Introduction
Gastrointestinal stromal tumors (GISTs) are the most
frequently diagnosed mesenchymal tumor of the GI tract, accounting
for 1–3% of all malignant GI tumors (1). They are defined pathologically as
c-Kit-positive mesenchymal spindle cell or epithelioid neoplasms
(1). GISTs usually arise from the
stomach (60–70%), followed by the small intestine (20–30%), the
rectum (5%) and the esophagus (<5%), with an estimated
prevalence of 11–14 cases per 1,000,000 individuals (2,3). Only
70% of patients with GIST are symptomatic. While 20% are
asymptomatic and the tumors are detected incidentally, the
remainder of cases are detected during autopsy. The most common
symptoms of GISTs are nausea, vomiting, abdominal discomfort,
bleeding from the GI tract and weight loss (4). The most common metastatic sites are
the liver and peritoneum, whereas GISTs are rarely found to
metastasize intracranially or to the lymph nodes, lungs or
subcutaneous tissue, as described in a limited number of previous
case studies (5–7). Although a small number of studies have
described bone metastases originating from GISTs (6,8–14), the
true prevalence remains unknown (15,16).
At present, the standard therapy for GIST is surgical complete
resection, which is is possible in ~85% of patients. However, GIST
recurrences and metastases are identified in 50% of patients
following complete resection. Thus, for the treatment of these
advanced or unresectable GISTs, the selective tyrosine kinase
inhibitors, imatinib or sunitinib, are used (4,17). The
overall survival rates for patients with advanced or unresectable
GISTs who are treated with imatinib is 55% (18). The present study describes two cases
of bone metastasis in patients with GISTs and reviews the relevant
literature. Written informed consent was obtained from both
patients.
Case reports
Case one
A 78-year-old male had previously undergone surgical
removal of a stomach GIST in 2004. The patient subsequently fell
and fractured the left femoral neck in September 2009. Although
osteosynthesis of the femoral neck was performed at a previous
hospital, union of the femoral neck was not achieved and the
patient reported a painful, swollen mass in the left buttock. Plain
radiographs revealed non-union at the surgical site and a mass in
the soft-tissue of the buttock. Magnetic resonance imaging of the
hip identified a giant mass spreading around the left buttock from
the femoral neck (Fig. 1). A biopsy
was performed at the previous hospital, and a diagnosis of synovial
sarcoma was suggested. The patient was admitted to Toyama
University Hospital (Toyama, Japan) in 2009, where wide resection
of the tumor and reconstruction of the hip joint using a tumor
prosthesis were performed (Fig. 2).
Histopathological examination revealed a hypercellular spindle-cell
neoplasm, with a severe mitotic index of 70 per 50 high-power
fields (Fig. 3A).
Immunohistochemically, the tumor cells were positive for c-KIT and
cluster of differentiation (CD)34, but negative for S-100 protein
(Fig. 3B–D). Chemotherapy was
administered, which consisted of treatment with imatinib (300 mg,
daily) for four years. The patient remains alive at four years
post-surgery and is able to walk unaided.
Case two
A 41-year-old male had previously undergone surgical
removal of a GIST of the rectum at Nagoya University Hospital
(Nagoya, Japan) in 1997. A local recurrence was identified and
resected in 2004. Positron emission tomography (PET)-computed
tomography (CT) revealed a metastatic bone lesion in the left
fourth rib. The bony lesion was completely resected in April 2007
at Toyama University Hospital and the patient was treated with
imatinib (400 mg, daily) for three years. A stable disease state
was subsequently maintained with the administration of imatinib
(400 mg, daily) for five years. However, metastatic renal and liver
lesions were identified and completely resected at Fukui-ken
Saiseikai Hospital (Fukui, Japan) in 2012. Following resection, the
patient was administered imantinib (400 mg, daily). In 2013, PET-CT
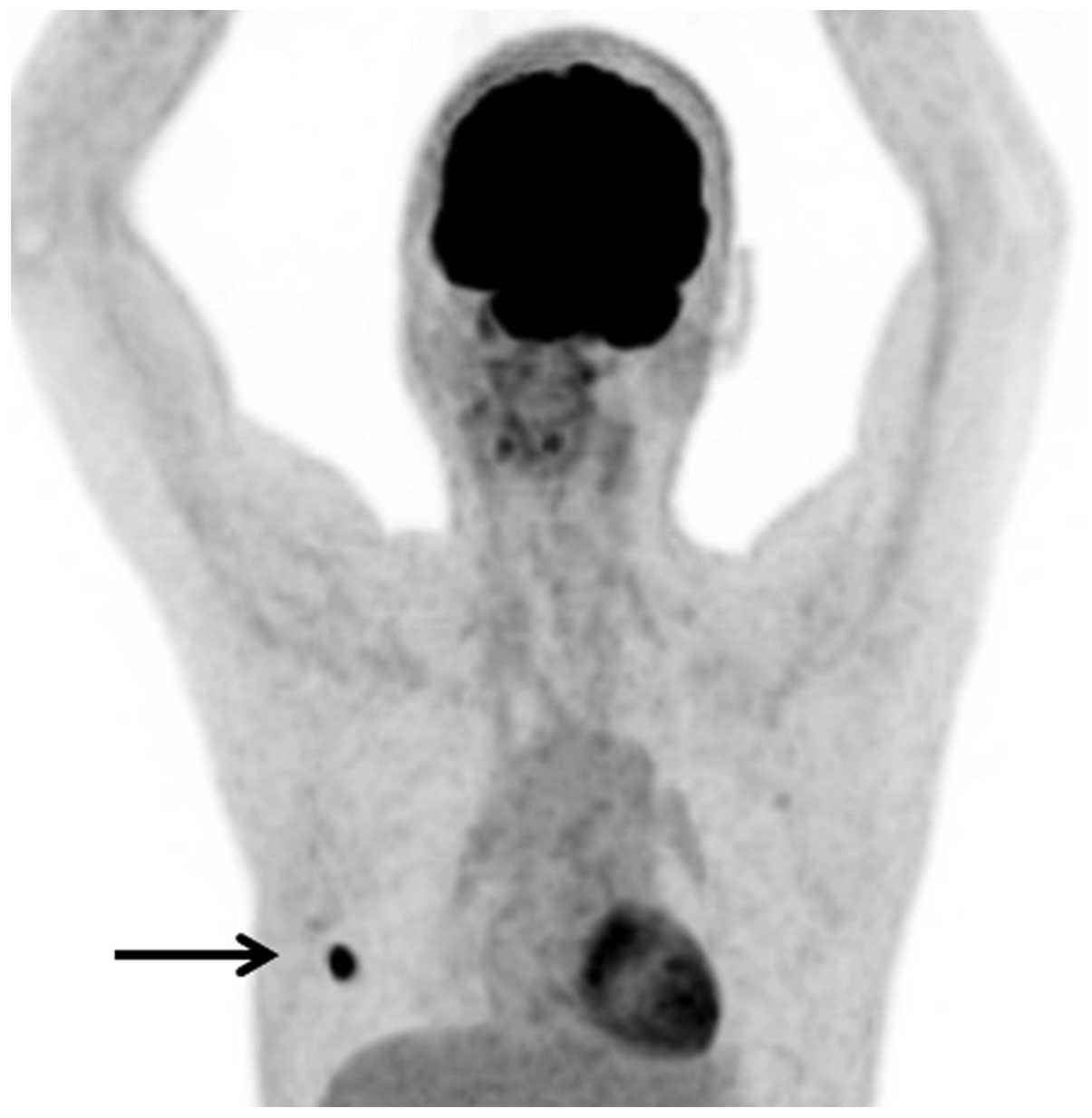
revealed the presence of metastasis in the right fifth rib
(Fig. 4), which was subsequently
completely resected at Toyama University Hospital in December 2013.
Histopathological examination revealed oval-shaped spindle cells
with fascicular proliferation. Immunohistochemical analysis
identified that the tumor cells were positive for c-Kit and CD34.
The patient remains alive 17 years after the initial diagnosis.
Discussion
GISTs are pleomorphic mesenchymal tumors of the GI
tract, consisting of spindle cells, epithelioid cells, or a
combination of these cells, expressing c-KIT protein and, in the
majority of cases, CD34 (4,19). The gold standard of therapy for
GISTs is surgical resection of the local disease. The aim of
treatment is complete resection of the lesion whilst avoiding tumor
rupture. Survival rates are determined by tumor size, and not the
presence of negative microscopic surgical margins. Complete
surgical resection is achieved in ~85% of patients, however, 50% go
on to develop recurrence or metastasis following the surgery
(17). The five-year survival rate
for GIST patients is ~50%, while the median time to recurrence
following resection of a primary high-risk GIST is two years
(4). However, since the
introduction of imatinib as a molecular-targeted therapy, marked
improvements have been made in the rates of progression-free and
overall survival. Despite this, GISTs have a high-risk of
metastatic relapse.
The most common metastatic sites are the liver (65%)
and peritoneal surface (50%) (17),
while GISTs rarely metastasizes to the bone. In a series of studies
concerning metastatic GISTs, the incidence of bone metastasis was
~3% (15,16,20).
However, the specific characteristics of patients with bone
metastasis have not yet been identified. In total, only 12 cases of
GISTs with metastasis to the bone, including those in the present
study, have been reported in the English literature (Table I) (6,8–14). In
the 12 literature cases, the most common site of bone metastasis
was the spine (6/12; 50%). Six cases that presented with metastases
to the spine did not undergo resection of the lesions. This
suggests that metastatic spinal lesions are typically unresectable
due to the infiltration of surrounding nervous tissue. As a result,
four of the six patients succumbed to the disease within 17–90
months (mean, 51.5 months). In total, 11 of the 12 cases (92%),
excluding case 11 (case one of the present study), exhibited liver
metastases at the time that the bone metastases were identified.
Therefore, in the event that liver metastasis is identified during
clinical follow-up, an examination for the presence of bone
metastases should also be performed. The time between initial
diagnosis and the development of bone metastasis varies. The
location of the primary site also varies. In the present study,
evidence of bone metastasis originating from a GIST was identified
five and nine years after the diagnoses of primary stomach and
rectal GISTs, respectively.
 | Table IClinical characteristics of patients
with bone metastases from a gastrointestinal stromal tumor. |
Table I
Clinical characteristics of patients
with bone metastases from a gastrointestinal stromal tumor.
| Case no. (ref.) | Age,
years/gender | Primary site | Site of
metastases | Period of bone
metastasesa, months | Site of bone
metastases | Therapy for bone
metastases | Outcomea, months |
|---|
| 1 (5) | 57/M | Rectum | Liver, bone | At initial
diagnosis | Spine | TKI | DOD, 17 |
| 2 (7) | 58/M | Small intestine | Liver, bone | 28 | Clavicle, spine | TKI, radiation | AWD, 47 |
| 3 (8) | 54/M | Rectum | Liver | 24 | Scapula | TKI, resection | AWD, 120 |
| 4 (9) | 57/M | Small intestine | Liver | 49 | Humerus | TKI, resection,
radiation | DOD, 55 |
| 5 (10) | 62/M | Small intestine | Liver, bone | At initial
diagnosis | Spine, pelvis,
rib | TKI, radiation,
zoledronic acid | DOD, 33 |
| 6 (10) | 82/F | Stomach | Liver, bone | At initial
diagnosis | Spine, pelvis | TKI | AWD, 48 |
| 7 (10) | 54/F | Small intestine | Liver | 84 | Spine, pelvis,
rib | TKI, zoledronic
acid | DOD, 96 |
| 8 (11) | 83/M | Rectum | Liver, bone | 12 | Femur | TKI, radiation,
zoledronic acid | AWD, NA |
| 9 (12) | 53/M | Esophagus | Lung, bone | At initial
diagnosis | Humerus | TKI | AWD, 2 |
| 10 (13) | 69/F | Stomach | Liver, bone | 12 | Spine | TKI | DOD, 60 |
| 11 (Present
study) | 78/M | Stomach | Bone | 48 | Femur | TKI, resection | NED, 72 |
| 12 (Present
study) | 41/M | Rectum | Liver, bone,
kidney | 108 | Rib | TKI, resection | NED, 204 |
A diagnosis of bone metastases originating from a
GIST is usually based upon clinical findings, bone fractures or
pain. An early diagnosis of bone metastases is desirable in order
to avoid a marked reduction in quality of life. PET-CT is useful
for the early diagnosis of bone metastases that originate from
GISTs. Upon PET-CT, metastatic bone lesions exhibit high
18F-fluorodeoxyglucose avidity, similar to that of the
liver and other metastatic sites (16). In the present study, PET-CT proved
useful in achieving a diagnosis.
Imatinib has revolutionized the treatment of
advanced GISTs (17), enabling the
long-term survival of patients. However, the optimal treatment for
bone metastases originating from GIST is yet to be elucidated. Of
the 12 patients with GIST-induced bone metastases described to
date, four were treated with imatinib alone, four received
radiotherapy and four underwent surgical resection of the lesion
(Table I). The choice of therapy
alters according to a number of factors. In the event that multiple
lesions develop despite the use of imatinib, another tyrosine
kinase inhibitor (TKI) may be administered. Treatment options for
patients with progressive disease or widespread systemic disease
and a good performance status include continuation of imatinib at
the same dose, dose escalation to 800 mg/day in the absence of
severe adverse drug reactions, or switching to sunitinib, a
multi-targeted receptor TKI (4). In
addition to TKIs, radiotherapy can be used in patients with bone
metastases for palliative purposes (12). Bisphosphonates are also effective
for the management of bone metastases (11). If the metastatic lesion is isolated
and occurs in a resectable site, imatinib may prove useful. In
cases where the tumor remains resectable, imatinib can be continued
indefinitely until there is evidence of tumor progression following
resection. In fact, long-term survival was achieved in the present
study by resecting an isolated bone lesion.
In conclusion, although bone metastases originating
from GISTs are rare, the likelihood of identifying metastases in
unusual sites is increasing due to the prolonged survival of
patients with the tumors and the introduction of imatinib therapy.
The risk of bone metastases from GISTs should be considered during
long-term follow-up, particularly in the presence of liver
metastases. Furthermore, since side-effects from TKIs are common,
bone metastases should be completely surgically excised if
possible.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research (C) 24592227 (KAKENHI). The authors would
like to thank Dr Ayumu Hosokawa, Third Department of Internal
Medicine, Toyama University Hospital (Toyama, Toyama, Japan) for
providing support during the clinical follow-up.
References
|
1
|
Miettinen M, El-Rifai W, Sobin HL and
Lasota J: Evaluation of malignancy and prognosis of
gastrointestinal stromal tumors: a review. Hum Pathol. 33:478–483.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fletcher CD, Berman JJ, Corless C, et al:
Diagnosis of gastrointestinal stromal tumors: a consensus approach.
Human Pathol. 33:459–465. 2002. View Article : Google Scholar
|
|
3
|
Joensuu H and Kindblom LG:
Gastrointestinal stromal tumors: a review. Acta Orthop Scand Suppl.
75:62–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rammohan A, Sathyanesan J, Rajendran K, et
al: A gist of gastrointestinal stromal tumors: A review. World J
Gastrointest Oncol. 5:102–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nannini M, Biasco G, Di Scioscio V, et al:
Clinical, radiological and biological features of lung metastases
in gastrointestinal stromal tumors (Case reports). Oncol Rep.
25:113–120. 2011.
|
|
6
|
Barrière J, Thariat J, Vandenbos F, et al:
Diplopia as the first symptom of an aggressive metastatic rectal
stromal tumor. Onkologie. 32:345–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamada S, Itami A, Watanabe G, et al:
Intracranial metastasis from an esophageal gastrointestinal stromal
tumor. Inter Med. 49:781–785. 2010. View Article : Google Scholar
|
|
8
|
Feki J, Bouzguenda R, Ayedi L, et al: Bone
metastases from gastrointestinal stromal tumors: a case of report.
Case Rep Oncol Med. 5098452012.
|
|
9
|
Selcukbiricik F, Tural D, Ozturk MA, et
al: Gastrointestinal stromal tumor of the rectum with scapular
metastasis: a case report. J Med Case Rep. 6:1452012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abuzakhm SM, Acre-Lara CE, Zhao W, et al:
Unusual metastases of gastrointestinal stromal tumor and genotypic
correlates: Case report and review of the literature. J
Gastrointest Oncol. 2:45–49. 2011.PubMed/NCBI
|
|
11
|
Di Scioscio V, Greco L, Pallotti MC, et
al: Three cases of bone metastases in patients with
gastrointestinal stromal tumors. Rare Tumors. 3:e172011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tezcan Y and Koç M: Gastrointestinal
stromal tumor of the rectum with bone and liver metastasis: a case
study. Med Oncol. 28(Suppl 1): S204–S206. 2011. View Article : Google Scholar
|
|
13
|
Ozan E, Oztekin O, Alacacioğlu A, et al:
Esophageal gastrointestinal stromal tumor with pulmonary and bone
metastases. Diagn Interv Radiol. 16:217–220. 2010.
|
|
14
|
Kroep JR, Bovée JV, van der Molen AJ, et
al: Extra-abdominal subcutaneous metastasis of a gastrointestinal
stromal tumor: report of a case and a review of the literature. J
Cutan Pathol. 36:565–569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burkill GJ, Badran M, Al-Muderis O, et al:
Malignant gastrointestinal stromal tumor: distribution, imaging
features and pattern of metastatic spread. Radiology. 226:527–532.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jati A, Tatli S, Morgan JA, et al: Imaging
features of bone metastases in patients with gastrointestinal
stromal tumors. Diagn Interv Radiol. 18:391–396. 2012.PubMed/NCBI
|
|
17
|
Stamatakos M, Douzinas E, Stefanaki C, et
al: Gastrointestinal stromal tumor. World J Surg Oncol. 7:612009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blanke CD, Demetri GD, von Mehren M, et
al: Long-term results from a randomized phase II trial of standard-
versus higher-dose imatinib mesylate for patients with unresectable
or metastatic gastrointestinal stromal tumors expressing KIT. J
Clin Oncol. 26:620–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors - definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dematteo RP, JoLewis JJ, Leng D, Mudan SS,
Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal
tumors: Recurrence patterns and prognostic factors for survival.
Ann Surg. 231:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|