Introduction
The erythropoietin-producing hepatocyte (Eph) family
of receptors is the largest family of receptor tyrosine kinases
(RTKs) in humans (1). This family
comprises 14 members associated with eight ephrin ligands. These
receptors and ligands are divided into A and B classes based on
their sequence homology and their affinity for their corresponding
receptor/ligand (2–6). Many Eph receptors and ephrin ligands
are known to be involved in the development or progression of
malignant tumors: Upregulation of EphA2, A7, A10, and ephrinA2 and
B3 is thought to be involved in tumorigenesis and/or invasiveness,
while downregulation of EphA1, A3, A4, A8, B3, B4, B6, and ephrin
A1 and B1 may be particularly important in tumor invasiveness
(7). EphB6 is a clinically
significant Eph receptor, as indicated by its loss in the most
aggressive forms of melanoma and neuroblastoma (8–10).
Loss of EphB6 is associated with angiogenesis and tumor vasculature
in several types of human cancer (7,11,12).
However, the reports with regard to the role of Eph RTK members,
particularly EphB6, in prostate cancer, are insufficient.
In the present study, the expression of EphB6
receptor in normal and prostate cancer tissue, and the association
between EphB6 expression, clinicopathological findings and
progression of prostate cancer was investigated. Additionally, the
potential association between the expression of EphB6 and
proliferating-cell nuclear antigen (PCNA), an independent
postoperative prognostic marker for prostate cancer patients
(13), was assessed.
Materials and methods
Tissue samples
The protocol was approved by the ethics committee of
Kurume University (Kurume, Japan). Between 2003 and 2005, 46
patients were enrolled in the study and underwent radical
prostatectomy for prostate cancer at Kurume University Hospital
(Kurume, Japan). Following a full explanation of the protocol,
written informed consent for the use of tissue samples was obtained
from all patients prior to enrollment. Patients with clinically
localized prostate cancer who underwent radical retropubic
prostatectomy were enrolled, however, patients treated with
hormones, irradiation or transuretheral resection prior to surgery
were excluded.
Prostatectomy specimens were evaluated using the
following technique, with sectioning performed at 3 mm intervals.
The grade of each tumor was determined according to the Gleason
system of five grades (14). In
each patient, the volume of the cancer was determined using a
computer-assisted image analysis system (15). Seminal vesicle invasion and positive
regional lymph nodes were recorded. A tissue microarray of the
prostate was constructed as previously described (16). Briefly, one donor block, which
included normal and tumor regions, was selected from 10–15 blocks
of formalin-fixed, paraffin-embedded prostate tissue from each
patient. Tissue cylinders, with a diameter of 2 mm, were
subsequently punched from six regions of normal and cancer tissue
in each donor block, using a tissue microprocessor instrument
(KIN-type I, AZUMAYA, Tokyo, Japan), and inserted into a recipient
paraffin block. Tissue blocks from 46 prostatectomy specimens were
evaluated for the expression of EphB6 and PCNA using
immunohistochemistry.
Immunohistochemistry
Rabbit polyclonal antibody (EphB6 antibody, H-90;
cat. no. sc-25461; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) at a dilution of 1:500 and mouse monoclonal (PCNA antibody,
PC-10; cat. no. M0879; Dako Corporation, Glostrup, Denmark) IgG at
a dilution of 1:200 were used to evaluate the expression of EphB6
and PCNA, respectively, by immunohistochemistry. Briefly, 5 μm
thick sections of the selected paraffin blocks were de-paraffinized
in xylene, rehydrated in graded alcohol, and incubated in 0.5%
hydrogen peroxide/methanol for 20 min to block endogenous
peroxidase activity. Antigen retrieval was conducted by boiling the
sections in a microwave for 10 min using 10 mM citrate buffer (pH
6.0). The sections were subsequently incubated with anti-EphB6 or
anti-PCNA antibody overnight at 4°C. Following this incubation, the
sections were washed with 0.5% Tween-20/phosphate buffered saline
(PBS) prior to incubation with the corresponding polyclonal
peroxidase-labeled goat anti-rabbit and goat anti-mouse secondary
antibodies (dilution 1:100; Histofine, Nichirei, Tokyo, Japan) for
60 min. The sections were subsequently washed with 0.5%
Tween-20/PBS, and exposed to 3,3′-diaminobenzidine
tetrahydrochloride solution (Dako, Carpinteria, USA) to yield an
insoluble brown deposit. Finally, the sections were counterstained
with hematoxylin, washed in running water, dehydrated in graded
alcohol and conventionally mounted. Replacement of the primary
antibodies with PBS was used as a negative control for the
immunohistochemistry process.
Scoring of immune reactions
The immunoreactivity of EphB6 and PCNA molecules was
evaluated without prior knowledge of the clinicopathological
findings. The staining intensity of EphB6 was scored as 0
(negative) when immunoreactivity was absent or present in <10%
of cells, and as 1 (weak), 2 (moderate) and 3 (strong) when present
in 10–20%, 20–50% and >50% of cells, respectively. The PCNA
labeling index (LI) was determined by counting 1000 tumor cells at
×400 magnification in 10 randomly selected microscopic fields.
Brown, granular nuclear staining was considered positive staining.
The PCNA LI was calculated as the percentage of tumor cells with
positive nuclear staining for PCNA (13).
Statistical analysis
SPSS version 19.0 for Windows (IBM SPSS, Armonk, NY,
USA) was used for data analysis. P<0.05 was considered to
indicate a statistically significant difference. The change in
EphB6 expression in prostate cancer compared with that of
corresponding normal tissue was assessed using Wilcoxon’s signed
rank test. The frequency of a categorical observation was compared
between different groups using the χ2 and Fisher’s exact
tests, and the correlation between expression status of EphB6 and
other continuous variables was evaluated by Spearman’s ρ test.
Mann-Whitney U and Kruskal-Wallis tests were used to compare the
mean rank of continuous variables between different clinical groups
and Kaplan-Meier survival analysis was used to compare the duration
of prostate-specific antigen (PSA)-free survival between the
different groups.
Results
Patient characteristics
The demographic characteristics of the investigated
patients and pathological features of the tumors are summarized in
Table I. Patients were aged from
49–78 years (mean, 65.8 years; median, 66 years). Serum PSA was
elevated in all patients, with a minimum value of 4.4 ng/ml and a
maximum of 33.9 ng/ml (normal range, <4 ng/ml). The majority of
tumors (56.5%) were of Gleason score 7. Cancer volume in prostatic
specimens ranged from 0.2–15.2 cm3 (median, 3.7
cm3). Pathological T stages were pT2a in 16, pT2b in
seven, T2c in nine, pT3a in 10 and pT3b in four patients. Nodal or
distant metastasis was not detected in any of the patients.
 | Table IThe demographic characteristics of
patients and tumors. |
Table I
The demographic characteristics of
patients and tumors.
| Clinicopathological
factors |
|---|
| Patients, n | 46 |
| Age, years |
| Mean (SD) | 65.8 (6.2) |
| Median (range) | 66 (49–78) |
| Serum PSA level,
ng/ml |
| Mean (SD) | 13.2 (7.5) |
| Median (range) | 11.4 (4.4–33.9) |
| Prostatic weight,
gm |
| Mean (SD) | 27.5 (10.0) |
| Median (range) | 26.2 (11.8–60.8) |
| Gleason score, n
(%) |
| 6 | 17 (37) |
| 7 | 26 (56.5) |
| 8 | 1 (2) |
| 9 | 2 (4.5) |
| Pathological T stage,
n (%) |
| pT2a | 16 (35) |
| pT2b | 7 (15) |
| pT2c | 9 (20) |
| pT3a | 10 (21.5) |
| pT3b | 4 (8.5) |
| Cancer volume,
cm3 |
| Mean (SD) | 4.8 (3.9) |
| Median (range) | 3.7 (0.2–15.2) |
Expression of EphB6
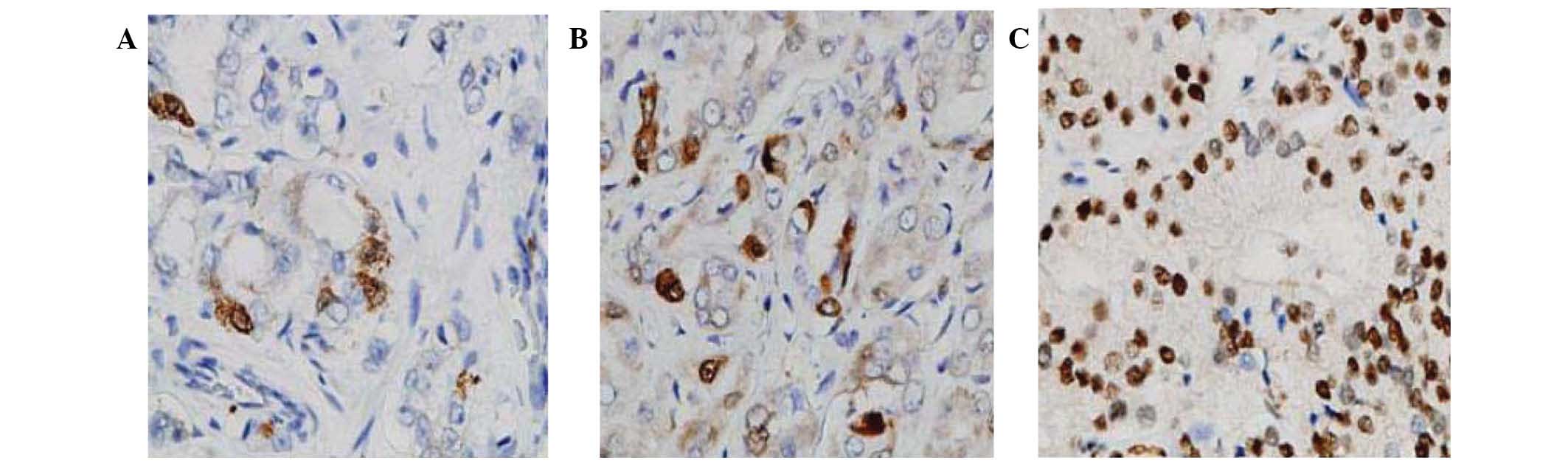
The expression of EphB6 was evaluated in normal and
prostate cancer tissues from each patient. In normal prostatic
tissues, EphB6 expression was observed in 100% of investigated
samples. EphB6 protein had a homogeneous cytoplasmic and membranous
distribution, and the immunoreactivity was either moderate or
strong (38% and 62% of samples, respectively; Fig. 1A and B). In prostate cancer tissue,
EphB6 expression was detected in the majority of cases (97.8%). The
distribution was also membranous and cytoplasmic, and the
expression level was negative or weak in a high proportion of cases
(26 cases, 56.5%) and moderate or strong in 17 (37%) and three
(6.5%) cases, respectively (Fig.
1C–F). Compared with corresponding normal tissue within the
same patient, prostate cancer cells showed a significantly
decreased expression level of EphB6 in 26 cases and retained a
similar expression level in the remaining cases (Wilcoxon signed
rank test, P<0.0001).
Expression of PCNA
The expression of PCNA, which is predominantly
nuclear, was detected in all investigated patients. The minimum LI
of PCNA was 1% and the maximum was 98%, with a median value of 22%.
Representative PCNA expressions in prostate cancer are shown in
Fig. 2. No significant association
between the expression status of PCNA and EphB6 was observed
(Spearman’s ρ=0.193, P=0.173).
Association between tumor characteristics
and EphB6 or PCNA expression
The association between tumor characteristics
(including Gleason score, cancer volume and pathological stage) and
EphB6 or PCNA expression were evaluated (Fig. 3). The results revealed that low
expression of EphB6 was significantly associated with a high volume
(≥4 cm3) of cancer (P=0.015) and advanced pathological
stage (pT3) (P=0.0007). However, none of the tumor characteristics
were associated with PCNA expression.
EphB6 expression and biochemical
progression-free survival
The effect of EphB6 expression on biochemical
progression-free (PSA-free) survival in prostate cancer patients
was evaluated (Fig. 4). The minimum
follow-up duration of the investigated patients was 12 months and
the maximum was 120 months (median, 47.5 months). According to the
Kaplan-Meier analysis, biochemical progression-free survival was
reduced in patients with negative or weak expression of EphB6,
compared with that of patients with mild or strong expression
(hazard ratio, 2.227; 95% CI, 0.7353–6.745; log-rank, P=0.157).
Discussion
An understanding of tumor pathogenesis and the
identification of prognostic and diagnostic molecules are crucial
in the management of prostate cancer. A number of studies have
reported that the Eph RTK family of receptors and their ephrin
ligands enhance tumor growth, invasion, metastasis and
neovascularization (17,18). Previous studies observed that
expression of EphB6 was diminished or lost in the most aggressive
forms of melanoma and neuroblastoma (8–10).
Furthermore, forced expression of EphB6 in neuroblastoma cells may
decrease their tumorigenicity in mouse xenograft models (9). In the current study, normal prostatic
tissue exhibited homogeneous moderate or strong expression of EphB6
in 15 (33%) and 31 (67%) of the investigated cases, respectively.
In addition, significantly reduced EphB6 expression was observed
within adjacent prostate cancer tissue in a considerable proportion
of cases (Wilcoxon’s signed rank test, P<0.0001). This is
consistent with previous semi-quantitative RT-PCR studies on
prostate cancer cell lines, which showed downregulation of EphB6
mRNA in a cell line derived from primary prostate cancer tissue,
compared with that in a cell line derived from normal prostatic
tissue from the same patient (19).
These findings support the hypothesis that EphB6 is a tumor
suppressor molecule in prostate cancer and that its expression is
correlated with favorable tumor prognosis. Additionally, data from
the current study suggested that EphB6 expression is gradually and
significantly reduced during the progression of prostate cancer
from a low volume to a high volume, or from pT2 stage to pT3.
Furthermore, no association was observed between EphB6 expression
and the expression of PCNA. Within the limits of the investigated
cases, the results indicate that EphB6 RTK has no
proliferation-stimulating effect in prostate cancer.
In apparent contradiction with the hypothesized
tumor suppressor effect of the EphB6 molecule in prostate cancer,
Fox et al (19) reported
that the invasive and metastasizing prostate cancer cell lines
DU145, PC-3 and PC-3ML exhibited upregulation of EphB6 mRNA
compared with that of cell lines derived from primary prostate
cancer or normal tissue. These controversial observations are not
fully understood; however, they may be associated with a change in
the subcellular localization of the EphB6 molecule. Additionally,
the regulation of EphB6 expression by promoter methylation may be
associated with altered expression in aggressive prostate cancer
cell lines (19).
In conclusion, although several members of the Eph
family are associated with the progression of cancer, the results
of the present study indicated that EphB6 may have a tumor
suppressor effect in prostate cancer, at least during early stages
of this disease. This provides new insight for the use of EphB6 RTK
as a potential diagnostic/prognostic marker for prostate cancer.
However, a significant limitation of the current study is the
inclusion of only early stages of prostate cancer. Further studies
of EphB6 protein and mRNA expression in later stage and metastatic
prostate cancer tissue are required in order to fully evaluate the
role of EphB6 in this disease, and to address the aforementioned
conflicting results from other studies.
Acknowledgements
This study was supported by a grant from the
Ministry of Health, Labor and Welfare of Japan (grant no. 22591782
to Professor Masanori Noguchi).
Abbreviations:
|
Eph
|
erythropoietin-producing
hepatocyte
|
|
LI
|
labeling index
|
|
PCNA
|
proliferating-cell nuclear antigen
|
|
PSA
|
prostate-specific antigen
|
|
RTK
|
receptor tyrosine kinase
|
|
TNA
|
tissue microarray
|
References
|
1
|
Manning G, Whyte DB, Martinez R, et al:
The protein kinase complement of the human genome. Science.
298:1912–1934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Q and Wilkinson DG: Eph-related
receptors and their ligands: mediators of contact dependent cell
interactions. J Mol Med (Berl). 75:576–586. 1997. View Article : Google Scholar
|
|
3
|
Nakamoto M: Eph receptors and ephrins. Int
J Biochem Cell Biol. 32:7–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holmberg J, Clarke DL and Frisén J:
Regulation of repulsion versus adhesion by different splice forms
of an Eph receptor. Nature. 408:203–206. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kullander K and Klein R: Mechanisms and
functions of Eph and ephrin signaling. Nat Rev Mol Cell Biol.
3:475–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Himanen JP and Nikolov DB: Eph receptors
and ephrins. Int J Biochem Cell Biol. 35:130–134. 2003. View Article : Google Scholar
|
|
7
|
Fox BP and Kandpal RP: Invasiveness of
breast carcinoma cells and transcript profile: Eph receptors and
ephrin ligands as molecular markers of potential diagnostic and
prognostic application. Biochem Biophys Res Commun. 318:882–892.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang XX, Zhao H, Robinson ME, et al:
Implications of EPHB6, EFNB2, and EFNB3 expressions in human
neuroblastoma. Proc Natl Acad Sci USA. 97:10936–10941. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang XX, Robinson ME, Riceberg JS, et al:
Favorable neuroblastoma genes and molecular therapeutics of
neuroblastoma. Clin Cancer Res. 10:5837–5844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hafner C, Bataille F, Meyer S, et al: Loss
of EphB6 expression in metastatic melanoma. Int J Oncol.
23:1553–1559. 2003.PubMed/NCBI
|
|
11
|
Hafner C, Schmitz G, Meyer S, et al:
Differential gene expression of Eph receptors and ephrins in benign
human tissues and cancers. Clin Chem. 50:490–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fox BP and Kandpal RP: Transcriptional
silencing of EphB6 receptor tyrosine kinase in invasive breast
carcinoma cells and detection of methylated promoter by methylation
specific PCR. Biochem Biophys Res Commun. 340:268–276. 2006.
View Article : Google Scholar
|
|
13
|
Taftachi R, Ahyan A, Ekici S, et al:
Proliferating-cell nuclear antigen (PCNA) as an independent
prognostic marker in patients after prostatectomy: a comparison of
PCNA and Ki-67. BJU Int. 95:650–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gleason DF: Histologic grading and
clinical staging of carcinoma of the prostate. Urologic Pathology:
The Prostate. Tannenbaum M: Lea & Febiger; Philadelphia, PA:
pp. 171–197. 1977
|
|
15
|
Noguchi M, Stamey TA, McNeal JE and Yemoto
CE: Assessment of morphometric measurements of prostate cancer
volume. Cancer. 89:1056–1064. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noguchi M, Yao A, Harada M, et al:
Immunological evaluation of neoadjuvant peptide vaccination before
radical prostatectomy for patients with localized prostate cancer.
Prostate. 67:933–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brantly-Sieders DM and Chen J: Eph
receptor tyrosine kinases in angiogenesis: from development to
disease. Angiogenesis. 7:17–28. 2004. View Article : Google Scholar
|
|
18
|
Pasquale EB: Eph receptors and ephrins in
cancer: bidirectional signaling and beyond. Nat Rev Cancer.
10:165–180. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fox BP, Tabone CJ and Kandpal RP:
Potential clinical relevance of Eph receptors and ephrin ligands
expressed in prostate carcinoma cell lines. Biochem Biophys Res
Commun. 342:1263–1272. 2006. View Article : Google Scholar : PubMed/NCBI
|