Introduction
Epithelial ovarian cancer is one of the most common
causes of mortality among females (1). The high mortality rate of ovarian cancer
patients (9.30 out of every 100,000 patients each year) is a
consequence of late-stage diagnosis, and the five-year survival
rate (<50% for patients >64 years) for the advanced stages is
extremely poor in the USA, Europe and Japan (2). A large tumor burden and extensive
metastatic lesions of the abdominal cavity also contribute to the
poor prognosis and the high rate of mortality of this disease
(3). Tumor cell migration/invasion is
a complex process involving cytoskeletal reorganization and
membrane ruffling. The suppression of cytoskeletal reorganization
and the redistribution of actin fibers may lead to the formation of
non-adhesive membrane protrusions and therefore, dysregulated
cellular adhesion capacity; this has been hypothesized to improve
the survival of patients with ovarian cancer (4).
The actin cytoskeleton is essential for cell
motility and cell invasion (5,6).
Serine/threonine p21-activated kinases (PAKs) are effector proteins
for the Rho GTPases Cdc42 and Rac, which are important for cell
morphology and cytoskeletal reorganization (7,8). PAK4 was
initially identified due to its regulation of cytoskeletal
reorganization (9,10). Subsequent studies indicated that PAK4
is a key integrator of cell migration, invasion and apoptosis
(11,12). Furthermore, PAK4 is upregulated in the
majority of cancer cell lines, while previous studies have revealed
that PAK4 is strongly linked to the progression of ovarian tumors
and breast cancer. Additionally, overexpression of PAK4 in mammary
epithelial cells leads to tumorigenesis in mice. Therefore, this
protein may be a valuable molecular prognostic marker and
therapeutic target in a number of cancers (13–16).
microRNAs (miRNA/miR), are non-coding RNAs of ~22
nucleotides, and are involved in various cellular processes,
including proliferation, differentiation, apoptosis and invasion
(17–19). miR-126 originates from a common
precursor structure located within the EGFL7 gene, and its
expression levels have been reported to vary in a number of human
cancers; patients with low miR-126 expression exhibit poor survival
compared with patients with high miR-126 levels (20–23). It
has been proposed that miR-126 is essential in the inhibition of
the invasive growth of cancer cells. Thus, the current study
investigated whether the up- or downregulation of miR-126 modulates
PAK4 expression in human ovarian cancer cells.
Materials and methods
Cell culture
SKOV3 cells (American Type Culture Collection,
Rockville, MD, USA) were used as the ovarian cancer cells in the
present study. The cells were maintained and propagated in
vitro by serial passage in Dulbecco's modified Eagle's medium
(DMEM; Gibco, Life Technologies Corporation, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco, Life
Technologies Corporation), 100 IU/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C. All procedures were performed according to the
internationally accepted ethical guidelines and approved by the
Institutional Review Board of the Second Affiliated Hospital,
School of Medicine, Zhejiang University (Hangzhou, China).
Plasmid construction, lentivirus
packaging and cell infection
pGLV3/H1/green fluorescent protein (GFP)+Puro
(pGLV3; Shanghai GenePhama Co., Ltd., Shanghai, China), a
lentiviral vector, was used to construct the pGLV3-miR-126 plasmid.
The miR-126 mimic, miR-126 inhibitor and negative control (NC)
oligonucleotides were chemosynthesized by Shanghai GenePhama Co.,
Ltd. The oligonucleotide sequences were as follows: miR-126,
5′-TCGTACCGTGAGTAATAATGCG-3′; hsa-miR-126 inhibitor,
5′-CGCATTATTACTCACGGTACGA-3′; and microRNA NC,
5′-TTCTCCGAACGTGTCACGT-3′. The miR-126 small hairpin (sh)DNA double
chain template sequence was synthesized artificially, and inserted
into the pGLV3-miRNA lentivirus plasmid. The miR-126 mimic sequence
was constructed as follows: (Forward) hsa-miR-126-BamHI,
GATCCGTCGTACCGTGAGTAATAATGCGTTCAAGAGACGCATTATTACTCACGGTACGACTTTTTTG;
(reverse) hsa-miR-126-EcoRI,
AATTCAAAAAAGTCGTACCGTGAGTAATAATGCGTCTCTTGAACGCATTATTACTCACGGTACGACG.
The miRNA-126 inhibitor sequence was constructed as follows:
(Forward) hsa-miR-126-BamHI,
GATCCGAGCATGGCACTCATTATTACGCTTCAAGAGAGCGTAATAATGAGTGCCATGCTCTTTTTTG;
(reverse) hsa-miR-126-EcoRI,
AATTCAAAAAAGAGCATGGCACTCATTATTACGCTCTCTTGAAGCGTAATAATGAGTGCCATGCTCG.
pGLV3-shDNA-NC was used as a negative control, with the following
sequence: (Forward) NC-BamHI,
GATCCGTCGTACCGTGAGTAATAATGCGTTCAAGAGACGCATTATTACTCACGGTACGACTTTTTTG;
(reverse) shNC-EcoRI,
AATTCAAAAAAGTCGTACCGTGAGTAATAATGCGTCTCTTGAACGCATTATTACTCACGGTACGACG.
The 293T producer cell line (Cell Bank of Chinese
Academy of Science, Beijing, China) was maintained in DMEM, with
10% FBS, 4.0 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin. One day prior to transfection, the cells were seeded
into a 15-cm dish. pGLV3-miR-126 or pGLV3 vectors and packing
plasmids, including pGag/Pol, pRev and pVSV-G (Shanghai GenePhama
Co., Ltd.) were co-transfected using RNAi-mate (Shanghai GenePhama
Co., Ltd.), according to the manufacturer's instruction. At 72 h
post-transfection, the supernatant was harvested, cleared by
centrifugation (2,200 × g at 4°C for 4 min), passed through a
0.45-µm syringe filter, and cleared by centrifugation again (20,000
rpm at 4°C for 2 h). The titer of the virus was measured according
to the expression level of GFP, following the manufacturer's
instructions. The packaged lentiviruses were designated
LV3-hsa-miR-126, LV3-hsa-miR-126 inhibitor and LV3-NC. The
sequences of the resulting vectors were verified by sequence
analysis.
The SKOV3 cells were infected with LV3-hsa-miR-126,
LV3-has-miR-126 inhibitor or LV3-NC, at a multiplicity of infection
ratio of 15, in the presence of 5 µg/ml polybrene (Shanghai
GenePhama Co., Ltd.); the infection efficiency was 80–90%, as
assessed by microscopic analysis of GFP fluorescence.
Immunofluorescence staining and
western blot analysis
At 48 h post-transfection, the cells were fixed in
4% paraformaldehyde, washed three times with phosphate-buffered
saline (PBS), and incubated for 5 min at −20°C in 95% ethanol
(vol/vol in PBS). The cells were subsequently washed three times
with PBS, blocked for 1 h in 5% normal goat serum in PBS with 0.1X
Triton X-100, and incubated overnight with polyclonal rabbit
anti-human PAK4 antibodies (Abcam, Cambridge, MA, USA; dilution,
1:200) at 4°C. The following day, the cells were washed three times
with PBS and incubated for 40 min at 37°C with the corresponding
secondary antibody [polyclonal goat anti-rabbit immunoglobulin
(Ig)G (H+L)-tetramethylrhodamine (TRITC); dilution 1:200;
SouthernBiotech, Birmingham, AL, USA], then washed and mounted.
Immunostained SKOV3 cultures were examined under a laser scanning
confocal microscope (LSM 510 Meta; Carl Zeiss Microscopy GmbH,
Jena, Germany) for detection of the TRITC-fluorophore. Each group
was photographed at x400 magnification with the aid of a digital
camera attached to the microscope, and the expression of PAK4 was
assessed by calculating the percentage of positive cells and the
optical density, subsequent to defining a threshold for background
correction.
For the western blot analysis, proteins were
extracted from the SKOV3 cells, solubilized in
radioimmunoprecipitation assay buffer, separated on 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (Wuhan Boster
Ltd., Wuhan, China) and electro-transferred onto polyvinylidene
difluoride membranes (Invitrogen Life Technologies, Carlsbad, CA,
USA). The membranes were blocked in 5% skimmed milk powder prepared
in Tris-buffered saline with Triton X-100 (TBS-T) for 30 min. For
PAK4 detection, the membranes were incubated at 4°C overnight with
anti-PAK4 antibodies (Abcam; dilution 1:500). The membranes were
washed three times for 10 min in TBS-T and incubated with a 1:5,000
dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG
for 2 h. Finally, the membranes were washed six times for 20 min
each in TBS-T, prior to development with a standard enhanced
chemiluminescence kit (KeyGEN Biotech, Nanjing, China). The
densitometric analysis of the PAK4 and β-actin bands was assayed by
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Statistical analysis was performed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA)
for Windows. The significance of any differences between groups was
evaluated using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
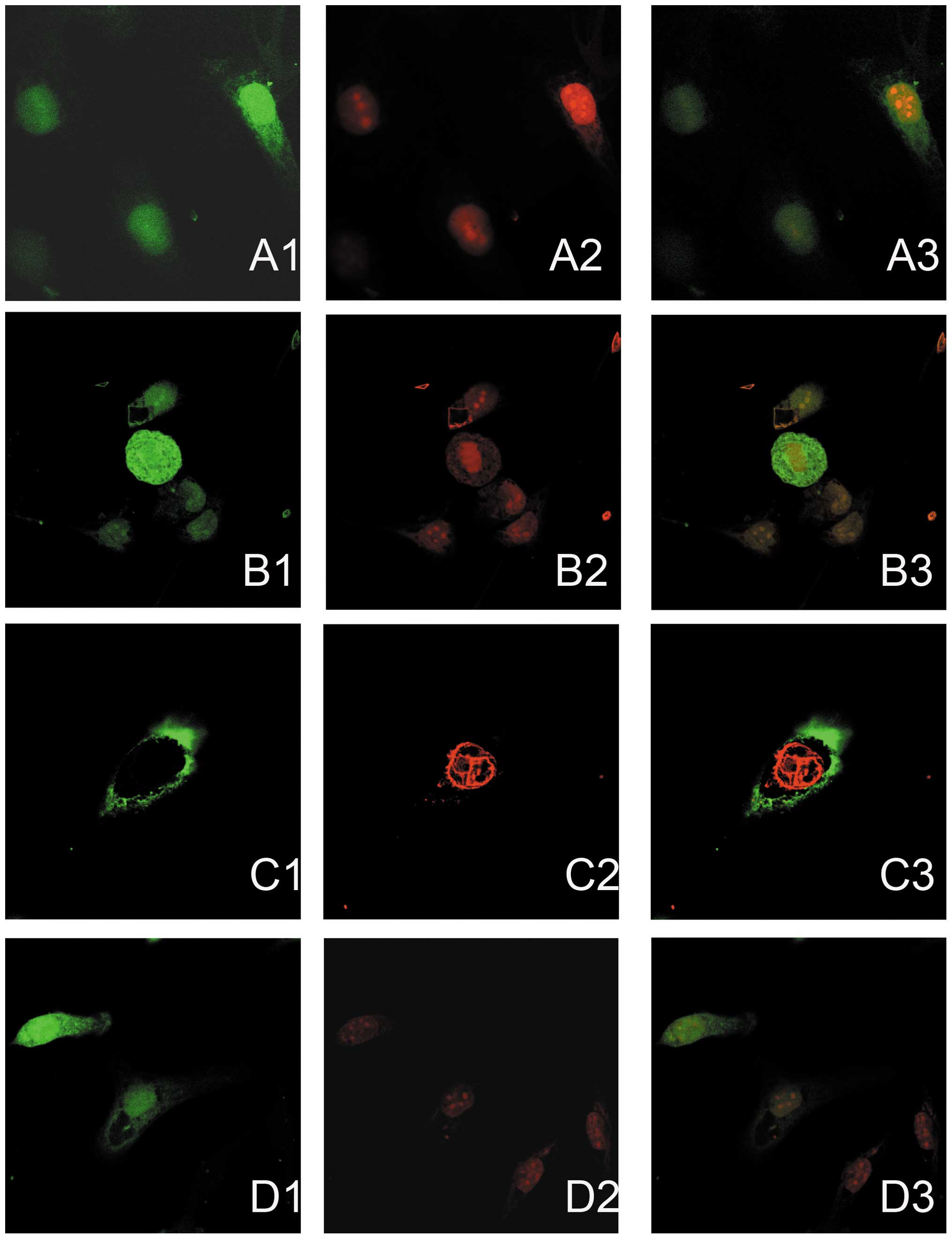
Immunofluorescence double staining and
semi-quantitative confocal laser scanning analysis detected the
expression of the miRNA vectors and PAK4 in the following four
groups of SKOV3 cells: Untransfected cells, LV3-NC-transfected
cells, LV3-hsa-miR-126-transfected cells and LV3-hsa-miR-126
inhibitor-transfected cells. The expression of PAK4 was indicated
by red immunofluorescence staining, and the GFP expressed by the
miRNA vectors (green fluorescence) highlighted successfully
transfected cells; green fluorescence was detected in all of the
nuclei, but only in certain cytoplasmic regions of the SKOV3 cells
in the NC, miR-126 inhibitor and miR-126 mimic groups. The mean
immunofluorescence intensity of PAK4 in the miR-126 inhibitor group
was significantly higher (Fig. 1, C2)
compared with that of the untransfected SKOV3 cells (Fig. 1, A2). Furthermore, the expression
level of PAK4 was effectively decreased by the overexpression of
miR-126 in the LV3-hsa-miR-126-transfected cells (Fig. 1, D2) compared with that of the
untransfected SKOV3 cells (Fig. 1,
A2), and particularly compared with that of LV3-hsa-miR-126
inhibitor-transfected cells (Fig. 1,
C2). Furthermore, as shown in Fig. 1
C3, the cells transfected with LV3-hsa-miR-126 inhibitor
(green) exhibited greater expression of PAK4 (red), whilst cells
transfected with LV3-hsa-miR-126 (green) exhibited reduced
expression of PAK4 (red) (Fig. 1
D3).
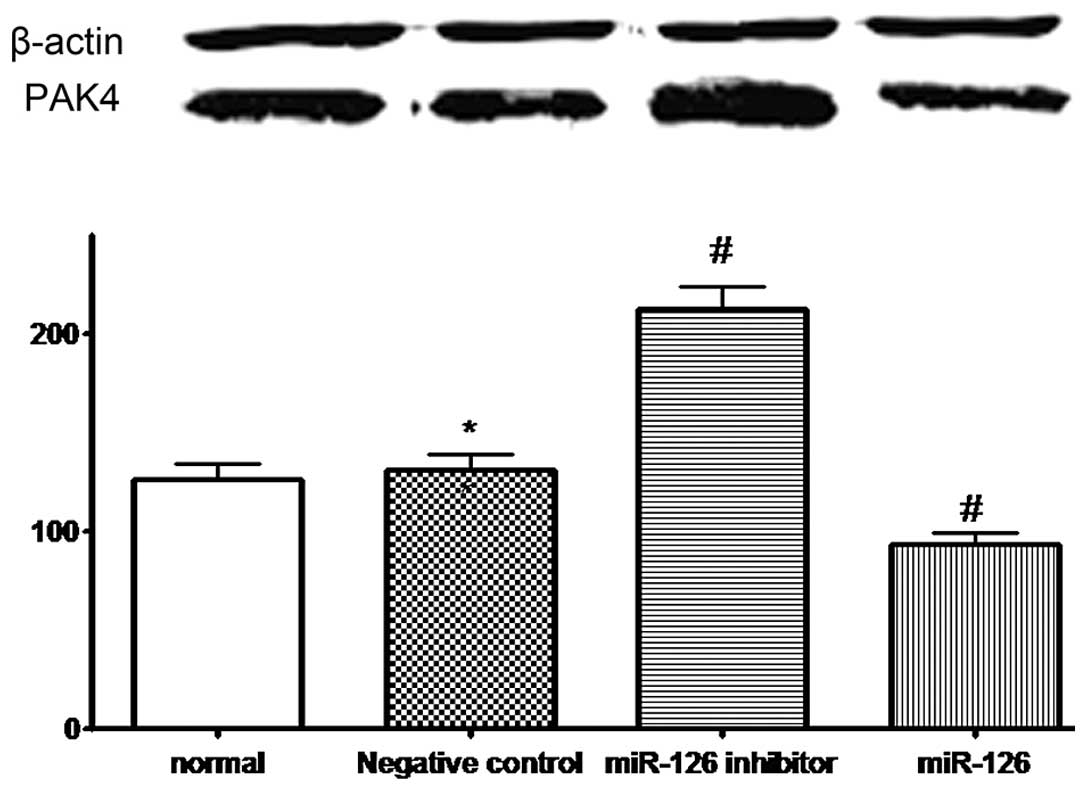
PAK4 protein expression in the four groups of cells
was also evaluated by western blotting. PAK4 was visible as bands
of ~64 kDa. A densitometric analysis of the PAK4/β-actin bands
revealed a significant increase in PAK4 expression in the SKOV3
cells transfected with LV3-hsa-miR-126 inhibitor (mean ± SD,
215.1±10.5 vs. 128.6±8.2%; P=0.001) and a decrease in PAK4
expression in the SKOV3 cells transfected with LV3-hsa-miR-126
(mean ± SD, 91.6±7.7 vs. 128.6±8.2%; P=0.002), compared with the
untransfected SKOV3 cells (Fig. 2).
No significant difference was observed between the expression in
the SKOV3 cells in the NC group and those that were untransfected
(mean ± SD, 130.9±9.1 vs. 128.6±8.2%; P=0.706; Fig. 2). Therefore, it is proposed that
LV3-has-miR-126 inhibitor increases the expression of PAK4, whereas
LV3-hsa-miR-126 attenuates this expression.
Discussion
In the present study, changes in PAK4 expression
were demonstrated in ovarian cancer cells with up- or downregulated
miR-126 (induced by the transfection of LV-miR-126 or
LV-has-miR-126 inhibitor) when compared with normal ovarian cancer
cells. The SKOV3 cells transfected with LV-hsa-miR-126 exhibited
reduced expression of PAK4, while the cells transfected with
LV-hsa-miR-126 inhibitor exhibited increased expression. These
findings suggest that miR-126 is a potential tumor suppressor, with
the ability to decrease the level of PAK4 in ovarian cancer SKOV3
cells.
The invasive ability of malignant cancer cells
depends upon the altered regulation of cell migration by the
membrane protrusion formation in response to chemotactic and
migratory stimuli (6). Membrane
protrusions are formed by polymerization of submembrane actin
filaments. The PAK family comprises important signaling proteins
that are indicated to be involved in a variety of cellular
functions, including cell proliferation, migration and cytoskeletal
organization (7,24). The family consists of six members,
categorized into two groups: Group A, PAKs 1, 2 and 3; and group B,
PAKs 4, 5 and 6 (7,25). PAK4 has been indicated to be involved
in several types of cancer, and strong links have been observed
between PAK4 and ovarian cancer (26). Analysis of cell migration and invasion
in in vitro and in vivo studies has highlighted the
contribution of PAK4 to the progression and metastasis of ovarian
cancer; this is consistent with the role of PAK4 in the
reorganization of the cytoskeleton and the migration of cells,
which is at least in part executed in the cytoplasm (26). PAK4 expression and activation are
important in cancer progression, and increased PAK4 expression has
been shown to be associated with metastasis, progression to late
stages of the disease, reduced patient survival and increased
resistance to chemotherapy (13,14,27). The
mechanisms by which PAK4 affects ovarian cancer cell progression
include the control of cell migration, invasion and proliferation.
PAK4 may act via the regulation of c-Src, mitogen-activated protein
kinase kinase/extracellular signal-regulated kinases 1/2, matrix
metalloproteinase-2, and c-Src/epidermal growth factor receptor.
Inhibition of PAK4 may therefore be a potentially valuable
therapeutic target (16,28).
miR-126 is a non-coding RNA that is involved in
various cellular processes, including proliferation,
differentiation, apoptosis and invasion (17,21,29).
miRNAs that are upregulated in cancer may function as oncogenes
through the negative regulation of tumor suppressor genes, whilst
miRNAs that are downregulated may function as tumor suppressor
genes and inhibit cancer by regulating oncogenes (30,31).
miR-126 acts as a metastatic suppressor in a number of human
cancers (21,23). However, the expression and function of
miR-126 in ovarian cancer remains unclear. In the present study,
the association between miR-126 and PAK4 was investigated in
ovarian cancer cells. The results demonstrated that transfection
with LV3-miR-126 may efficiently reduce the expression of PAK4 in
SKOV3 cells. Furthermore, the LV-miR-126 inhibitor was observed to
upregulate the expression of PAK4 in these cells.
In conclusion, as PAK4 is essential for ovarian
cancer cell invasion, the present study provides an experimental
foundation for the use of miR-126 as a potential tumor suppressor;
this miRNA may potentially be used to decrease expression levels of
PAK4, leading to the inhibition of ovarian cancer cell invasion.
However, further studies are required to elucidate the mechanisms
involved in the suppression of PAK4 by miR-126.
Acknowledgements
The authors would like to thank Dr Li Yu and Dr
Hongya Wang for their excellent assistance. This study was
supported by the National Natural Science Foundation of China
(grant no. 81371881) and the Science and Technology Department of
Zhejiang Province, China (grant no. 2011C23093).
References
|
1
|
Buys SS, Partridge E, Black A, et al: PLCO
Project Team: Effect of screening on ovarian cancer mortality: The
Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening
Randomized Controlled Trial. JAMA. 305:2295–2303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuda A and Katanoda K: Five-year
relative survival rate of ovarian cancer in the USA, Europe and
Japan. Jpn J Clin Oncol. 44:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malek JA, Mery E, Mahmoud YA, et al: Copy
number variation analysis of matched ovarian primary tumors and
peritoneal metastasis. PLoS ONE. 6:e285612011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brandhagen BN, Tieszen CR, Ulmer TM, Tracy
MS, Goyeneche AA and Telleria CM: Cytostasis and morphological
changes induced by mifepristone in human metastatic cancer cells
involve cytoskeletal filamentous actin reorganization and
impairment of cell adhesion dynamics. BMC Cancer. 13:352013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Nandelstadh P, Gucciardo E, Lohi J, Li
R, Sugiyama N, Carpen O and Lehti K: Actin-associated protein
palladin promotes tumor cell invasion by linking extracellular
matrix degradation to cell cytoskeleton. Mol Biol Cell.
25:2556–2570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rane CK and Minden A: P21 activated
kinases: Structure, regulation, and functions. Small GTPases.
5:52014. View Article : Google Scholar
|
|
8
|
Zhu J, Attias O, Aoudjit L, Jiang R,
Kawachi H and Takano T: p21-activated kinases regulate actin
remodeling in glomerular podocytes. Am J Physiol Renal Physiol.
298:F951–F961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abo A, Qu J, Cammarano MS, Dan C, Fritsch
A, Baud V, Belisle B and Minden A: PAK4, a novel effector for
Cdc42Hs, is implicated in the reorganization of the actin
cytoskeleton and in the formation of filopodia. EMBO J.
17:6527–6540. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bompard G, Rabeharivelo G, Cau J, Abrieu
A, Delsert C and Morin N: P21-activated kinase 4 (PAK4) is required
for metaphase spindle positioning and anchoring. Oncogene.
32:910–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paliouras GN, Naujokas MA and Park M:
PAK4, a novel Gab1 binding partner, modulates cell migration and
invasion by the Met receptor. Mol Cell Biol. 29:3018–3032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dart AE and Wells CM: P21-activated kinase
4 - not just one of the PAK. Eur J Cell Biol. 92:129–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wells CM, Whale AD, Parsons M, Masters JR
and Jones GE: PAK4: A pluripotent kinase that regulates prostate
cancer cell adhesion. J Cell Sci. 123:1663–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minden A: The pak4 protein kinase in
breast cancer. ISRN Oncol. 2012:6942012012.PubMed/NCBI
|
|
15
|
Guo Q, Su N, Zhang J, Li X, Miao Z, Wang
G, Cheng M, Xu H, Cao L and Li F: PAK4 kinase-mediated SCG10
phosphorylation involved in gastric cancer metastasis. Oncogene.
33:3277–3287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siu MK, Chan HY, Kong DS, et al:
p21-activated kinase 4 regulates ovarian cancer cell proliferation,
migration, and invasion and contributes to poor prognosis in
patients. Proc Natl Acad Sci USA. 107:18622–18627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tokarz P and Blasiak J: The role of
microRNA in metastatic colorectal cancer and its significance in
cancer prognosis and treatment. Acta Biochim Pol. 59:467–474.
2012.PubMed/NCBI
|
|
18
|
Zaman MS, Maher DM, Khan S, Jaggi M and
Chauhan SC: Current status and implications of microRNAs in ovarian
cancer diagnosis and therapy. J Ovarian Res. 5:442012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo J, Zhou J, Cheng Q, Zhou C and Ding Z:
Role of microRNA-133a in epithelial ovarian cancer pathogenesis and
progression. Oncol Lett. 7:1043–1048. 2014.PubMed/NCBI
|
|
20
|
Frampton AE, Krell J, Jacob J, Stebbing J,
Castellano L and Jiao LR: Loss of miR-126 is crucial to pancreatic
cancer progression. Expert Rev Anticancer Ther. 12:881–884. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: miR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cristóbal I, Aguilera O, García-Foncillas
J, Zazo S, Madoz-Gúrpide J and Rojo F: Clinical significance of
miR-126 in colorectal cancer. Genes Chromosomes Cancer. 53:8812014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Y, Bai Y, Zhang F, Wang Y, Guo Y and
Guo L: miR-126 inhibits non-small cell lung cancer cells
proliferation by targeting EGFL7. Biochem Biophys Res Commun.
391:1483–1489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Menges CW, Sementino E, Talarchek J, Xu J,
Chernoff J, Peterson JR and Testa JR: Group I p21-activated kinases
(PAKs) promote tumor cell proliferation and survival through the
AKT1 and Raf-MAPK pathways. Mol Cancer Res. 10:1178–1188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar R, Gururaj AE and Barnes CJ:
p21-activated kinases in cancer. Nat Rev Cancer. 6:459–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siu MK, Chan HY, Kong DS, et al:
p21-activated kinase 4 regulates ovarian cancer cell proliferation,
migration, and invasion and contributes to poor prognosis in
patients. Proc Natl Acad Sci USA. 107:18622–18627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu X, Feng J, Zeng D, Ding Y, Yu C and
Yang B: PAK4 confers cisplatin resistance in gastric cancer cells
via PI3K/Akt- and MEK/Erk-dependent pathways. Biosci Rep. 34:59–67.
2014. View Article : Google Scholar
|
|
28
|
Zhang J, Wang J, Guo Q, Wang Y, Zhou Y,
Peng H, Cheng M, Zhao D and Li F: LCH-7749944, a novel and potent
p21-activated kinase 4 inhibitor, suppresses proliferation and
invasion in human gastric cancer cells. Cancer Lett. 317:24–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu
XL and Zhou SH: Mesenchymal stem cells modified with miR-126
release angiogenic factors and activate Notch ligand Delta-like-4,
enhancing ischemic angiogenesis and cell survival. Int J Mol Med.
31:484–492. 2013.PubMed/NCBI
|
|
30
|
Li L, Huang K, You Y, Fu X, Hu L, Song L
and Meng Y: Hypoxia-induced miR-210 in epithelial ovarian cancer
enhances cancer cell viability via promoting proliferation and
inhibiting apoptosis. Int J Oncol. 44:2111–2120. 2014.PubMed/NCBI
|
|
31
|
Zhang Y, Wang X, Xu B, Wang B, Wang Z,
Liang Y, Zhou J, Hu J and Jiang B: Epigenetic silencing of miR-126
contributes to tumor invasion and angiogenesis in colorectal
cancer. Oncol Rep. 30:1976–1984. 2013.PubMed/NCBI
|