Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare
disorder that was previously referred to by a variety of synonyms,
including inflammatory pseudotumor, plasma cell granuloma,
fibroxanthoma, fibrous histiocytoma and xanthogranuloma (1). In 2002, the World Health Organization
classification scheme defined IMT as a ‘distinctive lesion composed
of myofibroblastic spindle cells accompanied by an inflammatory
infiltrate of plasma cells, lymphocytes and eosinophils’ (2). Despite its precise definition, IMT
remains controversial in regard to its nature and origin. With
alterations in the anaplastic lymphoma kinase (ALK) gene and an
overexpression of ALK protein reported in many cases of IMT, the
concept that IMT is a true neoplasm, rather than a reactive
process, has been increasingly accepted (2–4).
Furthermore, reported cases of IMTs exhibiting aggressive growth,
local invasion, recurrence and even distant metastasis, also
supports this concept (5–10).
IMT may occur at any age, but usually affects
children and adults <40 years old (6,11). IMT
primarily affects the lung, and accounts for 0.04–1% of all
reported lung tumors (12,13). Pulmonary IMT (PIMT) is the most
frequently diagnosed primary lung mass in children (14). Therefore, a large number of studies
regarding childhood PIMT exist in the literature. For adults,
particularly those >40 years old, a relatively small number of
studies concerning PIMT have been published, due to its rarity.
Furthermore, the most common malignant tumor of the lung, lung
cancer, predominantly affects this age group. Therefore, it is of
great importance to identify PIMT in patients over the age of
40.
PIMT is challenging to diagnose in the absence of
any pathological evidence, as few of its clinical manifestations
and laboratory results are specific. Imaging features of PIMT
remain poorly recognized, although a number of radiological studies
referring to chest X-ray, computed tomography (CT) and magnetic
resonance imaging have been published (12,15,16).
Recently, 18F-fluorodeoxyglucose (FDG) positron emission
tomography (PET)/CT has been widely used for the diagnosis and
differential diagnosis of lung masses. Therefore, a requirement
exists to extend the understanding of 18F-FDG PET/CT in
PIMT. Additionally, single-photon emission computed tomography
(SPECT) bone scans are frequently performed during the staging of
lung cancers, and also for the indirect confirmation of lung cancer
that has metastasized to the bone. The present study
retrospectively analyzed the CT, 18F-FDG PET/CT and
SPECT bone scan findings of PIMT occurring in patients >40 years
old.
Patients and methods
Patient characteristics
Between September 2004 and June 2013, 10 patients,
consisting of eight males and two females aged between 41 and 65
years, with a mean age of 56 years, were pathologically diagnosed
with PIMT following surgical resection or a biopsy at Jinling
Hospital, School of Medicine, Nanjing University (Nanjing, China).
Of the 10 patients, eight underwent CT and two underwent
18F-FDG PET/CT. In four of the 10 patients, a SPECT bone
scan using 99mTc-methylene diphosphonate (MDP) was
performed in order to determine the presence or absence of bone
metastasis. The clinical data of the patients were retrospectively
analyzed (Table I).
 | Table IClinical data of 10 cases of
pulmonary inflammatory myofibroblastic tumor. |
Table I
Clinical data of 10 cases of
pulmonary inflammatory myofibroblastic tumor.
| Patient | Gender | Age, years | Clinical
symptoms | Medical
history | Surgical
management |
|---|
| 1 | M | 54 | Cough,
expectoration, chest pain | Smoking,
hypertension | Wedge
resection |
| 2 | M | 62 | Cough,
expectoration, hemoptysis | Bronchiectasis,
smoking | Lobectomy |
| 3 | M | 56 | None | None | Lobectomy |
| 4 | M | 56 | Cough,
expectoration, hemoptysis | Smoking | Lobectomy |
| 5 | F | 54 | None | Systemic lupus
erythematosus | Lobectomy |
| 6 | F | 65 | Cough | Hypertension | Lobectomy |
| 7 | M | 58 | Chest pain | Hypertension | Lobectomy |
| 8 | M | 49 | Cough, fever, chest
pain | Smoking | Percutaneous
biopsy |
| 9 | M | 41 | Back pain,
fever | Smoking | Percutaneous
biopsy |
| 10 | M | 59 | Cough,
expectoration, fever | Smoking | Lobectomy |
CT examination
Of the eight patients who underwent CT examination,
seven underwent plain and contrast-enhanced chest CT images and one
underwent non-enhanced CT images alone. The scans were performed
using a dual-source CT (Somatom Definition; Siemens Healthcare,
Malvern, PA, USA) and a double-slice spiral CT scanners (Somatom
Spirit; Siemens Healthcare). The CT parameters were as follows: A
tube voltage of 120 kVp, a tube current of 150 mAs, a
reconstruction interval of 2 mm, a slice thickness of 2 mm, a field
of view of 250–350 mm and a matrix size of 512×512. The
contrast-enhanced CT scan was performed with an intravenous
injection of 100 ml iopamidol or 80 ml omnipaque at a rate of 2.5
ml/s, administered by a high-pressure autoinjector. CT enhancement
was obtained in the arterial and venous phases, 20 and 50 sec after
the injection of the contrast agent, respectively. The chest CT
images were evaluated by the consensus of two experienced
radiologists for the location, shape, size, density, margin and
contrast enhancement of the lesions.
18F-FDG PET/CT imaging
The patients were asked to fast for at least 6 h
prior to receiving an intravenous injection of ~370 MBq
18F-FDG. In addition, blood glucose was measured prior
to the injection to ensure that levels were <140 mg/dl. The
initial whole-body scan was carried out 60 min subsequent to the
injection using a PET/CT system (Biography Sensation 16; Siemens
Healthcare). The delayed scan was then localized to the lung and
performed 120 min subsequent to the injection. The PET emission
scan was performed with an acquisition time of 3 min for each bed.
Next, PET data were obtained with the attenuation correction
calculated from the coregistered CT images. Consequently, PET, CT
and fused images of the early scan were displayed, in addition to
those of the delayed imaging. The images were then visually
interpreted by the consensus of two experienced nuclear medicine
physicians for the location, shape, size, density, margin and
18F-FDG uptake pattern of the pulmonary lesions. The
maximal standard uptake values (SUVmax) of the
dual-time-point were also calculated.
SPECT bone scan
In four of the 10 patients, a SPECT bone scan (e.cam
Signature Series; Siemens Healthcare) was performed 3 h subsequent
to the intravenous administration of 1110 MBq 99mTc-MDP.
Anterior and posterior whole body planar images were acquired in a
continuous mode at a scan speed of 20 cm/min using parallel-hole,
low-energy, high-resolution collimators, with the patient in the
supine position. The matrix size was 256×1024, and the zoom was 1.0
during the total acquisition. The whole body planar images were
visually assessed by two experienced nuclear medicine physicians
for the presence or absence of bone metastasis.
Results
CT findings
The CT findings of PIMT in the present study were
based on 10 patients, consisting of eight who underwent routine CT
examination and two who underwent the unenhanced CT component of
PET/CT (Table II). CT revealed 10
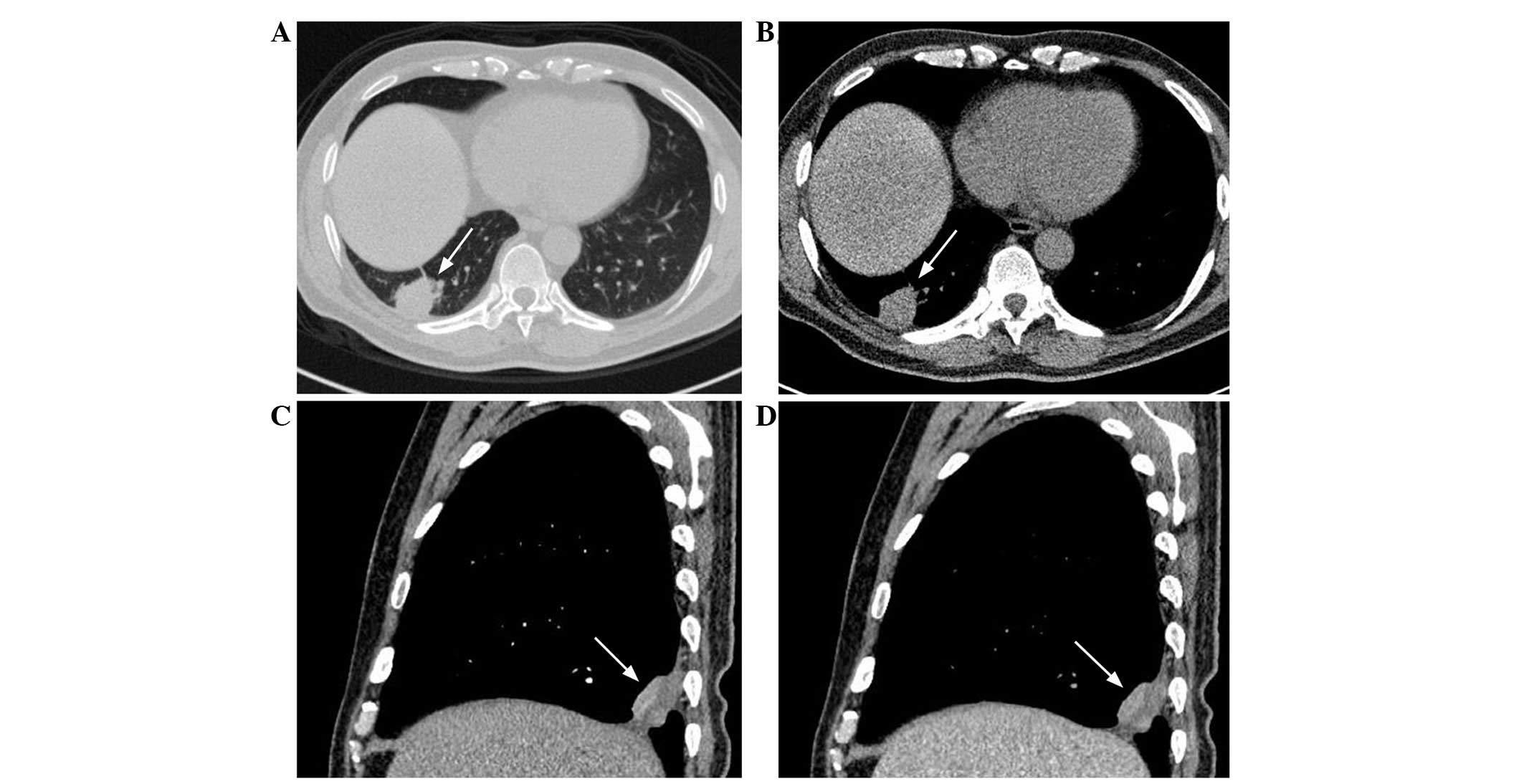
lesions, of which four were located in the upper lobe and six were
located in the lower lobe (Fig.
1A). In total, three lesions were located in the left lung and
seven involved the right lung. A central parenchymal lesion was
only identified in one patient, but the presence of a peripheral
parenchymal lesion was revealed in nine patients, of which six
presented with a sub-pleural mass (Fig.
1B). The maximum diameters of the ten lesions, comprising eight
masses and two nodules, ranged between 5 and 57 mm. The lesions
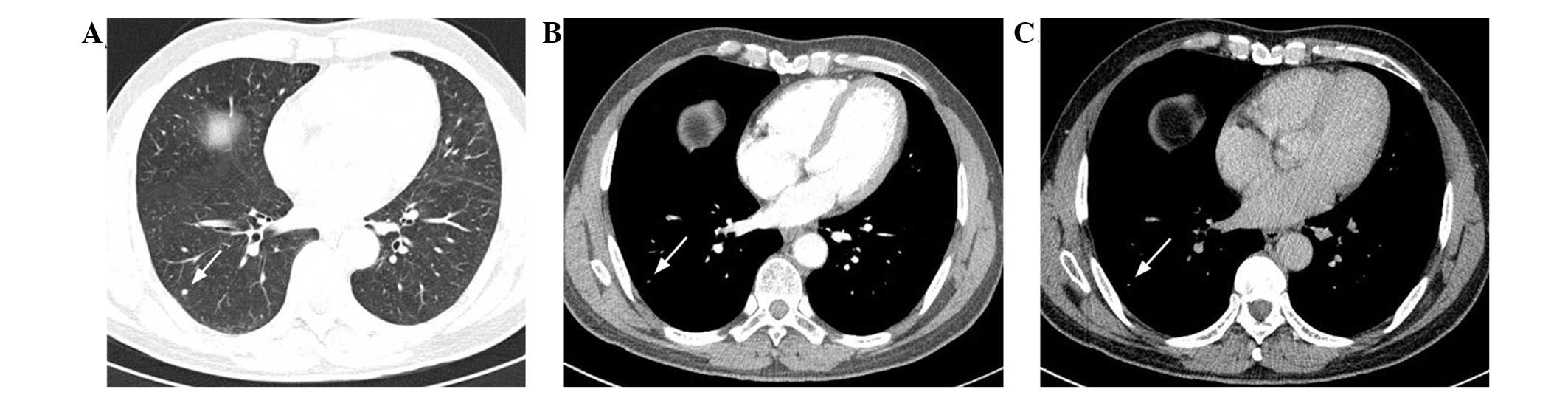
were either well- (n=4; Fig. 2A) or
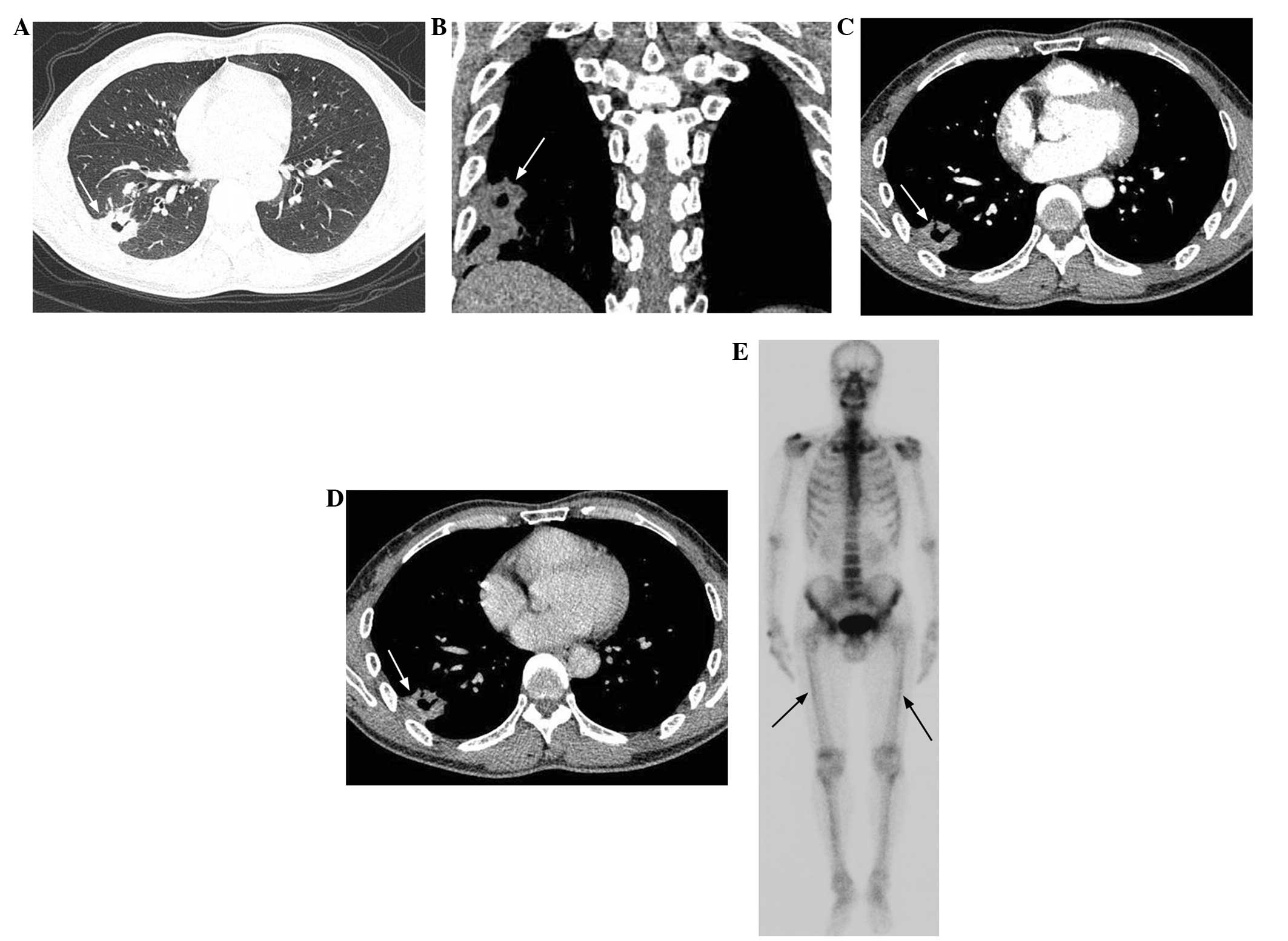
ill-defined (n=6; Fig. 3A) and
round to oval (n=5; Fig. 2A) or
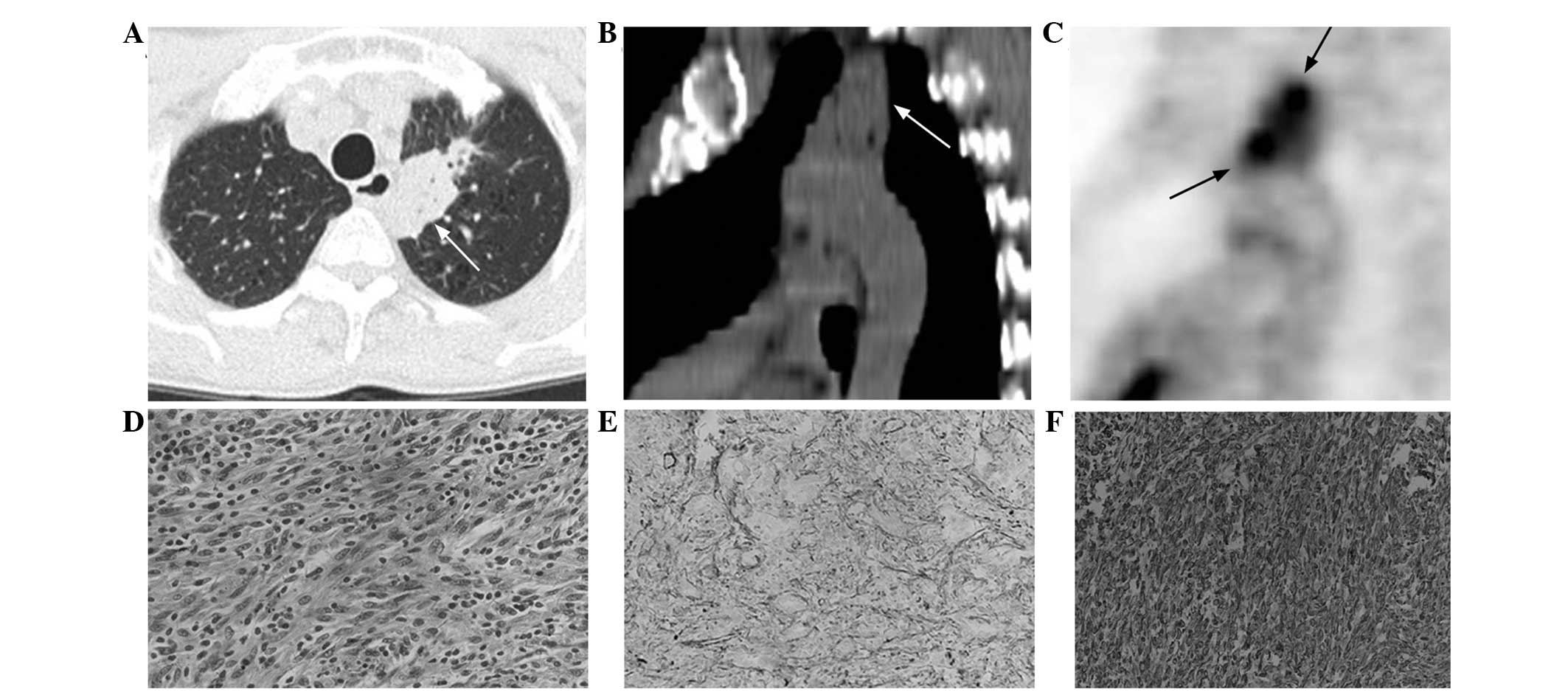
irregular (n=5; Fig. 4A and B) in
shape. The associated CT findings demonstrated calcification (n=3),
necrosis (n=6; Fig. 1C and D),
cavity (n=4; Fig. 3A and B), air
bronchogram (n=6; Fig. 4A and B)
and obstructive pneumonia (n=1). In four of the six lesions with
necrosis, peripheral necrosis was evident within the lesions
(Fig. 1C and D).
 | Table IIComputed tomograghy findings of eight
cases of pulmonary inflammatory myofibroblastic tumor. |
Table II
Computed tomograghy findings of eight
cases of pulmonary inflammatory myofibroblastic tumor.
| Patient | Location | Size, mm | Margin | Shape | AP NCE | VP NCE | Others |
|---|
| 1 | RLL, PP, SP | 44 | Well-defined | Oval | 37.7 | 34.0 | Calcification,
necrosis |
| 2 | LLL, PP | 34 | Ill-defined | Round | 12.0 | 30.4 | Necrosis,
cavity |
| 3 | RLL, PP, SP | 43 | Ill-defined | Irregular | 79.1 | 48.0 | Calcification,
cavity, air bronchogram |
| 4 | RUL, PP, SP | 38 | Ill-defined | Irregular | 44.2 | 57.9 | Cavity, necrosis,
air bronchogram |
| 5 | RLL, PP | 32 | Ill-defined | Irregular | 32.5 | 47.0 | Necrosis, air
bronchogram |
| 6 | RUL, PP | 30 | Ill-defined | Irregular | 25.3 | 47.8 | Cavity, necrosis,
air bronchogram |
| 7 | RLL, PP | 5 | Well-defined | Round | NA | NA | |
| 8 | RUL, CP | 26 | Well-defined | Oval | | | Necrosis,
obstructive pneumonia |
In total, seven patients underwent contrast-enhanced
CT, but one pulmonary lesion was unable to be evaluated by contrast
enhancement due to a small maximum diameter of 5 mm (Fig. 2B and C). The degree of contrast
enhancement was measured in six patients. The results revealed that
lesions increased in attenuation by between 12 and 79.1 Hounsfield
units (HU; mean, 38.5±22.8 HU) in the arterial phase, and between
30.4 and 57.9 HU (mean, 44.2±10.2 HU) in the venous phase (Fig. 3C and D).
18F-FDG PET/CT findings
In total, two patients with PIMT underwent
18F-FDG PET/CT imaging. In one patient, a mass-like high
FDG uptake was identified in the left lower lobe. The homogeneous
radioactive uptake following early and delayed imaging exhibited a
SUVmax of 6.0 and 6.9, respectively. The
SUVmax obtained from the delayed imaging exhibited a 15%
increase compared with the early imaging. A heterogeneous elevated
tracer uptake in the left upper lobe was observed in the other
patient. In total, two nodule-like radioactive foci were identified
within this lesion (Fig. 4C). The
nodules exhibited an early SUVmax of 5.4 and a delayed
SUVmax of 5.9, an increase of 9%. The PET/CT imaging
characteristics are presented in Table III.
 | Table III18F-fluorodeoxyglucose
positron emisission tomography/computed tomography findings of two
cases of pulmonary inflammatory myofibroblastic tumor. |
Table III
18F-fluorodeoxyglucose
positron emisission tomography/computed tomography findings of two
cases of pulmonary inflammatory myofibroblastic tumor.
| Pt. No. | Location | Size, mm | Margin | Shape | Others | UP | EI
SUVmax | DI
SUVmax |
|---|
| 9 | LLL, PP, SP | 52 | Well-defined | Oval | Air
bronchogram | Homogeneous | 6.0 | 6.9 |
| 10 | LUL, PP, SP | 57 | Ill-defined | Irregular | Calcification, air
bronchogram | Heterogeneous | 5.4 | 5.9 |
Bone scan findings
The patients that underwent SPECT were initially
suspected of having lung cancer prior to the surgery, which was
determined by the absence of bone metastases. Of the four patients,
two demonstrated no abnormal whole-body bone tracer uptake.
Overall, one patient demonstrated a mildly increased tracer uptake,
with a bilateral linear distribution along the cortex of the
femurs, which was suggestive of atypical hypertrophic pulmonary
osteoarthropathy (HPO) (Fig. 3E).
The other patient possessed three foci that were located in the
ribs, which were considered to demonstrate non-specific uptake in
combination with the corresponding CT images.
Pathology and immunohistochemistry
Microscopic analysis revealed that the tumors were
composed of bundles of spindle cells, including myofibroblasts and
fibroblasts, arranged in a fascicular or storiform manner, and
surrounded by chronic inflammatory cell infiltration (Fig. 4D). These inflammatory cells were
primarily composed of plasma cells, lymphocytes and granulocytes.
Specimens obtained from the five patients were subjected to
immunohistochemical examination. Positive staining for smooth
muscle actin (Fig. 4E) was
identified in all five cases, vimentin (Fig. 4F) in two cases, and cluster of
differentiation (CD)68, CD34, CD20 and CD3 in one case.
Discussion
PIMT is extremely uncommon. Overall, it is reported
that ~40% of all cases occur in adults >40 years old (15) who are more likely to be affected by
lung cancers. For this reason, a considerable number of PIMTs
occurring in this age group are misdiagnosed as lung cancer prior
to a biopsy or surgical resection (15,16).
In the present study, which included ten patients >40 years old
with PIMT, eight were originally suspected of having lung cancer
prior to a wedge resection or lobectomy. In general, PIMT affects
males and females equally (5,7,16,17).
However, there was a significant male predilection (8/10) in the
present study, which could be attributed to the older age group
included. Patients with PIMT are usually asymptomatic, or present
with non-specific symptoms, including coughing, hemoptysis,
dyspnea, fever and chest pain (16,18,19).
In the present study, two patients (2/10) were asymptomatic, and
one pulmonary lesion was detected incidentally on a routine health
check-up. Although the etiology of PIMT remains unknown, it has
been hypothesized that pulmonary infection may contribute to the
pathogenesis of the disorder, as prior pulmonary disease has only
been reported in 30% of patients (14,16).
The present study of 10 patients included only one patient with a
previous pulmonary disease, but included six patients with a
history of smoking, which indicates that smoking may be a factor
that contributes to PIMT.
According to a study by Kakitsubata et al
(16), no significant differences
were identified between PIMT involving the left or right side.
However, PIMT does exhibit a predilection for the lower lobes. In
the present study, the right (4/10) and left lower lobes (2/10)
were more frequently affected by PIMT compared with the right
(3/10) and left (1/10) upper lobes. In addition, lesions located in
the peripheral parenchyma (9/10) and sub-pleura (6/10) were
observed more often, which indicates that PIMT may also have a
predilection for these lung regions. It has been reported that the
size of PIMT usually ranges between 10 and 150 mm (14,16,20).
With the exception of one case, the size of the lesions in the
present study corresponded with those previously reported. The
exception in the present study had a maximum diameter of 5 mm. To
the best of our knowledge, this is the smallest reported PIMT,
which may aid in understanding the nature of PIMT.
At present, CT is the most widely used method for
the detection and differentiation of pulmonary masses, including
lung cancer, tuberculosis, inflammatory pseudotumors and PIMT
(21). However, due to its rarity,
major studies concerning CT delineations of PIMT are in the form of
case studies. In total, two previous studies have included only ~10
cases (15,16). Although classic PIMT is defined as a
slow-growing, solitary, round to oval-shaped and well-circumscribed
mass in the peripheral regions of the lower lobes (16), CT manifestations of PIMT are
generally diverse. A number of studies have reported that PIMTs
present as ill-defined or irregular lesions (6,16,22–24).
In addition, other CT findings, including calcification, cavity,
necrosis, obstructive atelectasis and pneumonia have also been
reported in several previous studies (8,9,15,16,18,20).
According to the results of Kakitsubata et al (16), the incidence of calcification and
cavities in cases of PIMT range between 4 and 17.5%, and 50 and
57%, respectively. The results of the present study are partly in
agreement with those of previous studies. In total, four cases
included in the present study were consistent with classic PIMT,
and the other six cases existed as ill-defined or irregular masses.
The presence of calcification and cavities was 30 (3/10) and 40%
(4/10), respectively. However, necrosis and air bronchogram (60%;
6/10) were relatively frequent, which are factors rarely mentioned
in previous studies.
The majority of PIMT cases reported in previous
studies have demonstrated homogeneous or heterogeneous CT contrast
enhancement (15,16,19,21).
In addition, the degree of enhancement has varied between them.
Calabrese et al (25)
reported two cases of PIMT with mild increases in density following
enhancement. Furthermore, Kim et al (15) assessed the degree of contrast
enhancement in seven lesions and identified an increase in
attenuation by 13–89 HU following contrast administration. Chen
et al (19) described a
patient with PIMT that demonstrated weak enhancement in the
arterial phase. According to the study by Takayama et al
(12), delayed enhancement was
evident in two cases of PIMT. In this study, early-phase images
with slight enhancement and delayed-phase images with heterogeneous
enhancement were obtained 70 and 300 sec after the injection of the
contrast medium, respectively. The presence of delayed enhancement
has also been confirmed in certain studies reporting IMT occurring
at other sites, including the heart, liver, kidney and the greater
omentum (26–30). However, there have been certain
cases of PIMT occurring in the absence of contrast enhancement, as
reported by Kakitsubata et al (16) and Dhouib et al (14). The present study evaluated the
degree of contrast enhancement in six patients with PIMT, which
included the arterial and venous phases at 20 and 50 sec after the
injection of the contrast agent, respectively. All lesions
demonstrated moderate to high contrast enhancement in each phase,
and four exhibited delayed enhancement.
To date, there have been a number of studies
concerning 18F-FDG uptake in PIMT (5,6,9,14,19,25,31,32).
These studies all demonstrated increased 18F-FDG uptake,
with the SUV values ranging between 2.8 and 25. The present study
included two cases of PIMT that were analyzed by 18F-FDG
PET/CT, and exhibited elevated 18F-FDG uptake with SUV
values above the normal range. Although 18F-FDG is known
to accumulate in a number of malignancies, including lung cancer,
it has also been observed to actively concentrate in certain benign
pulmonary diseases, such as pneumonia, Wegener’s granulomatosis,
tuberculosis, fungal infections and abscesses (32). Overall, two cases in the present
study were not identified as having lung cancer following PET/CT,
which confirmed the presence of pulmonary unifocal lesions without
metastatic disease. Furthermore, the two PIMT lesions underwent
delayed PET/CT imaging, which had not been performed in previous
case studies. In the two cases, the SUVmax of the
delayed imaging were higher than those of the initial imaging. A
number of previous studies have revealed that dual time-point
18F-FDG PET or PET/CT imaging may aid in distinguishing
malignant from benign processes (33–37).
Malignant diseases, including hepatocellular carcinoma, pancreatic
cancer, lung cancer and malignant lymphoma have demonstrated
increased 18F-FDG accumulation on delayed images
compared with early images. However, there have been other studies
that have identified a significant overlap in 18F-FDG
uptake patterns between benign and malignant lesions, particularly
for pulmonary lesions, and even on delayed time-point images
(38–42). As revealed by the present study, an
increased delayed imaging SUVmax of the lung cannot
guarantee the presence of malignancy. These 18F-FDG
PET/CT findings may aid in the diagnosis of PIMT, but further
studies that include larger patient populations are required in
order to expand these results.
The bone scan features of PIMT have not been
depicted in previous studies. The present study included four
patients who underwent SPECT for the detection of bone metastases.
Although there were no definitive bone metastases detected, SPECT
revealed atypical HPO in one PIMT case. HPO can occur secondary to
various neoplastic and non-neoplastic diseases, including primary
lung cancer, metastatic pulmonary disease and cystic fibrosis
(43). The ‘tram-line’ or
‘double-stripe’ sign, which represents abnormal periosteal bone
formation, is the classic appearance of HPO upon bone scanning
(43,44). With the exception of PIMT, CT did
not identify any other pulmonary diseases in the patient with HPO
in the present study. Based upon the chest CT findings, it was
hypothesized that the HPO detected by SPECT was caused by the PIMT.
To the best of our knowledge, this is the first study to
demonstrate that PIMT can lead to HPO.
PIMT occurring in patients >40 years old is
extremely rare and the symptoms often mimic those of lung cancer
(13,19). Certain imaging features are
relatively common in PIMT patients of this age group, such as being
located in the lower lobe and peripheral parenchyma, necrosis, air
bronchogram, moderate to high contrast enhancement or delayed
enhancement, increased 18F-FDG uptake with an elevated
SUVmax upon delayed imaging, and the absence of
definitive metastases. Although these imaging features remain
non-specific for the distinction between PIMT and lung cancer, they
may aid in enhancing the awareness of PIMT during the differential
diagnosis of lung masses. The combination of imaging modalities,
including CT, 18F-FDG PET/CT and SPECT bone scans, may
aid in successfully diagnosing PIMT, determining the extent of the
tumor and also managing the treatment.
References
|
1
|
Verbeke JI, Verberne AA, Den Hollander JC
and Robben SG: Inflammatory myofibroblastic tumour of the lung
manifesting as progressive atelectasis. Pediatr Radiol. 29:816–819.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swain RS, Tihan T, Horvai AE, et al:
Inflammatory myofibroblastic tumor of the central nervous system
and its relationship to inflammatory pseudotumor. Hum Pathol.
39:410–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen ST and Lee JC: An inflammatory
myofibroblastic tumor in liver with ALK and RANBP2 gene
rearrangement: combination of distinct morphologic,
immunohistochemical and genetic features. Hum Pathol. 39:1854–1858.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butrynski JE, D’Adamo DR, Hornick JL, et
al: Crizotinib in ALK-rearranged inflammatory myofibroblastic
tumor. N Engl J Med. 363:1727–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeda S, Onishi Y, Kawamura T and Maeda
H: Clinical spectrum of pulmonary inflammatory myofibroblastic
tumor. Interact Cardiovasc Thorac Surg. 7:629–633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carillo C, Anile M, De Giacomo T and
Venuta F: Bilateral simultaneous inflammatory myofibroblastic tumor
of the lung with distant metastatic spread. Interact Cardiovasc
Thorac Surg. 13:246–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ezzine-Baccari S, Bacha D, Sassi S, et al:
Inflammatory myofibroblastic tumor of the lung: a benign lesion
with aggressive behavior. Gen Thorac Cardiovasc Surg. 60:531–533.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma S, Sankhyan N, Kalra V, et al:
Inflammatory myofibroblastic tumor involving lung and brain in a
10-year-old boy: a case report. J Child Neurol. 24:1302–1306. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van den Heuvel DA, Keijsers RG, van Es HW,
et al: Invasive inflammatory myofibroblastic tumor of the lung. J
Thorac Oncol. 4:923–926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okiror L, Draaisma WA, Chinake C and
Harrison-Phipps K: Large pulmonary inflammatory myofibroblastic
tumour requiring extrapleural pneumonectomy and diaphragm
resection. Gen Thorac Cardiovasc Surg. 61:163–165. 2013. View Article : Google Scholar
|
|
11
|
Kim SJ, Kim WS, Cheon JE, et al:
Inflammatory myofibroblastic tumors of the abdomen as mimickers of
malignancy: imaging features in nine children. AJR Am J Roentgenol.
193:1419–1424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takayama Y, Yabuuchi H, Matsuo Y, et al:
Computed tomographic and magnetic resonance features of
inflammatory myofibroblastic tumor of the lung in children. Radiat
Med. 26:613–617. 2008. View Article : Google Scholar
|
|
13
|
Sakurai H, Hasegawa T, Watanabe S, et al:
Inflammatory myofibroblastic tumor of the lung. Eur J Cardiothorac
Surg. 25:155–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhouib A, Barrazzone C, Reverdin A, et al:
Inflammatory myofibroblastic tumor of the lung: a rare cause of
atelectasis in children. Pediatr Radiol. 43:381–384. 2013.
View Article : Google Scholar
|
|
15
|
Kim TS, Han J, Kim GY, et al: Pulmonary
inflammatory pseudotumor (inflammatory myofibroblastic tumor): CT
features with pathologic correlation. J Comput Assist Tomogr.
29:633–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kakitsubata Y, Theodorou SJ, Theodorou DJ,
et al: Myofibroblastic inflammatory tumor of the lung: CT findings
with pathologic correlation. Comput Med Imaging Graph. 31:607–613.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ochs K, Hoksch B, Frey U and Schmid RA:
Inflammatory myofibroblastic tumour of the lung in a five-year-old
girl. Interact Cardiovasc Thorac Surg. 10:805–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hammas N, Chbani L, Rami M, et al: A rare
tumor of the lung: inflammatory myofibroblastic tumor. Diagn
Pathol. 7:832012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CK, Jan CI, Tsai JS, et al:
Inflammatory myofibroblastic tumor of the lung-a case report. J
Cardiothorac Surg. 5:552010. View Article : Google Scholar
|
|
20
|
Cassivi SD and Wylam ME: Pulmonary
inflammatory myofibroblastic tumor associated with histoplasmosis.
Interact Cardiovasc Thorac Surg. 5:514–516. 2006. View Article : Google Scholar
|
|
21
|
Naidich DP, Bankier AA, MacMahon H, et al:
Recommendations for the management of subsolid pulmonary nodules
detected at CT: a statement from the Fleischner Society. Radiology.
266:304–317. 2013. View Article : Google Scholar
|
|
22
|
Lee MH, Lee HB, Lee YC, et al: Bilateral
multiple inflammatory myofibroblastic tumors of the lung
successfully treated with corticosteroids. Lung. 189:433–435. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaeffer CJ, Minai OA, Sharma N, et al:
Inflammatory myofibroblastic tumor of the lung: recurrence after
steroid treatment. J Thorac Imaging. 23:191–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura H, Kawasaki N, Taguchi M, et al:
Pulmonary inflammatory myofibroblastic tumor resected by
video-assisted thoracoscopic surgery: Report of a case. Surg Today.
37:137–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calabrese F, Zuin A, Brambilla E, et al:
Pulmonary inflammatory myofibroblastic tumour with unusual
octreoscan uptake: two reports. Eur Respir J. 35:448–450. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen L, Sun QR, Diao XW, et al: Renal
inflammatory myofibroblastic tumour with multiple calcifications.
Clin Radiol. 67:188–191. 2012. View Article : Google Scholar
|
|
27
|
Aptel S, Gervaise A, Fairise A, et al:
Abdominal inflammatory myofibroblastic tumour. Diagn Interv
Imaging. 93:410–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoey ET, Ganesh V, Gopalan D and Screaton
NJ: Cardiac inflammatory myofibroblastic tumor: evaluation with
dual-source CT. J Cardiovasc Comput Tomogr. 3:114–116. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
See TC, Davies SE, Appleton DS and Ng CS:
CT and angiographic features of hepatic inflammatory
myofibroblastic tumour. Clin Radiol. 60:718–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu JS, Park C, Kim JH, et al: Inflammatory
myofibroblastic tumors in the liver: MRI of two
immunohistochemically-verified cases. J Magn Reson Imaging.
26:418–421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okiror L, Draaisma WA, Chinake C, et al:
Large pulmonary inflammatory myofibroblastic tumour requiring
extrapleural pneumonectomy and diaphragm resection. Gen Thorac
Cardiovasc Surg. 61:163–165. 2013. View Article : Google Scholar
|
|
32
|
Howman-Giles R, London K, McCowage G, et
al: Pulmonary inflammatory myofibroblastic tumor after Hodgkin’s
lymphoma and application of PET imaging. Pediatr Surg Int.
24:947–951. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiu Y, Bhutani C, Dhurairaj T, et al:
Dual-time point FDG PET imaging in the evaluation of pulmonary
nodules with minimally increased metabolic activity. Clin Nucl Med.
32:101–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schillaci O, Travascio L, Bolacchi F, et
al: Accuracy of early and delayed FDG PET-CT and of
contrast-enhanced CT in the evaluation of lung nodules: a
preliminary study on 30 patients. Radiol Med. 114:890–906. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shinya T, Fujii S, Asakura S, et al:
Dual-time-point F-18 FDG PET/CT for evaluation in patients with
malignant lymphoma. Ann Nucl Med. 26:616–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishiyama Y, Yamamoto Y, Monden T, et al:
Evaluation of delayed additional FDG PET imaging in patients with
pancreatic tumour. Nucl Med Commun. 26:895–901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin WY, Tsai SC and Hung GU: Value of
delayed 18F-FDG-PET imaging in the detection of hepatocellular
carcinoma. Nucl Med Commun. 26:315–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen CJ, Lee BF, Yao WJ, et al: Dual-phase
18F-FDG PET in the diagnosis of pulmonary nodules with an initial
standard uptake value less than 2.5. AJR Am J Roentgenol.
191:475–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Laffon E, de Clermont H, Begueret H, et
al: Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary
lesions. Nucl Med Commun. 30:455–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Umeda Y, Demura Y, Morikawa M, et al:
Prognostic value of dual-time-point 18F-fluorodeoxyglucose positron
emission tomography in patients with pulmonary sarcoidosis.
Respirology. 16:713–720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng Z, Pan Y, Guo F, et al:
Multimodality FDG PET/CT appearance of pulmonary tuberculoma
mimicking lung cancer and pathologic correlation in a
tuberculosis-endemic country. South Med J. 104:440–445. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng G, Torigian DA, Zhuang H, et al:
When should we recommend use of dual time-point and delayed
time-point imaging techniques in FDG PET? Eur J Nucl Med Mol
Imaging. 40:779–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Narla VV, Rajagopalan MS, Kanderi T and
Muthukrishnan A: Atypical presentation of hypertrophic pulmonary
osteoarthropathy on Tc-99 m MDP bone scintigraphy. Clin Nucl Med.
33:702–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Russo RR, Lee A, Mansberg R and Emmett L:
Hypertrophic pulmonary osteoarthropathy demonstrated on SPECT/CT.
Clin Nucl Med. 34:628–631. 2009. View Article : Google Scholar : PubMed/NCBI
|