Introduction
Taxanes, along with anthracyclines, are a key
component in chemotherapy for breast cancer. The Taxanes include
paclitaxel and docetaxel. In Japan, weekly paclitaxel has been
widely used due to good efficacy and high tolerability (1). However, since paclitaxel is relatively
insoluble, polyoxyethylated castor oil (Cremophor®EL)
and ethanol have served as solvents to enhance solubility.
Consequently, patients must receive premedication with
corticosteroids, antihistamines and histamine-2 receptor
antagonists prior to administration of paclitaxel. Despite
premedication, however, ~40% patients exhibit mild hypersensitivity
reactions and ~3% have serious, life-threatening reactions
(2). Premedication with
polyoxyethylated castor oil may also result in peripheral
neuropathy and alter the pharmacokinetics of paclitaxel (3). Paclitaxel also has other
solvent-related problems: Only limited types of intravenous
infusion sets may be used and treating patients who exhibit alcohol
intolerance is difficult (4).
Nanoparticle albumin-bound (nab)-paclitaxel
(Abraxane®) is a solvent-free, colloidal suspension of
paclitaxel and human serum albumin. Compared with conventional
preparations of paclitaxel, nab-paclitaxel has a number of
advantages: i) No premedication to prevent hypersensitivity is
required; ii) any type of intravenous infusion set may be used
(with no requirement for in-line filters); iii) nab-paclitaxel may
be used even in patients who are sensitive to alcohol; and iv)
nab-paclitaxel may be administered at a higher dose over the course
of a shorter time period than paclitaxel (5). A phase III controlled study comparing
tri-weekly paclitaxel (175 mg/m2) with tri-weekly
nab-paclitaxel (260 mg/m2) in female breast cancer
patients, conducted outside of Japan, reported that nab-paclitaxel
was significantly superior to paclitaxel in terms of response rate
(33% versus 19%; P<0.001) and progression-free survival times
(23.0 versus 16.9 weeks; hazard ratio=0.75; P=0.006) (5). Nab-paclitaxel has thus overcome the
predominant disadvantages of paclitaxel, and exerts enhanced
antitumor activity. The present study reports the clinical
experience of female breast cancer patients treated with
nab-paclitaxel, and describes the adverse event management. Written
informed consent was obtained from all patients.
Patients and methods
Patients
Data regarding 22 women with advanced or recurrent
breast cancer who received nab-paclitaxel in the National Hospital
Organization Shikoku Cancer Center (Matsuyama, Japan) between
November 2010 and June 2012 were retrospectively analyzed. The
general condition of the patients who received nab-paclitaxel had
to satisfy the following conditions: i) A histologically confirmed
diagnosis of breast cancer; ii) an Eastern Cooperative Oncology
Group performance status of 0 to 2; iii) adequate bone marrow
function (white blood cell count, ≥4,000/μl; platelet count,
≥100×103/μl); iv) adequate liver function (bilirubin
levels, ≤1.5 mg/dl; aspartate aminotransferase and alanine
aminotransferase levels, ≤2.5-fold the institutional upper limit of
normal); v) adequate renal function (creatinine levels, ≤1.5
mg/dl); and vi) adequate cardiac function.
Treatment
Nab-paclitaxel was administered as a continuous
intravenous infusion over the course of 30 min every three weeks.
The patients did not receive any particular premedication.
Response and toxicity assessment
Computed tomography and magnetic resonance imaging
scans were performed at baseline and after three months to assess
the radiological response of each patient according to the Response
Evaluation Criteria in Solid Tumors, version 1.1 (6). The clinical benefit ratio (CBR) was
defined as the percentage of patients who had a complete response
(CR), partial response (PR) or stable disease. Adverse events were
evaluated according to the Common Terminology Criteria for Adverse
Events, Japanese version 4.0 (Japan Clinical Oncology Group/Japan
Society of Clinical Oncology edition) (7). Time to treatment failure (TTF) was
defined as the time period between the initiation of treatment and
the cessation of treatment for any reason, including progressive
disease, treatment-related toxicity and fatality, and was estimated
by the Kaplan-Meier method.
Countermeasures against adverse
events
In the National Hospital Organization Shikoku Cancer
Center, pharmacists provide patients with a detailed explanation
with regard to the time periods when greatest bone marrow
suppression occurs (nadir white-cell count), the countermeasures
against infection, and the management of fever prior to
chemotherapy and prior to discharge, using a patient compliance
manual. Subcutaneous injection of granulocyte colony-stimulating
factor (G-CSF) and treatment with antibacterial agents requires
consideration in patients with grade 3 or higher febrile
neutropenia, or grade 4 neutropenia.
In patients with peripheral neuropathy, treatment
withdrawal or dose reduction was performed as required, and
symptomatic treatment with vitamins, Gosha-jinki-gan, pregabalin
(Lyrica®) and/or analgesics, such as loxoprofen
(Loxonin®), was administered. Prophylactic treatment for
myalgia and arthralgia was not administered, but nonsteroidal
anti-inflammatory drugs (Loxonin and Mohrus®) were
prescribed as required. The patients were informed in advance with
regard to when these symptoms were most likely to occur and were
instructed to take the prescribed drugs, so as to avoid enduring
pain.
In our center, patients who receive nab-paclitaxel
monotherapy are not usually administered antiemetics. The initial
dose of nab-paclitaxel is administered during hospitalization, and
the second and subsequent doses are prescribed on an outpatient
basis. Pharmacists provide patients with drug management counseling
prior to treatment, including information regarding drug names,
treatment goals, treatment schedules, and potential adverse events
with possible times of onset and countermeasures. Pharmacists are
stationed in outpatient clinics and interview patients with regard
to adverse events.
Nurses at the center provide patients with guidance
concerning daily activities, accounting for the background
characteristics of each patient. The nurses also describe the
typical patient experience (development and management of adverse
events), thereby attempting to relieve anxiety. In addition, the
nurses provide patients with information regarding the severity of
adverse events that would require treatment withdrawal or dose
reduction, or the possibility of switching to other regimens, and
the patients may seek consultation at any time.
Results
Patients
The clinical characteristics of the patients are
shown in Table I. The median age at
the initiation of treatment was 59 years (range, 35 to 73). A total
of 18 patients exhibited postoperative recurrence and four had
stage IV disease. The hormone receptor (HR) and human epidermal
growth factor receptor-2 (HER2) protein expression status of
patients was as follows: HR-positive and HER2-negative in 14
patients, and HR-negative and HER2-negative in 8 patients. No
patient had HER2-positive tumors. The metastatic sites were the
lymph nodes in 15 patients, liver in 12, lung in 11, bone in seven,
pleura in five and skin in one. A total of 15 patients had
metastases to multiple organs. The starting dose of nab-paclitaxel
was the standard recommended dose (260 mg/m2) (8) in 13 patients and a reduced dose (179
to 240 mg/m2) in nine patients. Nab-paclitaxel was
administered as a first-line treatment for metastasis/recurrence in
10 patients, a second-line treatment in four, and a third-line or
subsequent treatment in eight. Prior to the nab-paclitaxel
treatment, five patients had received capecitabine, four received
gemcitabine, one received doxorubicin (Adriamycin®) plus
cyclophosphamide, one received eribulin, one received S-1, two
received paclitaxel and three received docetaxel.
 | Table IDemographic characteristics of females
with advanced breast cancer who received nab-paclitaxel between
November 2010 and June 2012. |
Table I
Demographic characteristics of females
with advanced breast cancer who received nab-paclitaxel between
November 2010 and June 2012.
| Clinical
parameter | No. of patients |
|---|
| Age, yearsa | 59.0 (35–73) |
| PS |
| 0 | 12 |
| 1 | 8 |
| 2 | 2 |
| HR, HER2 status |
| HR(+)/HER2(−) | 14 |
| HR(−)/HER2(−) | 8 |
| Metastasis |
| (+)/(−) | 22/0 |
| Liver | 12 |
| Lung | 11 |
| Lymph nodes | 10 |
| Bone | 7 |
| Pleura | 5 |
| Skin | 1 |
| Number of metastatic
sites |
| 1 | 7 |
| 2 | 4 |
| ≥3 | 11 |
| Therapy |
| 1st line | 10 |
| 2nd line | 4 |
| ≥3rd line | 8 |
Efficacy
Among the 22 patients, none had achieved a CR, but
six reached a PR (response rate, 27.3%; 95% confidence interval,
8.7–45.9%). The CBR was 31.8% (Table
II), and the median number of treatment courses was six (range,
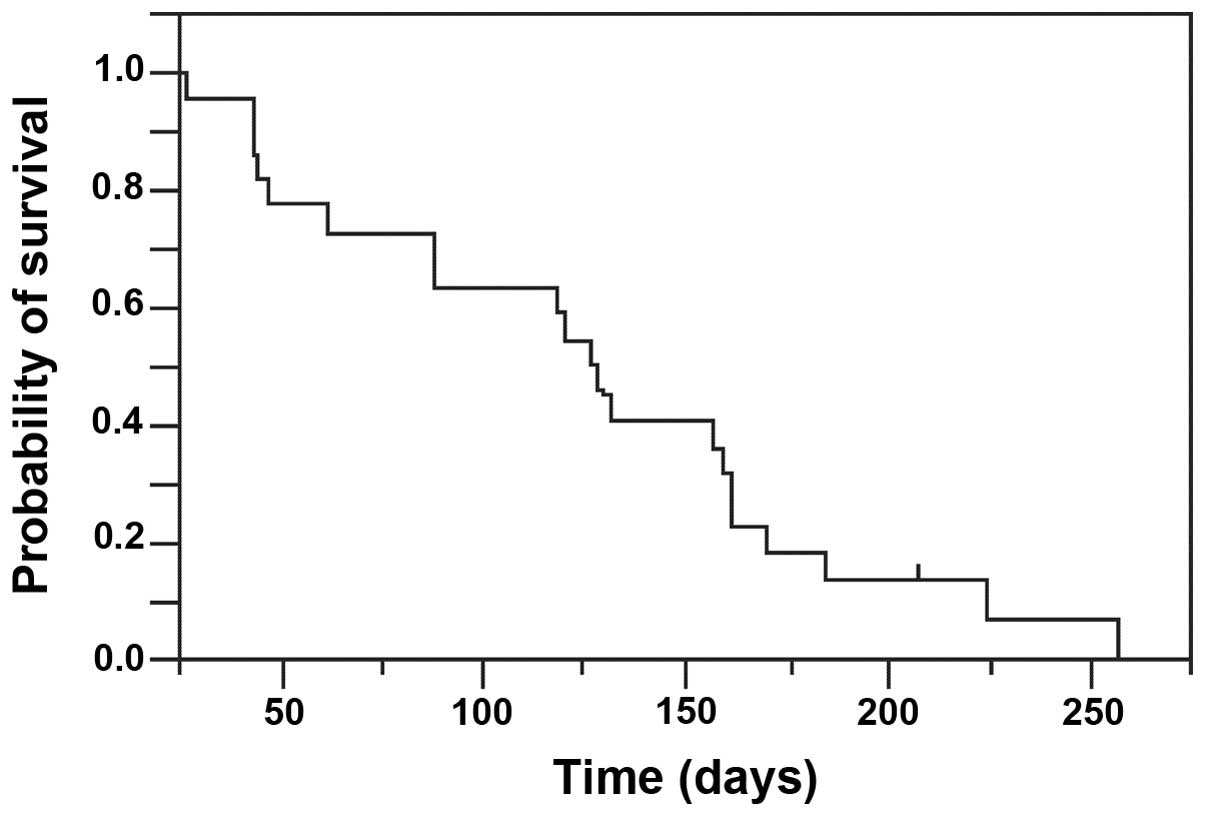
two to 12). The TTF was 127 days (range, 27 to 224), and treatment
is being continued in one patient (Fig.
1). With regard to the association between response and HR
expression status, the response rate did not differ between
patients with HR-positive tumors and those with HR-negative tumors.
The disease-free survival times did not differ between patients who
responded to treatment and those who did not, or between patients
who exhibited clinical benefits and those who did not. None of the
five patients who had previously received taxanes responded to
nab-paclitaxel. The response rate and CBR were markedly higher in
patients with metastasis to a single organ than in those with
metastases to multiple organs.
 | Table IIAntitumor effectiveness of
nab-paclitaxel in females with advanced breast cancer. |
Table II
Antitumor effectiveness of
nab-paclitaxel in females with advanced breast cancer.
| Response | No. of patients |
|---|
| Complete
response | 0 |
| Partial response | 6 |
| Stable disease | 1 |
| Progressive
disease | 11 |
| Not evaluable | 4 |
| Response rate
(%) | 27.3 |
| Clinical benefit rate
(%) | 31.8 |
Toxicity
The adverse events that developed during treatment
with nab-paclitaxel are shown in Table III. Peripheral neuropathy occurred
in 13 patients (59.1%), two of which exhibited grade 3 reactions.
The mean number of treatment cycles at the onset of peripheral
neuropathy was 2.5 (range, one to nine). Other adverse events
reported included arthralgia and myalgia in 13 patients each, fever
in four, rash in 10, nausea and vomiting in 11 patients each,
diarrhea in four and stomatitis in four. These adverse events were
mild and generally appeared only during the first course of
treatment. The hematological toxicity symptoms (grade 3 or higher)
reported included leukopenia and neutropenia in 12 patients (54.5%)
each, but no febrile neutropenia was detected. Treatment was
postponed in only one patient and G-CSF was used in only one
patient. No hypersensitivity reactions to nab-paclitaxel occurred,
despite the absence of premedication and shorter administration
time than paclitaxel (5). No nail
changes or rashes were observed.
 | Table IIIAdverse events following
nab-paclitaxel treatment in female patients with advanced breast
cancer. |
Table III
Adverse events following
nab-paclitaxel treatment in female patients with advanced breast
cancer.
| No. patients | Percentage of
patients |
|---|
|
|
|
|---|
| Gr1 | Gr2 | Gr3 | Gr4 | All grades | ≥Gr3 |
|---|
| Leukopenia | 4 | 5 | 8 | 4 | 95.4 | 54.5 |
| Neutropenia | 3 | 4 | 6 | 6 | 86.3 | 54.5 |
| Thrombocytopenia | 6 | 1 | 0 | 1 | 36.3 | 4.5 |
| Anemia | 8 | 5 | 3 | 0 | 72.7 | 13.6 |
| AST | 9 | 4 | 0 | 0 | 59.1 | 0.0 |
| ALT | 16 | 0 | 0 | 0 | 72.7 | 0.0 |
| Creatinine | 4 | 2 | 0 | 0 | 27.2 | 0.0 |
| Peripheral
neuropathy | 5 | 6 | 2 | 0 | 59.1 | 9.1 |
| Myalgia | 12 | 1 | 0 | - | 59.1 | 0.0 |
| Arthralgia | 7 | 6 | 0 | - | 59.1 | 0.0 |
| Malaise | 11 | 0 | - | - | 50.0 | - |
| Alopecia | 0 | 22 | - | - | 100.0 | - |
| Nausea | 9 | 2 | 0 | - | 50.0 | 0.0 |
| Vomiting | 9 | 2 | 0 | 0 | 50.0 | 0.0 |
| Diarrhea | 3 | 1 | 0 | 0 | 18.2 | 0.0 |
Reasons for discontinuation of treatment
and subsequent chemotherapy administered
Treatment was discontinued due to progressive
disease in 11 patients and adverse events in 10 patients, nine of
whom experienced peripheral neuropathy. Adverse events were the
primary reason for the withdrawal of first-line treatment, and
progressive disease was the predominant reason for the cessation of
second- and third-line, or subsequent treatment. Following the
withdrawal of nab-paclitaxel treatment, four patients received
capecitabine, three received vinorelbine, two received doxorubicin
plus cyclophosphamide, two received eribulin, two received S-1 and
one patient received paclitaxel. The CBR was 50% (2/4) in patients
who received capecitabine and 50% (1/2) in those who were
administered S-1. However, no clinical benefit was observed in
patients on other treatment regimens.
Discussion
Several large-scale phase III studies of paclitaxel
administered every three weeks have shown that the response rate
ranges between 11 and 29%, and that the median TTP ranges between
3.6 and 5.0 months (1,7,9–12). In
the present study, six patients exhibited a PR to nab-paclitaxel
with a response rate of 27.3%, a CBR of 31.8% and TTF of 127 days.
A total of 80% patients on first-line treatment received the full
dose of nab-paclitaxel, 50% of those on second-line treatment and
40% of those on third-line or later treatment. In the patients who
were receiving first- or second-line treatment, the response rate
was ~60%, indicating high antitumor effectiveness. Few patients
responded nab-paclitaxel administered as third-line or later
treatment. The proportion of patients who discontinued
nab-paclitaxel due to adverse events was 60% when the drug was
provided as first-line treatment, 0% for second-line treatment and
20% for third-line or later treatment. The finding that the CBR was
only marginally higher than the response rate may be due to the
high withdrawal rate of first-line treatment due to adverse events.
Peripheral neuropathy was the adverse event that most frequently
required the withdrawal of treatment. Improved methods of managing
peripheral neuropathy must therefore be established.
As peripheral neuropathy exerts a particularly
marked impact on quality of life, the management of this adverse
effect is a key determinant of the success and completion rates of
taxane treatment. Peripheral neuropathy is considered to involve
the binding of taxanes to microtubules in peripheral nerve cells,
promoting the aggregation of intracellular microtubules. This
abnormal aggregation of microtubules in neuronal cells is
considered to disturb sensory nerve function, resulting in
neuropathy, but a number of factors remain unclear (13,14).
Countermeasures against peripheral neuropathy include dose
reduction, cessation of treatment and supportive medical therapy.
One study evaluating low-dose nab-paclitaxel reported a response
rate of 51.2% and a progression-free survival time of 22.4 weeks in
patients who received 175 mg/m2 of nab-paclitaxel every
three weeks (15). Furthermore, the
low-dose regimen was not associated with grade 3 or higher
peripheral neuropathy. A number of studies have assessed the
effectiveness of various drugs as prophylactic or supportive
therapy for peripheral neuropathy. Several randomized, phase II
studies have reported that amifostine reduces the incidence of
grade 2 peripheral neuropathy, whereas other clinical trials have
demonstrated no apparent effect of this drug on the incidence of
peripheral neuropathy (16–21). Although glutamine (22), vitamins (23) and other agents have been shown to be
somewhat effective, treatment remains to be established and the
management of peripheral neuropathy is problematic. At present, the
early detection of peripheral neuropathy and dose reduction or
treatment withdrawal prior to the onset of severe symptoms remains
essential. In the National Hospital Organization Shikoku Cancer
Center, peripheral neuropathy is symptomatically treated with
vitamins or Gosha-Jinki-Gan. Since detecting symptoms of peripheral
neuropathy is difficult for healthcare providers, pharmacists and
nurses working in the outpatient chemotherapy clinic are encouraged
to actively ask patients regarding their condition to facilitate
the early detection and effective management of adverse events
before they become serious.
In Japan, breast cancer is the most common type of
cancer among females. The peak incidence of breast cancer occurs in
females in their late 40s to early 60s, which is somewhat earlier
than that of other types of cancer (24). As numerous chemotherapeutic regimens
for breast cancer may be administered on an outpatient basis, the
majority of patients want to receive treatment while continuing
life as usual. The management of adverse events during home care is
thus important. Since breast cancer therapy produces a number of
adverse effects that directly influence quality of life, such as
nausea and vomiting, peripheral neuropathy and hair loss, it is
important to reduce anxiety whenever possible by providing
appropriate advice with regard to the management of these events,
including the expected times of peak occurrence. Close
communication with patients is also essential to the early
detection of these adverse events, further increasing the
importance of patient interviews with pharmacists and nurses.
Information obtained by physicians, pharmacists, nurses and other
medical professionals should be shared to ensure that therapy is
delivered more effectively and safely.
Nab-paclitaxel may be used safely; our clinical
experience suggests that nab-paclitaxel is most likely to be
effective when provided as a second-line treatment for patients
with metastasis to a single organ. Even if the effectiveness of
nab-paclitaxel is only equivalent to that of other taxanes, this
drug offers numerous advantages for patients as well as medical
professionals, such as a shorter treatment time.
However, use of the standard recommended dose of
nab-paclitaxel for first-line therapy was associated with a high
rate of treatment withdrawal, due to peripheral neuropathy.
Therefore, the management of peripheral neuropathy remains
important. Countermeasures against peripheral neuropathy, including
the development of novel drugs, are required. Although experience
remains limited, the use of a lower dose of nab-paclitaxel has been
reported to be associated with a lower risk of peripheral
neuropathy, without compromising effectiveness (14). Maintaining a good balance between
treatment effectiveness and the quality of life of patients who
receive nab-paclitaxel is therefore a main aim of patient care; the
use of a lower dose should be considered whenever feasible.
References
|
1
|
Seidman AD, Berry D, Cirrincione C, et al:
Randomized phase III trial of weekly compared with every-3-weeks
paclitaxel for metastatic breast cancer, with trastuzumab for all
HER-2 overexpressors and random assignment to trastuzumab or not in
HER-2 nonoverexpressors: final results of Cancer and Leukemia Group
B protocol 9840. J Clin Oncol. 26:1642–1649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weiss RB, Donehower RC, Wiernik PH, et al:
Hypersensitivity reactions from taxol. J Clin Oncol. 8:1263–1268.
1990.PubMed/NCBI
|
|
3
|
Mielke S, Sparreboom A and Mross K:
Peripheral neuropathy: a persisting challenge in paclitaxel-based
regimes. Eur J Cancer. 42:24–30. 2006. View Article : Google Scholar
|
|
4
|
Taxol® (paclitaxel) injection
[packaging insert]. Princeton, NJ, USA: Bristol-Myers Squibb
Company; 2007
|
|
5
|
Gradishar JW, Tjulandin S, Davidson N, et
al: Phase III trial of nanoparticle albumin-bound paclitaxel
compared with polyethylated castor oil-based paclitaxel in women
with breast cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
7
|
Japan Clinical Oncology Group. Common
Terminology Criteria for Adverse Events version 4.0. http://www.jcog.jp/doctor/tool/CTCAEv4J_20140920.pdf.
Accessed December 10, 2014
|
|
8
|
Abraxane® for Injectable
Suspension (nab-paclitaxel) [packaging insert]. Summit, NJ, USA:
Celegene Corporation; 2013
|
|
9
|
Nabholtz JM, Gelmon K, Bontenbal M, et al:
Multicenter, randomized comparative study of two doses of
paclitaxel in patients with metastatic breast cancer. J Clin Oncol.
14:1858–1867. 1996.PubMed/NCBI
|
|
10
|
Jones SE, Erban J, Overmoyer B, et al:
Randomized phase III study of docetaxel compared with paclitaxel in
metastatic breast cancer. J Clin Oncol. 23:5542–5551. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albain KS, Nag SM, Calderillo-Ruiz G, et
al: Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in
patients with metastatic breast cancer and prior anthracycline
treatment. J Clin Oncol. 26:3950–3957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harris LN, Broadwater G, Lin NU, et al:
Molecular subtypes of breast cancer in relation to paclitaxel
response and outcomes in women with metastatic disease: results
from CALGB 9342. Breast Cancer Res. 8:R662006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rowinsky EK and Donehower RC: Paclitaxel
(Taxol). N Engl J Med. 332:1004–1014. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swain SM and Arezzo JC: Neuropathy
associated with microtubule inhibitors: diagnosis, incidence, and
management. Clin Adv Hematol Oncol. 6:455–467. 2008.PubMed/NCBI
|
|
15
|
Ibrahim NK, Samuels B, Page R, et al:
Nanoparticle paclitaxel (ABI-007) in metastatic breast cancer
(MBC): efficacy and evidence of dose-dependent activity in two
multicenter phase II studies. Proc Am Soc Clin Oncol.
21:2092002.
|
|
16
|
Hilpert F, Stähle A, Tomé O, et al;
Arbeitsgemeinschaft Gynäkologische Onkologoie (AGO) Ovarian Cancer
Study Group. Neuroprotection with amifostine in the first-line
treatment of advanced ovarian cancer with
carboplatin/paclitaxel-based chemotherapy - a double-blind,
placebo-controlled randomized phase II study from the
Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) Ovarian Cancer
Study Group. Support Care Cancer. 13:797–805. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Vos FY, Bos AM, Schaapveld M, et al: A
randomized phase II study of paclitaxel with carboplatin +/−
amifostine as first line treatment in advanced ovarian carcinoma.
Gynecol Oncol. 97:60–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lorusso D, Ferrandina G, Greggi S, et al;
Multicenter Italian Trials in Ovarian Cancer Investigators. Phase
III multicenter randomized trial of amifostine as cytoprotectant in
first-line chemotherapy in ovarian cancer patients. Ann Oncol.
14:1086–1093. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sevelda P, Sevelda U, Denison U and Kaider
A; Austrian Society of Gynecologic Oncology. Cytoprotection of
amifostine (A) in ovarian cancer patients receiving
paclitaxel/carboplatin (PC) first line chemotherapy in a
multicenter phase III trial. J Clin Oncol, 2004 ASCO Annual Meeting
Proc (Post-Meeting Edition). (July 15 Suppl)22:50212004.
|
|
20
|
Leong SS, Tan EH, Fong KW, et al:
Randomized double-blind trial of combined modality treatment with
or without amifostine in unresectable stage III non-small-cell lung
cancer. J Clin Oncol. 21:1767–1774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gelmon K, Eisenhauer E, Bryce C, et al:
Randomized phase II study of high-dose paclitaxel with or without
amifostine in patients with metastatic breast cancer. J Clin Oncol.
17:3038–3047. 1999.PubMed/NCBI
|
|
22
|
Jacobson SD, Loprinzi CL, Sloan JA, et al:
Glutamine does not prevent paclitaxel-associated myalgias and
arthralgias. J Support Oncol. 1:274–278. 2003.
|
|
23
|
Argyriou AA, Chroni E, Koutras A, et al:
Vitamin E for prophylaxis against chemotherapy-induced neuropathy:
a randomized controlled trial. Neurology. 64:26–31. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Center for Cancer Control and Information
Services. National Estimates of Cancer Incidence Based on Cancer
Registries in Japan (1975–2010). http://ganjoho.jp/pro/statistics/en/table_download.html#02.
Accessed December 10, 2014
|