Introduction
In carcinogenesis, hypoxia renders a more aggressive
phenotype, with increased invasiveness, proliferation and
metastasis and poorer survival rate (1,2).
Additionally, several clinical studies have demonstrated that
hypoxia is associated with poor response to radiation and
chemotherapy (3–5). Cellular adaptation to hypoxia
represents a crucial step in tumor progression.
Hypoxia-inducible factor 1 (HIF-1) is a
transcription factor that is critical in the adaptive cellular
response to hypoxia (1,6). HIF-1 is composed of the subunits
HIF-1α and HIF-1β, which are basic helix-loop-helix DNA binding
proteins. HIF-1α has been described as an endogenous hypoxic marker
(7), and its overexpression has
been demonstrated to correlate with a poorer survival rate in
patients with cancers of the cervix (8), lung (9), colon (7,10–12),
endometrium (13) and ovary
(14).
HIF-1α is able to induce the expression of more than
30 genes that are involved in cellular metabolism, angiogenesis,
proliferation and survival. These gene products include carbonic
anhydrase 9 (CA9), glucose transporter-1 (GLUT1) and vascular
endothelial growth factor (VEGF) (15). CA9 is a transmembrane glycoprotein
important for maintaining extracellular pH by catalyzing the
reversible hydration of carbonic dioxide to carbonic acid. CA9 is
overexpressed in a wide spectrum of human cancers, and has been
proposed to be a potential intrinsic marker of hypoxia (10–12).
The membrane-bound glycoprotein GLUT1 is responsible for
facilitating glucose transport (16). Several studies have demonstrated an
association between GLUT1 expression and carcinogenesis, in
addition to an unfavorable prognosis in various cancers (17,18).
Younes et al (17) reported
that in bladder cancer, tumors with >10% GLUT1-positive cancer
cells were more likely to have higher stage than tumors with
<10% GLUT1-positive cells. These results suggested that GLUT1
expression is a marker of aggressive biological potential in
patients with bladder cancer (17).
Furthermore, a positive association between GLUT1 and depth of
invasion, lymphatic permeation, venous invasion, lymph node
metastasis, hepatic metastasis, and carcinoma stage has been
reported in gastric cancers (18).
VEGF acts as a potent inducer of angiogenesis, and its
overexpression is also associated with a higher rate of metastases
and poor outcome in a variety of human cancers. Tumors expressing
high levels of VEGF were significantly more prevalent in advanced
stage cancer and associated with poorer survival in ovarian and
endometrial carcinomas (19,20).
Soft tissue sarcomas (STS) comprise less than 1% of
all malignant tumors and consist of more than 50 histopathologic
subtypes (21), many with different
biological behaviors. STS is locally aggressive, and recurrence and
distant metastasis are often observed. A number of prognostic
factors determine tumor progression and patient outcome, including
tumor grade, size, location, depth, histological type, tumor stage
and presence of local relapse (22). Numerous different biological
prognostic factors have been studied in STS (23). Several reports have indicated that
tumor hypoxia correlates with distant metastatic spread and poor
prognosis in STS. These studies measured tumor oxygenation using
polarographic oxygen-sensitive electrodes, and reported that higher
median pO2 in samples was associated with an increased
risk of developing metastases, and with poorer survival (1,6). Other
studies have investigated hypoxic markers in several human cancers
using immunohistochemical methods as an alternative approach. Using
immunohistochemistry, Maseide et al (24) demonstrated that the hypoxic marker
CA9 indicated poor prognosis in patients with high grade STS and
may be a useful marker in retrospective studies of
paraffin-embedded material.
The current study aimed to determine the expression
of hypoxic markers, including HIF-1α, CA9, GLUT1, and VEGF, in STS
using immunohistochemistry, and to analyze the impact of
overexpression on the clinicopathological features of tumor
aggressiveness.
Materials and methods
STS tissue samples
Formalin-fixed, paraffin-embedded samples were
obtained from 55 patients with STS who had undergone surgical
resection at Pusan National University Hospital (Busan, Korea)
between 1998 and 2007. Diagnoses were confirmed by pathological
analysis using the diagnostic criteria defined in the World Health
Organization (WHO) classification. Among the cases, 19 liposarcomas
(LPS), 16 malignant fibrous histiocytomas (MFH), seven
rhabdomyosarcomas (RMA), five leiomyosarcomas (LMS), six synovial
sarcomas (SS), and two malignant peripheral nerve sheath tumors
(MPNST) were recorded. Each case was evaluated according to the
French Federation of Cancer Centers (FNCLCC) sarcoma group grading
system and the staging system of the American Joint Committee on
Cancer (AJCC) (21). Clinical
information was obtained from medical records. The overall survival
(OS) was calculated from the date of surgery to the date of
mortality or last follow-up visit. The progression-free survival
(PFS) was calculated from the date of surgery to the date of tumor
relapse or progression. Written informed consent from the patients
and approval from the Institutional Ethics of Pusan National
University Hospital were obtained prior to the use of these
materials and informed consent was obtained from all patients.
Samples and clinical information were anonymized prior to
statistical analysis.
Immunohistochemistry
Each slide was deparaffinized and rehydrated
according to the standard procedure (14), and was subsequently treated with
0.01 mol/l sodium citrate buffer (Ventana-Bio Tek solutions,
Tucson, AZ, USA) in a laboratory microwave at 120°C for 15 min.
Immunohistochemical staining was performed using the avidin-biotin
peroxidase complex method with diaminobenzidine as a chromogen,
using the Vectastain ABC elite kit (Vector laboratories,
Burlingame, CA, USA). Rabbit polyclonal antibodies for CA9 (1:1000;
Abcam, Cambridge, UK; catalog no. ab15086) and GLUT1 (1:200;
Neomarkers, Fremont, CA, USA; catalog no. RB9052), and mouse
monoclonal antibodies for HIF-1α (1:1000; Abcam; catalog no.
ab8366) and VEGF (1:50; Neomarkers; catalog no. MS350) were used as
primary antibodies. Specimens of colon adenocarcinoma and renal
cell carcinoma were used as positive controls for HIF-1α and CA9,
respectively, due to the known strong expression of these markers.
Tumor capillaries were considered to be an internal positive
control for GLUT1 and VEGF.
Immunohistochemical staining was evaluated by two
independent pathologists who were blinded to the specific diagnosis
and prognosis for each individual case. Expression of HIF-1α was
assessed by analyzing ≥1000 tumor cells from tumor fields, and the
labeling index was calculated as the percentage of labeled nuclei
per total number of tumor cells that were counted. The
immunoreactivity of HIF-1α was graded from 0–3+ (0, no staining;
1+, 1–25%; 2+, 26–50%; 3+, 50% nuclear staining) according to the
nuclear expression, and only a grade of 3+ (>50% nuclear
staining) was considered to be a positive immunohistochemical
result (24,25). For GLUT1 and CA9, cases were
considered positive if >10% of their cells showed distinct
membranous staining. For VEGF, cases were considered positive if
>10% of their cells showed distinct cytoplasmic staining
(8,14).
Statistical analysis
A statistical analysis was conducted using SPSS 17.0
software (SPSS, Chicago, IL, USA). The associations between
clinicopathological variables and the expression of HIF-1α, CA9,
GLUT1 and VEGF were assessed using Pearson’s χ2 test. OS
and PFS were calculated using the Kaplan-Meier log-rank test. A
multivariate analysis to assess their independent prognostic values
was conducted using the Cox regression method. P<0.05 was
considered to indicate a statistically significant difference.
Results
In total, data from 55 patients were collected (mean
age, 57 years; range, 1–82). The clinicopathological features
observed within this sample are summarized in Table I. Stage I was classified as early
stage, and stages II–IV were classified as advanced stage. None of
the patients had received prior chemotherapy. In total, 31 patients
(56.4%) developed either local recurrence or metastasis
(progression group), whereas 24 patients (43.6%) were free of
progression (progression-free group). With a median follow-up time
of 38 months (range, two to 187 months), the overall survival rate
was 50.9%. Chemotherapy following surgical resection was received
by 27 patients. Chemotherapy consisted of mensa, doxorubicin, and
ifosfamide (mesna 1.2 g/m2/day, doxorubicin 25
mg/m2/day and intravenous ifosfamide 2.0
g/m2/day on days 1–3; repeated every 3 weeks).
 | Table IClinicopathological data in 55 cases
of STS. |
Table I
Clinicopathological data in 55 cases
of STS.
| Clinicopathological
data | Cases |
|---|
| Age, years |
| Range (median) | 1–82 (57) |
| Gender, n |
| Male | 29 |
| Female | 26 |
| Histological type,
n |
| LPS | 19 |
| MFH | 16 |
| RMS | 7 |
| LMS | 5 |
| SS | 6 |
| MPNST | 2 |
| Site, n |
| Thigh | 16 |
| Upper arm | 8 |
| Retroperitoneum | 6 |
| Forearm | 4 |
| Lower leg | 4 |
| Head and neck | 4 |
| Back | 4 |
| Buttock | 3 |
| Others | 6 |
| Location, n |
| Superficial | 14 |
| Deep | 41 |
| Tumor size, n
(cm) |
| <10 | 32 |
| ≥10 | 23 |
| FNCLCC grade,
n |
| 1 | 13 |
| 2 | 20 |
| 3 | 22 |
| AJCC stage, n |
| I | 12 |
| II–IV | 43 |
| Disease
progression, n |
|
Progression-free | 24 |
| Progression | 31 |
| Overall survival,
n |
| Alive | 28 |
| DOD | 27 |
| Chemotherapy,
n |
| Yes | 27 |
| No | 28 |
| Total | 55 |
Overexpression of HIF-1α, CA9, GLUT1, and VEGF was
observed in 54.5% (30/55), 32.7% (18/55), 52.7% (29/55) and 25.5%
(14/55) of STS samples, respectively. HIF-1α expression was
recognized through the nuclear staining of positive cells, whereas
CA9 and GLUT1 staining were distinct in the cell membrane. VEGF
expression was observed in the cytoplasm. Representative cases of
immunohistochemical staining of all markers are shown in Fig. 1.
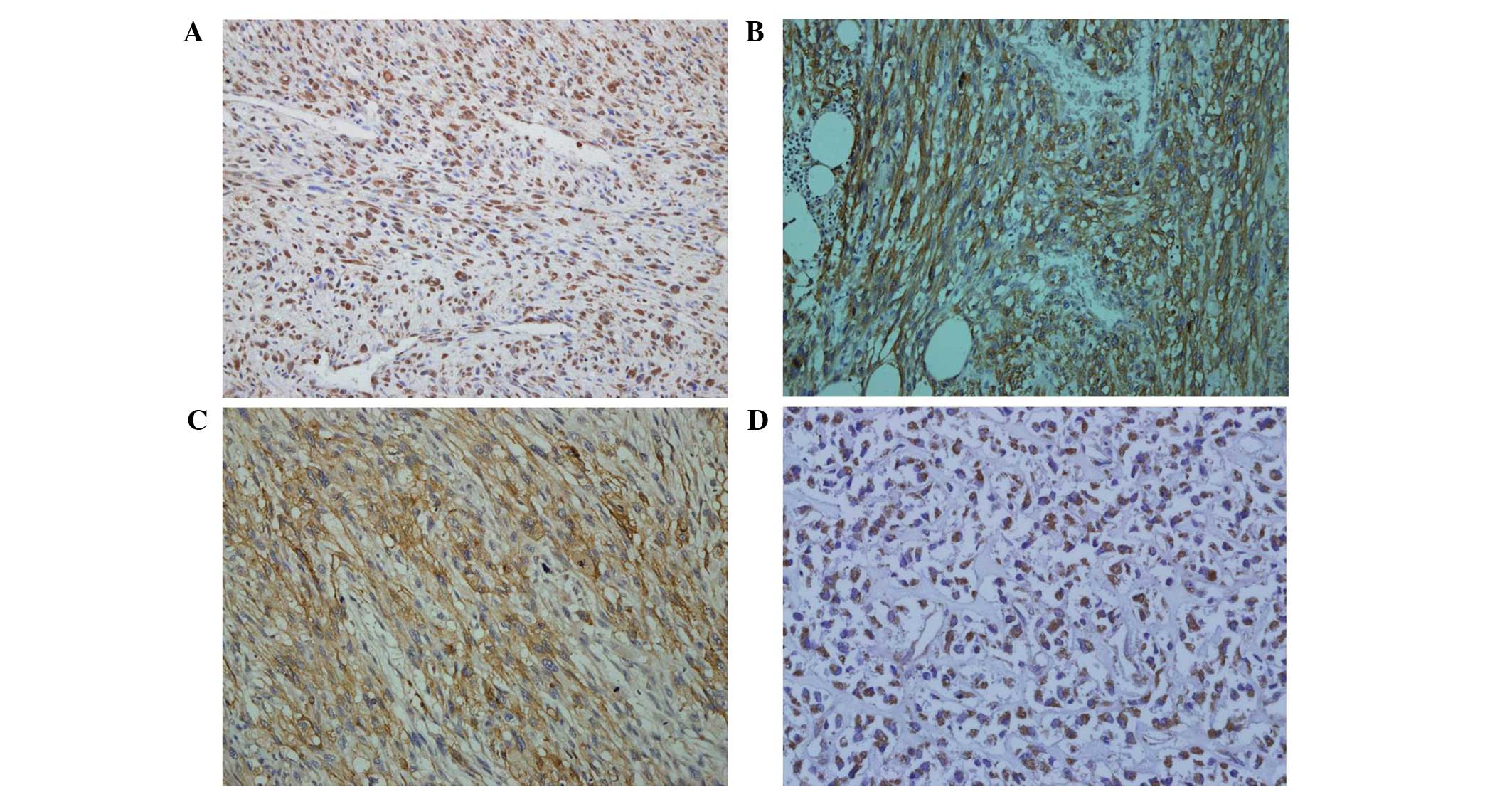 | Figure 1Immunohistochemical staining of
HIF-1α, CA9, GLUT1 and VEGF in high grade soft tissue sarcoma.
Representative cases are shown: (A) HIF-1α (magnification, ×200);
(B) CA9 (magnification, ×200); (C) GLUT1 (magnificiation, ×200) and
(D) VEGF (magnification, ×200). HIF-1α, hypoxia-inducible factor
1α; CA9, carbonic anhydrase 9; GLUT1, glucose transporter-1; VEGF,
vascular endothelial growth factor. |
The correlations between clinicopathological
variables and expression of HIF-1α, CA9, GLUT1 and VEGF are shown
in Table II. Immunohistochemical
analysis revealed a difference in the expression of HIF-1α, CA9,
GLUT1 and VEGF between histological types. The rate of expression
was significantly lower in LPS (HIF-1α, 36.8; CA9, 5.3; GLUT1,
15.8; and VEGF, 10.5%) compared with other types of STS, whereas
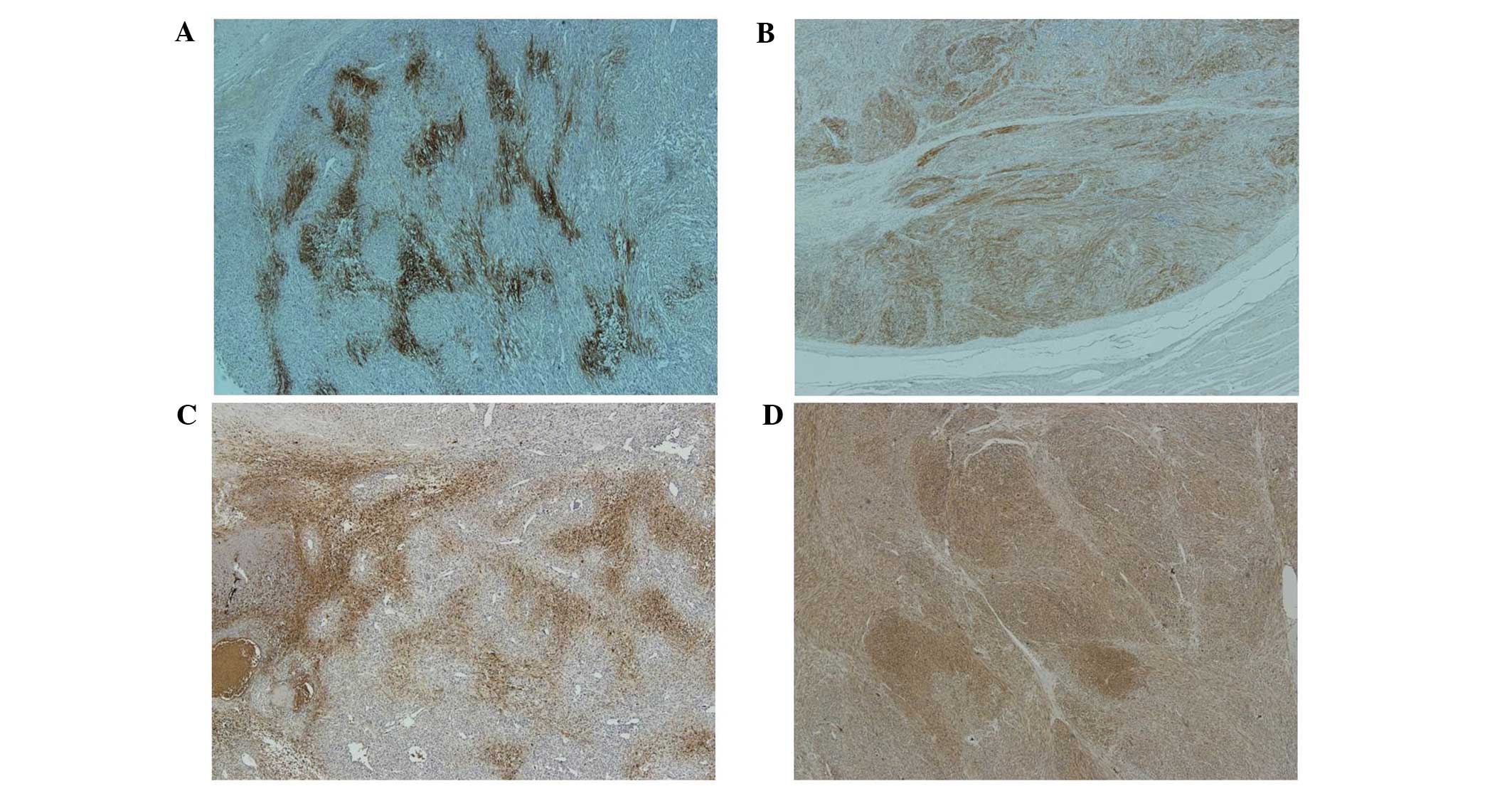
MFH and LMS exhibited a higher rate of expression. The CA9 and
GLUT1 positive cells were typically identified adjacent to the
necrotic regions in cases of MFH with necrosis, but CA9 and GLUT1
were diffusely expressed in cases of LMS cases where no necrosis
was present (Fig. 2). The
expression of HIF-1α, CA9, GLUT1, and VEGF was significantly
associated with a higher histological grade and advanced AJCC stage
in the total cases of STS. No significant correlation between the
expression of HIF-1α, CA9, GLUT1 and VEGF and tumor size or
location was observed.
 | Table IIAssociation between HIF-1α, CA9,
GLUT1 and VEGF expression status and clinicopathological variables
(n=55). |
Table II
Association between HIF-1α, CA9,
GLUT1 and VEGF expression status and clinicopathological variables
(n=55).
| HIF-1α | CA9 | GLUT1 | VEGF |
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Positive
(n=30) | Negative
(n=25) | P-value | Positive
(n=18) | Negative
(n=37) | P-value | Positive
(n=29) | Negative
(n=26) | P-value | Positive
(n=14) | Negative
(n=41) | P-value |
|---|
| Histological type,
n (%) | | | 0.329 | | | 0.007 | | | 0.004 | | | 0.301 |
| LPS | 7 (36.8) | 12 (63.2) | | 1 (5.3) | 18 (94.7) | | 3 (15.8) | 16 (84.2) | | 2 (10.5) | 17 (89.5) | |
| MFH | 11 (68.8) | 5 (31.2) | | 8 (50) | 8 (50) | | 12 (75) | 4 (25) | | 6 (37.5) | 10 (62.5) | |
| RMS | 3 (42.9) | 4 (57.1) | | 1 (14.3) | 6 (85.7) | | 4 (57.1) | 3 (42.9) | | 3 (42.9) | 4 (57.1) | |
| LMS | 4 (80) | 1 (20) | | 4 (80) | 1 (20) | | 4 (80) | 1 (20) | | 2 (40) | 3 (60) | |
| SS | 4 (66.7) | 2 (33.3) | | 3 (50) | 3 (50) | | 4 (66.7) | 2 (33.3) | | 1 (16.7) | 5 (83.3) | |
| MPNST | 1 (50) | 1 (50) | | 1 (50) | 1 (50) | | 2 (100) | 0 (0) | | 0 (0) | 2 (100) | |
| Tumor size, n
(cm) | | | 0.172 | | | 0.774 | | | 0.422 | | | 0.547 |
| <10 | 14 | 17 | | 11 | 20 | | 18 | 13 | | 9 | 22 | |
| ≥10 | 16 | 8 | | 7 | 17 | | 11 | 13 | | 5 | 19 | |
| Location, n | | | 0.762 | | | 0.514 | | | 0.215 | | | 0.494 |
| Superficial | 7 | 7 | | 5 | 9 | | 5 | 9 | | 3 | 11 | |
| Deep | 23 | 18 | | 13 | 28 | | 24 | 17 | | 11 | 30 | |
| Histological grade,
n | | | 0.012 | | | 0.005 | | | 0.001 | | | 0.024 |
| Low | 3 | 10 | | 0 | 13 | | 1 | 12 | | 0 | 13 | |
| High | 27 | 15 | | 18 | 24 | | 28 | 14 | | 14 | 28 | |
| AJCC stage, n | | | 0.026 | | | 0.005 | | | 0.001 | | | 0.025 |
| I | 3 | 9 | | 0 | 12 | | 1 | 11 | | 0 | 12 | |
| II to IV | 27 | 16 | | 18 | 25 | | 28 | 15 | | 14 | 29 | |
Of the 30 patients exhibiting HIF-1α expression, 22
(73.3%) showed disease progression and 23 (76.7%) died from the
disease, compared with only nine (36%) and four (16%),
respectively, of the 25 patients who did not display HIF-1α
expression. These differences were statistically significant
(P=0.007 and 0.042, respectively). However, the expression of GLUT1
and VEGF was not associated with disease progression and survival
(Table III). To investigate the
prognostic impact of these markers in STS, Kaplan-Meier survival
analyses were conducted and the differences in survival between the
groups were examined. The Kaplan-Meier survival curves (Fig. 3) indicated that HIF-1α and CA9
expression had a significant impact on disease free survival
(P=0.001 and 0.006) and OS (P=0.001 and 0.003). Multivariate
analyses revealed that advanced AJCC stage (P=0.011) and HIF-1α
expression (P=0.006) were independent prognostic markers for OS
compared with early AJCC stage and no HIF-1α overexpression
(Table IV). In the group receiving
chemotherapy (n=27), HIF-1α expression was independently associated
with shorter survival, and was an independent prognostic factor on
multivariate analysis (P=0.010) (Table
V).
 | Table IIIAssociation between HIF-1α, CA9,
GLUT1 and VEGF expression status and clinical behavior of patients
with soft tissue sarcoma (n=55). |
Table III
Association between HIF-1α, CA9,
GLUT1 and VEGF expression status and clinical behavior of patients
with soft tissue sarcoma (n=55).
| HIF-1α | CA9 | GLUT1 | VEGF |
|---|
|
|
|
|
|
|---|
| Parameters | Positive
(n=30) | Negative
(n=25) | P-value | Positive
(n=18) | Negative
(n=37) | P-value | Positive
(n=29) | Negative
(n=26) | P-value | Positive
(n=14) | Negative
(n=41) | P-value |
|---|
| Disease
progression, n (%) | | | 0.007 | | | 0.042 | | | 0.422 | | | 0.067 |
| Progression
free | 8 (33.3) | 16 (66.7) | | 4 (16.7) | 20 (83.3) | | 11 (45.8) | 13 (54.2) | | 3 (12.5) | 21 (87.5) | |
| Progression | 22 (71) | 9 (29) | | 14 (45.2) | 17 (54.8) | | 18 (58.1) | 13 (41.9) | | 11 (45.8) | 20 (54.2) | |
| OS | | | 0.001 | | | 0.004 | | | 0.180 | | | 0.547 |
| Alive | 7 | 21 | | 4 | 24 | | 12 | 16 | | 6 | 22 | |
| DOD | 23 | 4 | | 14 | 13 | | 17 | 10 | | 8 | 19 | |
 | Table IVMultivariate analysis of prognostic
factors in patients with soft tissue sarcomas (n=55). |
Table IV
Multivariate analysis of prognostic
factors in patients with soft tissue sarcomas (n=55).
| Variables | Grouping | P-value | Ratio of risk | 95% CI |
|---|
| HIF-1α | Overexpression vs.
no overexpression | 0.006 | 0.165 | 0.046–0.601 |
| CA9 | Positive vs.
negative | 0.514 | 0.745 | 0.308–1.802 |
| Histological
grade | G2–G3 vs. G1 | 0.208 | 1.822 | 0.716–4.636 |
| AJCC stage | II–IV vs. I | 0.011 | 8.096 | 2.480–42.707 |
 | Table VMultivariate analysis of prognostic
factors in patients with soft tissue sarcomas who received
chemotherapy (n=27). |
Table V
Multivariate analysis of prognostic
factors in patients with soft tissue sarcomas who received
chemotherapy (n=27).
| Variables | Grouping | P-value | Ratio of risk | 95% CI |
|---|
| HIF-1α | Overexpression vs.
no overexpression | 0.010 | 0.103 | 0.018–0.582 |
| CA9 | Positive vs.
negative | 0.620 | 0.700 | 0.171–2.865 |
| Histological
grade | G2–G3 vs G1 | 0.507 | 2.381 | 0.184–30.849 |
| AJCC stage | II–IV vs. I | 0.442 | 0.539 | 0.111–2.607 |
Discussion
Tumor hypoxia is known to affect patient prognosis
as it leads to a more aggressive phenotype, with increased
invasiveness, proliferation and metastasis, resulting in a poorer
survival rates (1,2). HIF-1α is essential for adapting the
cellular environment to hypoxia by inducing the expression of
various hypoxia response molecules, including CA9, GLUT1 and VEGF.
The evidence for these molecules as reliable markers of hypoxia has
been reviewed elsewhere (7,10–12)
and the overexpression of HIF-1α, CA9, GLUT1 and VEGF in various
malignant tumors has also been demonstrated (8–14,17–20).
In cervical carcinogenesis, it has been reported that HIF-1α is a
marker for hypoxia-induced proliferation in the initial stage, and
overexpression of GLUT1 and CA9 represent early and later events,
respectively (8). Each contributes
to tumor progression, greatly impacting the prognosis (11,27).
Overexpression of HIF-1α and CA9 have also been shown to be
powerful prognostic factors in colorectal cancers (10). Additionally, Chen et al
(28) reported that HIF-1α affects
tumor progression during breast carcinogenesis, and that GLUT1 and
CA9 expression may indicate an aggressive phenotype.
Few reports have indicated that hypoxia may be a
predictor of metastasis in patients with STS. Brizel et al
(1) observed that disease-free
survival was increased for patients with median tumor
pO2 values of >10 mm Hg compared with those with
median pO2 values of <10 mm Hg, suggesting that tumor
hypoxia may be a useful marker for biologically aggressive forms of
the disease. In addition, Nordsmark et al (6) demonstrated that patients with hypoxic
tumors with a median pO2 of <19 mm Hg had poorer
survival than those with well-oxygenated tumors, and that hypoxia
was an indicator for poorer disease-specific and OS rates in
patients with STS. Maseide et al (24) investigated the association between
hypoxia and metastasis in a larger number of STS cases by
conducting immunohistochemical analyses for CA9, a reliable marker
of hypoxia, in paraffin-embedded tissue sections, and subsequently
quantifying the CA9-positive area fraction by image analysis. The
data indicated that the disease-specific and OS rates were
significantly lower for patients with CA9-positive tumors than for
those with CA9-negative tumors.
To the best of our knowledge, this study is the
first to investigate the expression patterns of multiple hypoxic
markers and their prognostic significance in STS using
immunohistochemistry. Approaches to immunohistochemical evaluation
for the scoring of hypoxic markers vary and may be complicated;
simple and commonly used criteria were selected for use in this
study in order to improve the reliability and consistency of
interpretation. The overexpression of HIF-1α, CA9, GLUT1 and VEGF
was observed to be significantly associated with high FNCLCC grades
and high AJCC stages. The overexpression of HIF-1α and CA9 was also
associated with shorter OS and shorter PFS. Furthermore, on
multivariate analysis, HIF-1α overexpression exhibited independent
prognostic significance.
The various HIF-1α expression patterns have
different prognostic implications in certain types of cancer. In
breast cancer, patients with a diffuse HIF-1α staining pattern have
been demonstrated to have a significantly better prognosis than
patients with perinecrotically overexpressed HIF-1α (29). Seeber et al (27) suggested that perinecrotic HIF-1α
expression was significantly associated with a shorter disease-free
survival compared with diffuse HIF-1α expression in endometrioid
endometrial carcinoma. This significance of expression pattern
could be explained by the fact that perinecrotic HIF-1α expression
is thought to be hypoxia driven, whereas diffuse HIF-1α expression
may rather be due to non-hypoxic stimuli. The results of the
current study revealed a difference in the expression of HIF-1α,
CA9, GLUT1 and VEGF between different histological types. The rate
of expression of these molecules was significantly lower in LPS,
compared with higher expression in MFH and LMS compared with other
histological types. In particular, CA9 and GLUT1 expression was
typically identified adjacent to the necrotic regions in cases of
MFH with necrosis, but were diffusely expressed in cases of LMS
where no necrosis was present. However, these expression patterns
had no significant association with prognosis in STS. Further
investigation is required to determine the mechanisms that result
in the differing expression patterns between histological
types.
Hypoxic malignant cells are more resistant to
radiotherapy and chemotherapy (3–5). In
advanced stage ovarian carcinoma, GLUT1 expression has been
reported to be an independent prognostic factor of response to
chemotherapy (30). CA9 may also be
an important marker in the prediction of drug responsiveness in
tongue cancer chemotherapy (31).
The present study attempted to analyze the effect of HIF-1α
overexpression in a group receiving chemotherapy following surgical
resection, demonstrating that HIF-1α overexpression was
independently associated with shorter OS in patients with STS who
received chemotherapy, particularly on multivariate analysis. To
the best of our knowledge, this study is the first aimed at
evaluating the prognostic significance of hypoxic markers in a
series of STS patients receiving chemotherapy.
Numerous studies have investigated the selective
application of new treatment modalities based on targeting tumor
hypoxia (15,19), reporting that hypoxic markers,
including HIF-1α, CA9 and VEGF, may be specific and favorable
therapeutic targets. The present study demonstrated that the
expression of these molecules was common in STS. HIF-1α, CA9,
GLUT1, and VEGF may therefore be useful markers to indicate
aggressive phenotypes and predict prognosis, and are also potential
therapeutic targets.
In conclusion, the expression of hypoxic markers,
including HIF-1α, CA9, GLUT1 and VEGF is common in patients with
STS and is strongly associated with tumor progression, as indicated
by the significant association of their expression with higher
histological grade and advanced tumor stage. In addition, the
results suggest that HIF-1α overexpression is an independent
unfavorable prognostic factor in STS, and may predict poor response
to chemotherapy. Additional investigation of hypoxic markers,
including HIF-1α, as biomarkers of aggressive tumor behavior and as
novel therapeutic targets, is warranted.
Acknowledgements
This study was supported by a Medical Research
Institute Grant (grant no. 2011–25), Pusan National University
Hospital and by a grant from the National Research and Development
Program for Cancer Control, Ministry for Health, Welfare and Family
affairs, Republic of Korea (grant no. 0920050).
References
|
1
|
Brizel DM, Scully SP, Harrelson JM, et al:
Tumor oxygenation predicts for the likelihood of distant metastases
in human soft tissue sarcoma. Cancer Res. 56:941–943.
1996.PubMed/NCBI
|
|
2
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subarsky P and Hill RP: The hypoxic tumour
microenvironment and metastatic progression. Clin Exp Metastasis.
20:237–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Unruh A, Ressel A, Mohamed HG, et al: The
hypoxia-inducible factor-1 alpha is a negative factor for tumor
therapy. Oncogene. 22:3213–3220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jubb AM, Buffa FM and Harris AL:
Assessment of tumour hypoxia for prediction of response to therapy
and cancer prognosis. J Cell Mol Med. 14:18–29. 2010. View Article : Google Scholar
|
|
6
|
Nordsmark M, Alsner J, Keller J, et al:
Hypoxia in human soft tissue sarcomas: adverse impact on survival
and no association with p53 mutations. Br J Cancer. 84:1070–1075.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quintero M, Mackenzie N and Brennan PA:
Hypoxia-inducible factor 1 (HIF-1) in cancer. Eur J Surg Oncol.
30:465–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee WY, Huang SC, Hsu KF, Tzeng CC and
Shen WL: Roles for hypoxia-regulated genes during cervical
carcinogenesis: somatic evolution during the
hypoxia-glycolysis-acidosis sequence. Gynecol Oncol. 108:377–384.
2008. View Article : Google Scholar
|
|
9
|
Enatsu S, Iwasaki A, Shirakusa T, et al:
Expression of hypoxia-inducible factor-1 alpha and its prognostic
significance in small-sized adenocarcinomas of the lung. Eur J
Cardiothorac Surg. 29:891–895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korkeila E, Talvinen K, Jaakkola PM, et
al: Expression of carbonic anhydrase IX suggests poor outcome in
rectal cancer. Br J Cancer. 100:874–880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirkpatrick JP, Rabbani ZN, Bentley RC, et
al: Elevated Caix Expression Is Associated With An Increased Risk
Of Distant Failure In Early-Stage Cervical Cancer. Biomark
Insights. 1:45–55. 2008.
|
|
12
|
Robertson N, Potter C and Harris AL: Role
of carbonic anhydrase IX in human tumor cell growth, survival, and
invasion. Cancer Res. 64:6160–6165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seeber LM, Horrée N, van der Groep P, van
der Wall E, Verheijen RH and van Diest PJ: Necrosis related
HIF-1alpha expression predicts prognosis in patients with
endometrioid endometrial carcinoma. BMC Cancer. 19:3072010.
View Article : Google Scholar
|
|
14
|
Kim K, Park WY, Kim JY, et al: Prognostic
Relevance Of The Expression Of Ca Ix, Glut1, And Vegf In Ovarian
Epithelial Cancers. Korean J Pathol. 46:532–540. 2012. View Article : Google Scholar
|
|
15
|
Poon E, Harris AL and Ashcroft M:
Targeting the hypoxia-inducible factor (HIF) pathway in cancer.
Expert Rev Mol Med. 27:e262009. View Article : Google Scholar
|
|
16
|
Younes M, Lechago LV, Somoano JR, Mosharaf
M and Lechago J: Wide expression of the human erythrocyte glucose
transporter Glut1 in human cancers. Cancer Res. 56:1164–1167.
1996.PubMed/NCBI
|
|
17
|
Younes M, Juarez D, Lechago LV and Lerner
SP: Glut 1 expression in transitional cell carcinoma of the urinary
bladder is associated with poor patient survival. Anticancer Res.
21:575–578. 2001.PubMed/NCBI
|
|
18
|
Kawamura T, Kusakabe T, Sugino T, et al:
Expression of glucose transporter-1 in human gastric carcinoma:
association with tumor aggressiveness, metastasis, and patient
survival. Cancer. 92:634–641. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duncan TJ, Al-Attar A, Rolland P, et al:
Vascular endothelial growth factor expression in ovarian cancer: a
model for targeted use of novel therapies? Clin Cancer Res.
14:3030–3035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozbudak IH, Karaveli S, Simsek T, Erdogan
G and Pestereli E: Neoangiogenesis and expression of
hypoxia-inducible factor 1alpha, vascular endothelial growth
factor, and glucose transporter-1 in endometrioid type endometrium
adenocarcinomas. Gynecol Oncol. 108:603–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: WHO classification of tumors of soft tissue and
bone. 4th edition. IARC; Lyon: 2013
|
|
22
|
Zagars GK, Ballo MT, Pisters PW, Pollock
RE, Patel SR and Benjamin RS: Prognostic factors for
disease-specific survival after first relapse of soft-tissue
sarcoma: analysis of 402 patients with disease relapse after
initial conservative surgery and radiotherapy. Int J Radiat Oncol
Biol Phys. 57:739–747. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ottaiano A, De Chiara A, Fazioli F, et al:
Biological prognostic factors in adult soft tissue sarcomas.
Anticancer Res. 25:4519–4526. 2005.PubMed/NCBI
|
|
24
|
Måseide K, Kandel RA, Bell RS, et al:
Carbonic anhydrase IX as a marker for poor prognosis in soft tissue
sarcoma. Clin Cancer Res. 10:4464–4471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS
and Wu KJ: Prognostic significance of hypoxia-inducible
factor-1alpha, TWIST1 and Snail expression in resectable non-small
cell lung cancer. Thorax. 64:1082–1089. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang MH, Wu MZ, Chiou SH, et al: Direct
regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell
Biol. 10:295–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seeber LM, Horrée N, Vooijs MA, et al: The
role of hypoxia inducible factor-1alpha in gynecological cancer.
Crit Rev Oncol Hematol. 78:173–184. 2011. View Article : Google Scholar
|
|
28
|
Chen CL, Chu JS, Su WC, Huang SC and Lee
WY: Hypoxia and metabolic phenotypes during breast carcinogenesis:
expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch.
457:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vleugel MM, Greijer AE, Shvarts A, et al:
Differential prognostic impact of hypoxia induced and diffuse
HIF-1alpha expression in invasive breast cancer. J Clin Pathol.
58:172–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cantuaria G, Fagotti A, Ferrandina G, et
al: GLUT1 expression in ovarian carcinoma: association with
survival and response to chemotherapy. Cancer. 92:1144–1150. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng G, Zhou M, Ou X, et al:
Identification of carbonic anhydrase 9 as a contributor to
pingyangmycin-induced drug resistance in human tongue cancer cells.
FEBS J. 277:4506–4518. 2010. View Article : Google Scholar : PubMed/NCBI
|