each mass, and the
longest dimension was obtained for each transducer position.
Whenever possible, the longest dimension was obtained collinear to
the ultrasound beam. The present study was performed with the
approval of the Institutional Ethical Committee of Kangnam CHA
University Hospital (approval number, KNC13-016).
Statistical analysis
All statistical analyses were performed using SPSS
version 14.0 (SPSS Inc., Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant difference. The
tumor sizes that were measured using USG and histology were
calculated and analyzed by paired t-tests. Bivariate simple
correlation analysis of the tumor size was performed using
Pearson’s correlation coefficient. Pearson’s correlation
coefficient was analyzed between the tumor size in the final
pathological result subsequent to surgery and the pre-operative
tumor size on USG.
Results
In total, 209 patients were enrolled in the present
study. These patients were classified by the ultrasound BI-RADS
categorization as shown in Table I.
A comparison in size between the clinical T staging performed using
USG and the final pathological T stage subsequent to surgery
revealed that the pathological tumor size was smaller than the
USG-determined size in the majority of cases (114 out of 209
patients; 54.5%). The pathological tumor size and USG-determined
size were equal in 34 cases (16.3%), while the pathological tumor
size was larger than the USG-determined size in 61 cases (29.2%)
(Table II). Further analysis of
these results by T staging revealed that the pathological tumor
size was smaller than the USG-determined size in 12 out of 13 pT1a
cases (92.3%). In addition, the pathological tumor size was smaller
than the USG-determined size in 37 of the 49 pT1b cases (75.5%).
This was also reported in 34 out of 77 pT1c cases (44.2%) and in 31
out of 65 pT2 cases (47.7%), but was not observed in the three pT3
cases (0.0%).
 | Table IBreast imaging reporting and data
system usltrasonography categories of invasive breast cancer
diagnosed by vacuum-assisted breast biopsy. |
Table I
Breast imaging reporting and data
system usltrasonography categories of invasive breast cancer
diagnosed by vacuum-assisted breast biopsy.
| Category | Patients, n (%) |
|---|
| 3 | 7 (3.3) |
| 4a | 36 (17.2) |
| 4b | 44 (21.1) |
| 4c | 47 (22.5) |
| 5 | 75 (35.9) |
| Total | 209 (100) |
 | Table IIOverall comparison between the
post-operative permanent pathological size and the initial
USG-determined size. |
Table II
Overall comparison between the
post-operative permanent pathological size and the initial
USG-determined size.
| | Pathological size vs.
USG-determined size, n (%) | |
|---|
| |
| |
|---|
| T Stage | Total, n | Larger | Equal | Smaller | P-value |
|---|
| pT1a | 13 | 0 (0.0) | 1 (7.7) | 12 (92.3) | 0.003 |
| pT1b | 49 | 4 (8.2) | 8 (16.3) | 37 (75.5) | 0.0001 |
| pT1c | 77 | 29 (37.7) | 14 (18.2) | 34 (44.2) | 0.0161 |
| pT2 | 65 | 24 (36.9) | 10 (15.4) | 31 (47.7) | 0.9337 |
| pT3 | 5 | 4 (80.0) | 1 (20.0) | 0 (0.0) | 0.2829 |
| Total | 209 | 61 (29.2) | 34 (16.3) | 114 (54.5) | 0.0001 |
Taken together, these findings indicate that the
larger the size of the primary tumor, the lower the possibility of
histological underestimation. This is likely due to the volume of
specimens excised by VABB being relatively small, while the
residual lesion of larger primary tumors exists in a wide range,
making pathological measurement easier.
Analysis on ultrasound BI-RADS categorization
revealed that in 27 of 43 category 3–4a cases (62.8%), in which
complete excision by VABB, the pathological tumor size was smaller
than the USG-determined size, while only 10 cases (23.3%) revealed
the opposite result (Table III).
An analysis of the aforementioned results by T staging also
demonstrated that the pathological tumor size was smaller than the
USG-determined size in 100% of pT1a cases, 77.8% of pT1b cases,
33.3% of pT1c cases, 66.7% of pT2 cases and 0% of pT3 cases, again
indicating that the bigger the pathological tumor size, the less
likely it is that histological underestimation occurs. However, 88
out of 164 cases (53.7%) in category 4b-5, where an incisional
biopsy by VABB was performed, revealed that the pathological tumor
size was smaller than the USG-determined size (Table IV). Further analysis of the
category 4b-5 results by tumor-node-metastasis (TNM) staging showed
that the pathological tumor size was smaller than the
USG-determined size in 88.9% of pT1a cases, 82.8% of pT1b cases,
46.8% of pT1c cases, 45.8% of pT2 cases and 0.0% of pT3 cases,
confirming that the larger the pathological tumor size, the less
likely it is that histological underestimation takes place. Simple
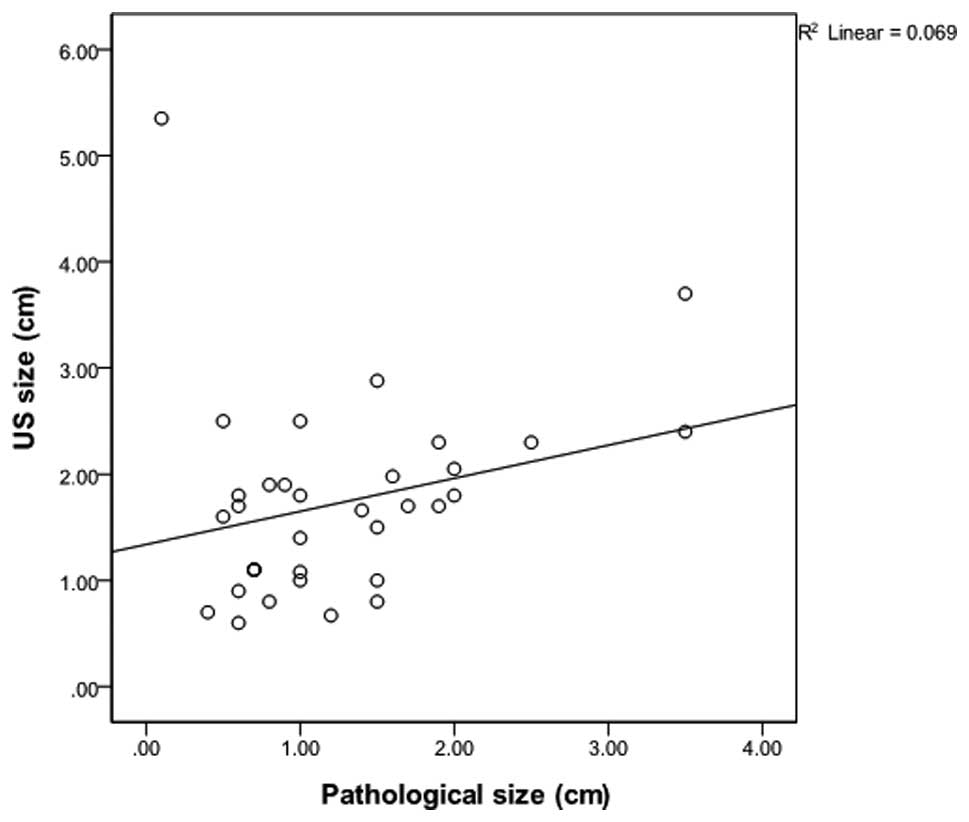
correlation analysis on the category 3–4a and 4b-5 groups revealed
that the correlation coefficient of the category 3–4a group was
0.262 (P=0.129), which was lower than the coefficient of 0.502
(P<0.01) identified in the category 4b-5 group (Figs. 1 and 2). These findings indicate that
histological underestimation occurs more commonly when a target
lesion is confirmed as malignant following complete excision of the
USG category 3 or 4a lesion using VABB compared with incisional
biopsy only for lesions in USG category 4b or above.
 | Table IIIComparison between the post-operative
permanent pathological size and initial USG-determined size in USG
category 3–4a lesions. |
Table III
Comparison between the post-operative
permanent pathological size and initial USG-determined size in USG
category 3–4a lesions.
| | Pathological size vs.
USG-determined size, n (%) | |
|---|
| |
| |
|---|
| T Stage | Total, n | Larger | Equal | Smaller | P-value |
|---|
| pT1a | 4 | 0 (0.0) | 0 (0.0) | 4 (100.00) | 0.141 |
| pT1b | 18 | 0 (0.0) | 4 (22.2) | 14 (77.8) | 0.0001 |
| pT1c | 15 | 8 (53.3) | 2 (13.3) | 5 (33.3) | 0.862 |
| pT2 | 6 | 2 (33.3) | 0 (0.0) | 4 (66.7) | 0.441 |
| pT3 | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Total | 43 | 10 (23.3) | 6 (14.0) | 27 (62.8) | 0.01 |
 | Table IVComparison between the post-operative
permanent pathological size and the initial USG-determined size in
USG category 4b-5 lesions. |
Table IV
Comparison between the post-operative
permanent pathological size and the initial USG-determined size in
USG category 4b-5 lesions.
| | Pathological size vs.
USG-determined size, n (%) | |
|---|
| |
| |
|---|
| T Stage | Total, n | Larger | Equal | Smaller | P-value |
|---|
| pT1a | 9 | 0 (0.0) | 1 (11.1) | 8 (88.9) | 0.016 |
| pT1b | 29 | 1 (3.4) | 4 (13.8) | 24 (82.8) | 0.002 |
| pT1c | 62 | 21 (33.9) | 12 (19.3) | 29 (46.8) | 0.015 |
| pT2 | 52 | 22 (37.3) | 10 (16.9) | 27 (45.8) | 0.783 |
| pT3 | 5 | 4 (80.0) | 1 (20.0) | 0 (0.0) | 0.283 |
| Total | 164 | 48 (29.3) | 28 (17.1) | 88 (53.7) | 0.001 |
Discussion
Tumor size is essential not only for determining the
clinical stage prior to surgery, assessing the requirement for
pre-operative chemotherapy and deciding whether to perform sentinel
lymph node biopsy, but is also crucial for determining the stage of
tumors, the prognosis for the patient and the necessary
post-operative adjuvant therapy (4,10,11).
Tumor size can be measured by physical examination, mammography,
USG and MRI prior to surgery and by pathological T staging
subsequent to surgery. Physical examination is an easy, simple and
economical measurement method that yields immediate results.
However, it is limited in cases involving nonpalpable breast masses
that are clinically latent deep within the breast tissue (5), and the assessment can be subjective
based on factors such as the examiner and obesity (12). Mammography is a more objective
measurement for even nonpalpable breast cancer and it is also less
affected by either the patient or the examiner (6). However, the determined tumor size can
be inconsistent depending on the distance between the breast tumor
and the X-ray film, and the maximal diameter of the tumor can be
inaccurate in the plane dimension (7). Accurate measurement is also limited in
young and premenopausal women with dense breast tissues, or in
cases of tumors with long and slender cytoplasmic processes and
unclear boundaries (6–8). By contrast, breast sonography is known
to be more useful in patients with dense breast tissues (8), and its usage has been increasing.
Breast USG allows for the measurement of the longest dimension of a
breast mass from various directions, with no artificially enlarged
image of a mass and no radiation exposure. However, breast USG is a
relatively subjective procedure that depends on the examiner,
resulting in non-reproducible results. In addition, the boundary of
the mass must be clear to ensure accurate measurement (6,7).
Despite these limitations, USG measurements of the
mass size are usually more accurate than physical examination or
mammography (13–15). Forouhi et al reported that
the USG-determined size demonstrated an improved correlation with
the histological size (correlation coefficient, 0.89) compared to
the size obtained by physical examination or mammography (9). Other studies have also reported that
breast ultrasonography can measure the mass size with the greatest
accuracy, demonstrating correlation coefficients with physical
examination or mammography of 0.84 and 0.80, respectively (6,16).
Choi et al demonstrated that the correlation coefficient
with the histological size was 0.83, which is significantly higher
compared with the value obtained by physical examination or
mammography, demonstrating that breast USG is the most accurate
measurement of breast masses (7).
With the increased used of VABB for the diagnosis of breast cancer,
measurement of the histopathological size has become increasingly
challenging. The present study aimed to identify the impact of VABB
on tumor size measurements by comparing the pre-operative
USG-determined size with the post-operative histopathological size
in 209 patients with invasive breast cancer who underwent surgery
subsequent to tissue biopsy by VABB.
The USG-determined breast mass size strongly
correlates with the histological tumor size, although it tends to
underestimate the pathological size (7,17–19).
Several studies have reported that mass size has been
underestimated in ~80% of cases by breast USG (6,9,16).
Breast USG particularly underestimates tumors 2 cm in size. In
addition, Lee et al reported that physical examination,
mammography and USG tended to underestimate tumor size (20). This was attributed to the fact that
patients involved in the study possessed relatively large tumors
that were all clinically palpable.
In the present study, the USG-determined and
pathological tumor sizes were compared, revealing 148 cases (70.8%)
in which the tumor sizes were equal in size between USG and
pathological analysis or overestimated by USG, while numerous other
studies have revealed a tendency of underestimation in USG. This is
due to the measured pathological size being smaller than the
primary lesion, since the pathological size represents the
measurement of the residual lesion subsequent to biopsy using VABB.
In comparison with the USG-determined size, the pathological size
was found to be smaller in 114 cases (54.5%) in the present study.
The pathological tumor size was smaller than the USG-determined
size in 92.3% of pT1a cases, 75.5% of pT1b cases, 44.2% of pT1c
cases and 47.7% of pT2 cases, indicating that the measurement of
tumor size is smaller pathologically compared with USG when the
primary tumor appears smaller clinically. By contrast, the
histopathological tumor size was larger than the USG-determined
size in 61 cases (29.2%) and equivalent in 34 cases (16.3%). This
may be due to a large portion of the small primary lesion being
removed by VABB and it may have been challenging to exactly measure
the pathological T size with the fragmented specimens.
The VABB procedure varies depending on the USG
BI-RADS category. Usually complete excision biopsy is performed in
category 3–4a cases. However, incisional biopsy is performed to
obtain 3–5 core tissue samples in category 4b-5 cases. As a result,
the present study divided the cases into two groups by procedure,
category 3–4a and category 4b-5. The USG measurement and the
pathological measurement were compared in each group. In category 3
and 4a, 27 cases (62.8%) exhibited a pathological size that was
smaller than the USG-determined size. The pathological size
measurement was smaller than the USG-determined size in 100% of
pT1a, 77.8% of pTlb, 33.3% of pT1c and 66.7% of pT2 cases. In
category 4b-5, 88 cases (53.7%) had a pathological size that was
smaller than the USG-determined size. The percentage of cases was
88.9% in the pT1a, 82.8% in the pTlb, 46.8% in the pT1c and 45.8%
in the pT2 categories. In these two groups, the pathological size
was also smaller than the USG-determined size when the size of the
primary lesion was small. Simple correlational analysis of the
pathological and USG-determined sizes revealed that the correlation
coefficient in categories 3–4a was 0.262 (P=0.129), which was not
significant, although a weak-positive linear correlation was
observed. By contrast, the correlation coefficient in category 4b-5
was 0.502 (P<0.01), which demonstrated a clear positive linear
correlation.
The square of the correlation coefficient is
described in terms of explanatory power, which refers to the
variable importance or impact of each variable. Using this,
dependence between the pathological size and the USG-determined
size can be demonstrated. The explanatory power of categories 3–4a
and categories 4b-5 was 6% and 25%, respectively. In categories
3–4a, where a complete excision was performed, the dependence
between the pathological size and the USG-determined size is low
due to the pathological underestimation. In the case of a large
tumor, the residual lesion did not demonstrate a large difference,
as the amount of the lesion removed by VABB is not relatively
large. However, in the case of a small tumor, a possibility remains
that the size of the residual lesion can be measured as smaller
than the original size. In these cases, histopathological T staging
is underestimated, possibly influencing whether to implement
adjuvant therapy in the future. Taken together, these data
demonstrate that the smaller the primary tumor in lesions
classified as category 3–4a, the higher the likelihood of
pathological underestimation between pre-operative USG and
post-operative histopathological tumor sizes. This underestimation
can lead to omission of necessary adjuvant chemotherapy and
underlines the importance of considering the size of clinical
lesions properly when staging tumors.
Acknowledgements
This abstract was presented at the 8th European
Breast Cancer Conference, 21 March 2012–24 March 2012, Vienna,
Austria and published as abstract no. 507 in Eur J Cancer (Suppl 1,
S2–S242), 2012.
References
|
1
|
Veronesi U, Galimberti V, Zurrida S,
Pigatto F, et al: Sentinel lymph node biopsy as an indicator for
axillary dissection in early breast cancer. Eur J Cancer.
37:454–458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cowen D, Jacquemier J, Houvenaeghel G, et
al: Local and distant recurrence after conservative management of
‘very low-risk’ breast cancer are dependent events: a 10-year
follow-up. Int J Radiat Oncol Biol Phys. 41:801–807. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Dongen JA, Bartelink H, Fentiman IS,
et al: Factors influencing local relapse and survival and results
of salvage treatment after breast-conserving therapy in operable
breast cancer: EORTC trial 10801, breast conservation compared with
mastectomy in TNM stage I and II breast cancer. Eur J Cancer.
28A:801–805. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter CL, Allen C and Henson DE: Relation
of tumor size, lymph node status, and survival in 24,740 breast
cancer cases. Cancer. 63:181–187. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hieken TJ, Harrison J, Herreros J and
Velasco JM: Correlating sonography, mammography, and pathology in
the assessment of breast cancer size. Am J Surg. 182:351–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fornage BD, Toubas O and Morel M:
Clinical, mammographic, and sonographic determination of
preoperative breast cancer size. Cancer. 60:765–771. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi KH, Bae JW, Lee JB and Koo BH:
Clinical, mammographic, and ultrasonographic assessment of breast
cancer sizes. J Korean Breast Cancer Soc. 2:167–173. 1999.
View Article : Google Scholar
|
|
8
|
Fei SA: Breast masses. Mammographic and
sonographic evaluation. Radiol Clin North Am. 30:67–92. 1992.
|
|
9
|
Forouhi P, Walsh JS, Anderson TJ and
Chetty U: Ultrasonography as a method of measuring breast tumour
size and monitoring response to primary systemic treatment. Br J
Surg. 81:223–225. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clark GM: Integrating prognostic factors.
Breast Cancer Res Treat. 22:187–191. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maehle BO and Skjaerven R: Prediction of
prognosis in axillary lymph node positive breast cancer patients: a
statistical study. Br J Surg. 71:459–462. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dixon JM, Senbanjo RO, Anderson TJ,
Forrest AP and Elton RA: Clinical assessment of tumour size in
primary breast carcinoma. Clin Oncol. 10:117–121. 1984.PubMed/NCBI
|
|
13
|
Lambie RW, Hodgden D, Herman EM and
Kopperman M: Sonomammographic detection of lobular carcinoma not
demonstrated on xeromammography. J Clin Ultrasound. 11:495–497.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Warwick DJ, Smallwood JA, Guyer PB,
Dewbury KC and Taylor I: Ultrasound mammography in the management
of breast cancer. Br J Surg. 75:243–245. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishimura S, Matsusue S, Koizumi S and
Kashihara S: Size of breast cancer on ultrasonography, cut-surface
of resected specimen, and palpation. Ultrasound Med Bio. 14(Suppl
1): 139–142. 1988. View Article : Google Scholar
|
|
16
|
Pierie JP, Perre CI, Levert LM and de
Hooge P: Clinical assessment, mammography and ultrasonography as
methods of measuring the size of breast cancer: a comparison. The
Breast. 7:247–250. 1998. View Article : Google Scholar
|
|
17
|
Hwang KT, Kim HY, Chung JK, et al: A
comparative study between the preoperative diagnostic tumor size
and the postoperative pathologic tumor size in patients with breast
tumors. J Breast Cancer. 13:187–197. 2010. View Article : Google Scholar
|
|
18
|
Bosch AM, Kessels AG, Beets GL, Rupa JD,
Koster D, van Engelshoven JM and von Meyenfeldt MF: Preoperative
estimation of the pathological breast tumour size by physical
examination, mammography and ultrasound: a prospective study on 105
invasive tumours. Eur J Radiol. 48:285–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meden H, Neues KP, Röben-Kämpken S and
Kuhn W: A clinical, mammographic, sonographic and histologic
evaluation of breast cancer. Int J Gynaecol Obstet. 48:193–199.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee CS, Bong JG, Park JH, et al: The
accuracy of the physical examination, mammography, and
ultrasonography in the assessment of tumor size and axillary lymph
node metastasis in breast cancer patients. J Korean Breast Cancer
Soc. 6:87–94. 2003. View Article : Google Scholar
|