Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common malignant neoplasm and the third most common cause of
cancer-associated mortalities worldwide (1,2). At
present, the combination of α-fetoprotein (AFP) with
ultrasonography or computed tomography (CT) is widely used in
clinical practice for the early diagnosis of HCC. However, a
previous study identified that the sensitivity of AFP was only
25–65%. In addition, a number of patients with chronic hepatitis,
liver cirrhosis and other liver diseases or gastrointestinal
cancers have been found to exhibit elevated levels of AFP (3). Therefore, identifying novel and
promising makers that may improve the diagnostic accuracy of HCC is
required. Survivin, also known as baculoviral inhibitor of
apoptosis repeat containing 5 (BIRC 5), is an inhibition of
apoptosis protein. It was initially separated by researchers from
Yale University, and has since been identified to be involved in
the inhibition of apoptosis (4) and
the regulation of mitosis (5).
Previous studies have revealed that survivin is
highly expressed in the majority of human tumors and fetal tissues,
but is normally undetectable in normal human tissues (6). This suggests that survivin is associated
with the occurrence and progression of carcinomas. A study by Ito
et al (7) was the first to
report the expression of survivin in HCC tissues. Those authors
demonstrated that survivin mRNA expression was upregulated in HCC
tissues and in four HCC cell lines using reverse
transcription-polymerase chain reaction (RT-PCR) analysis. In
addition, survivin protein expression was also found to be elevated
in HCC tissues following immunohistochemical staining. By contrast,
survivin mRNA and protein were undetectable in adjacent
paraneoplastic tissues (7). Since
then, a number of studies have also identified an elevation in the
level of survivin in HCC tissues using immunohistochemical staining
(8–10)
and RT-PCR analysis (11,12). These findings indicate the potential
value of survivin in the diagnosis of HCC.
To the best of our knowledge, only two studies have
investigated the expression of survivin in the serum of patients
with HCC. El-Attar et al (13)
revealed that the concentrations of survivin in the serum of HCC
patients infected with the hepatitis C virus were not significantly
different to those in the serum of healthy humans. A study by
Matteucci et al (14) failed
to detect the expression of serum survivin in 262 patients using
ELISA. However, the two studies had limitations concerning the
selection of patients and controls. Therefore, the expression of
survivin in the serum of HCC patients remains to be fully
elucidated. The present study recruited healthy controls and
controls with nonmalignant chronic liver disease, and used two
different commercially-available ELISA kits in order to detect the
levels of serum survivin and assess the diagnostic role of survivin
in HCC. In addition, the study aimed to identify which ELISA kit
performed best in detecting the levels of serum survivin.
Materials and methods
Patients and samples
The present study included 60 patients with liver
diseases who had been admitted to the Tianjin Third Center
Hospital, Tianjin Medical University (Tianjin, China) between
January 2010 and August 2013. In addition, 20 healthy individuals
were included, who had a normal liver biochemical function, no
history of liver diseases, no viral hepatitis and no history of any
other tumors or malignant disease. The participants were classified
into four groups (n=20 each) as follows: i) patients with HCC; ii)
cirrhotic patients without HCC; iii) patients with chronic
hepatitis B (HBV) infection; and iv) control individuals. The
clinical characteristics of the patients are shown in Table I.
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
|
| AFP, ng/ml |
|---|
|
|
|
|---|
| Group | n | Median age,
years | Gender, M/F | HBsAg, +/− | Child-Pugh score,
A:B:C | <20 | ≥20 |
|---|
| HCC | 20 | 58.6±8.1 | 16/4 | 12/8 | 14:3:3 | 4 | 16 |
| LC | 20 | 60.0±11.3 | 12/8 | 0/20 | 10:7:3 | 15 | 5 |
| CHB | 20 | 44.6±13.8 | 11/9 | 20/0 | - | 20 | 0 |
| NC | 20 | 51.2±9.6 | 7/13 | 0/20 | - | 20 | 0 |
The diagnosis of HCC was established by the clinical
characteristics in combination with imaging evidence (ultrasound,
CT or magnetic resonance imaging findings) and biochemistry
(including the AFP level and liver function). Following resection,
histopathological analysis was used to confirm the final diagnosis
according to guidelines provided by the American Association for
the Study of Liver Diseases (15).
All the HCC patients were initially admitted to hospital without
receiving any surgery or intervention therapy. The tumor stage was
defined according to the Barcelona Clinic Liver Cancer (BCLC)
staging system (16). Early-stage HCC
was defined as BCLC stage 0+A. The patients with hepatitis
infection were diagnosed on the basis of a biochemical function
examination, a positive hepatitis B surface antigen result that had
been apparent for the previous 6 months, and HBV DNA concentrations
>103 IU/ml. Patients with cirrhosis were diagnosed
using histopathological analysis of liver biopsy samples.
Evaluation of liver reserve function used the Child-Pugh score
system, and liver function were divided into grade A, B and C.
Written informed consent was obtained from all the patients at the
time of recruitment.
All the serum samples were obtained using serum
separator tubes at the initial presentation prior to any treatment.
The samples were left to clot for 30 min at room temperature,
followed by centrifugation for 15 min at 1000 × g, and then stored
at −80°C until use.
Detection of serum survivin level
Serum survivin levels were detected by two
independent researchers who had no access to the patients' clinical
information. The assays were performed using two different ELISA
kits: The Quantikine® ELISA Human Survivin Immunoassay (cat. no.
DSV00; R&D Systems, Inc., Minneapolis, MN, USA) and the BIRC5
Human ELISA (cat. no. KA0441; Abnova Corporation, Taipei City,
Taiwan) kits, according to the manufacturer's instructions.
The levels of serum survivin were detected using the
R&D kit (recombinant human survivin; range, 31.2–2000 pg/ml).
Briefly, 100 µl RD1-9 assay diluent and 100 µl of standards were
added to each well. The specimen serum to be detected and the
appropriate controls were added to the appropriate wells, which
were precoated with a mouse anti-human monoclonal antibody specific
for survivin (catalog no. MAB886; R&D Systems, Inc.,
Minneapolis, MN, USA). The plates were then incubated for 2 h at
room temperature on a horizontal orbital microplate shaker (0.12″
orbit) set at 1,000 × g. Next, 200 µl survivin conjugate was added
to each well and incubated for 2 h at room temperature on the
shaker. Subsequently, 200 µl substrate solution was added to each
well and incubated for 30 min at room temperature, whilst protected
from light. Finally, the stop solution was added and the optical
density was determined at 450 nm and referenced to 540 nm on a
microplate reader (Multiskan 3, Thermo Fisher Labsystems, Waltham,
MA USA). All the measurements were performed in duplicate.
The levels of serum survivin were also detected
using the Abnova kit (recombinant human survivin; range, 62.5–4000
pg/ml). Briefly, 100 µl of standards, the specimen serum to be
detected and the controls were added into the appropriate wells,
which were precoated with a rabbit anti-human polyclonal antibody
specific for survivin (catalog no. PAB0272; Abnova Corporation,
Taipei, Taiwan). The plates were then incubated at 37°C for 90 min.
Next, 100 µl biotinylated mouse anti-human polyclonal survivin
antibody (catalog no. H00000332-B01P; Abnova Corporation) was added
to each well and incubated for 1 h at 37°C, followed by addition of
100 µl ABC working solution to each well and incubation at 37°C for
90 min. Subsequently, 90 µl TMB color developing agent was added to
each well and incubated at 37°C for 20 min. Finally, the stop
solution was added and the optical density was determined. All the
measurements were performed in duplicate.
Statistical analysis
All the statistical analyses were performed using
SPSS version 19.0 software (IBM Corporation, Armonk, NY, USA). The
data are expressed as the mean ± standard deviation or median for
nonparametric data. Differences between two independent groups were
compared using the Mann-Whitney U test (continuous variables and
nonparametric analyses). The Spearman correlation coefficient was
used to analyze any associations between the results detected by
the R&D and Abnova kits. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum survivin levels detected by two
different kits for the diagnosis of HCC
In order to detect the serum survivin levels with
the R&D kit, a standard curve was constructed using serially
diluted recombinant human survivin (Fig.
1A). The kit was able to detect survivin with a linear range
from 0–2000 pg/ml. The regression coefficients
(r2) were 0.9962 and 0.9995. Survivin was
undetectable in 73 out of 80 serum samples (91.25%). The
survivin-positive samples (n=7; median level, 11.0 pg/ml; range,
0–39.8 pg/ml) corresponded to 1 HCC patient, 1 cirrhosis patient, 1
chronic HBV patient and 4 healthy controls (Table II). The levels of serum survivin were
not significantly higher in the patients with HCC and chronic HBV
compared with the normal controls (P<0.05). Only patients with
cirrhosis exhibited an elevation in serum survivin compared with
the healthy controls (P=0.038).
 | Table II.Comparative analysis of serum survivin
levels in 80 patients, as detected by R&D and Abnova ELISA
kits. |
Table II.
Comparative analysis of serum survivin
levels in 80 patients, as detected by R&D and Abnova ELISA
kits.
|
|
| R&D kit | Abnova kit |
|---|
|
|
|
|
|
|---|
| Group | n | Range, pg/ml | Positive, n (%) | Z | P-valuea | Range, pg/ml | Positive, n (%) | Z | P-valuea |
|---|
| HCC | 20 | 0.0–39.8 | 1 (5) | -1.459 | 0.144 | 0.0–93.5 | 5 (25) | -0.756 | 0.449 |
| LC | 20 | 0.0–11.0 | 1 (5) | -2.078 | 0.038 | 0.0–107.0 | 4 (20) | -0.818 | 0.413 |
| CHB | 20 | 0.0–13.5 | 1 (5) | -1.023 | 0.306 | 0.0–553.5 | 3 (20) | -0.888 | 0.375 |
| NC | 20 | 0.0–26.6 | 4 (20) | - | - | 0.0–437.0 | 6 (30) | - | - |
The standard curves for the assays performed using
the Abnova kit are shown in Fig. 1B.
The kit was able to detect survivin with a linear range from 0–4000
pg/ml, using the regression coefficients (r2)
0.9964 and 0.9931. Survivin was detectable in 22.5% (18/80) of the
serum samples (median level, 67.0 pg/ml; range, 2.0–553.5 pg/ml),
which corresponded to 5 HCC patients, 4 cirrhosis patients, 3
chronic HBV patients and 6 healthy controls (Table II). No statistically significant
differences were observed between the patients with HCC, cirrhosis
and chronic HBV and the healthy controls (P>0.05).
Association between R&D and Abnova
kits in detecting survivin in the same serum samples
As shown in Fig. 2,
the Spearman correlation coefficient was 0.178 (P=0.114), which
indicated that there were no associations between the results of
the R&D and Abnova kits in detecting the level of serum
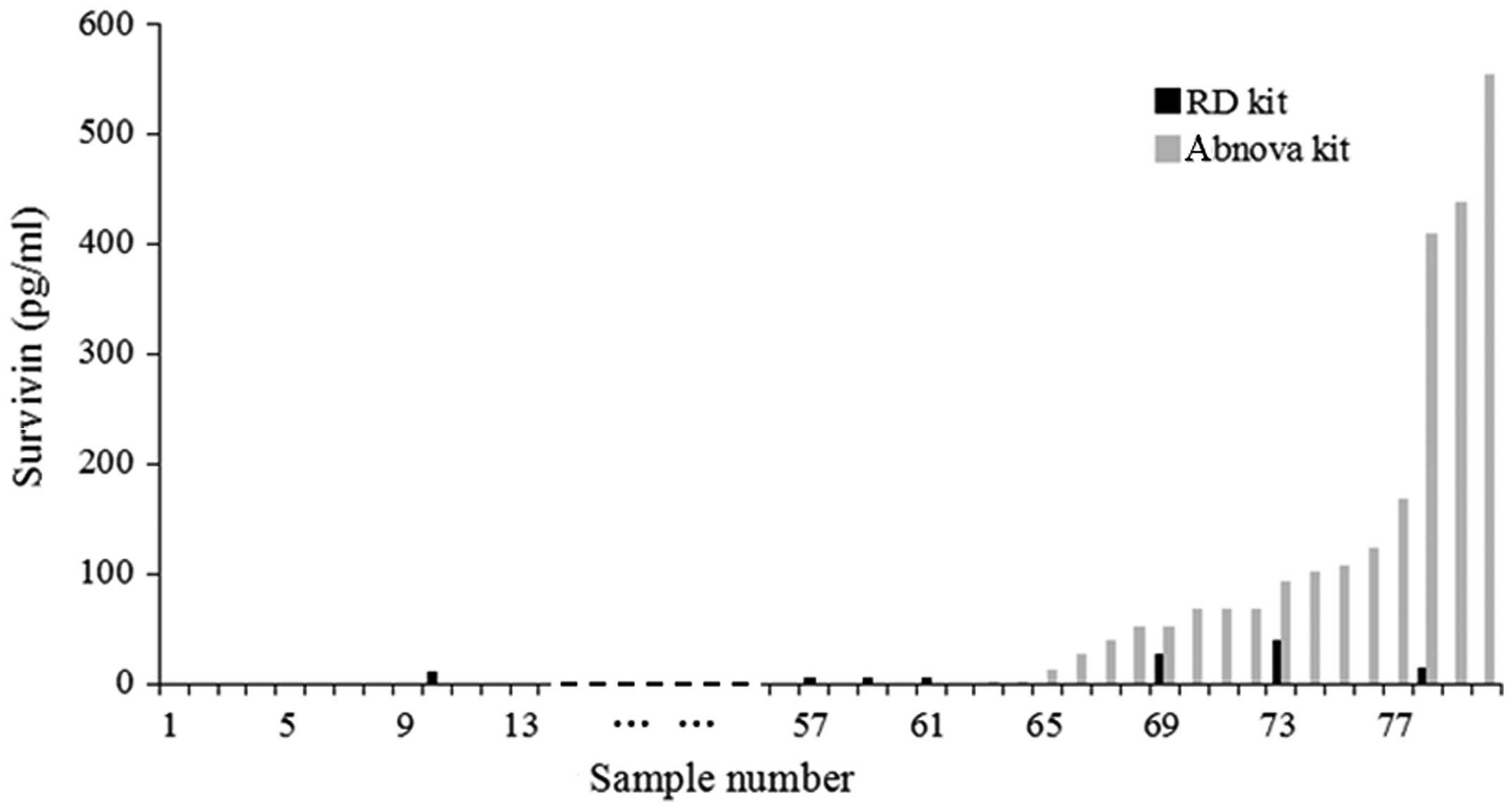
survivin. A histogram was constructed in order to compare the serum
survivin levels of each sample analyzed by the R&D and Abnova
kits (Fig. 3). A statistically
significant difference was identified between the results of the
two kits (P=0.002), which suggested that the sensitivity of the
Abnova kit was higher compared to that of the R&D kit.
Discussion
Survivin, which is a member of the inhibition of
apoptosis protein family, is important in the inhibition of
apoptosis and the regulation of mitosis (4,5). Previous
studies reported that survivin was highly-expressed in the majority
of human tumors and fetal tissues, but was undetectable in normal
human tissue (6). This suggests that
survivin is associated with the occurrence and progression of
carcinomas. Ito et al (7)
first evaluated the expression of survivin in HCC tissues using
immunohistochemical and RT-PCR analyses. It was revealed that the
level of survivin protein was elevated in 70% (14/20) of the HCC
tissues and that survivin mRNA was present in all eight types of
HCC tissues, as well as the HepG2, Huh7, SK-Hep1 and HLE cell
lines. By contrast, survivin mRNA and protein were undetectable in
adjacent paraneoplastic tissues (7).
Studies by Yang et al (8), Zhu
et al (9) and Hui et al
(10) that used immunohistochemical
staining, as well as studies by Chau et al (11) and Augello et al (12) that used RT-PCR analysis, confirmed the
elevated levels of survivin in HCC tissues. These studies indicated
that survivin protein was a promising histological tumor marker.
However, whether survivin can be used as a serological marker for
HCC remains to be elucidated. To the best of our knowledge, only
two studies have investigated the serum expression of survivin
protein (7,14). However, the conclusions of these
studies were unclear and limited by the absence of healthy controls
or controls with nonmalignant chronic liver diseases. The
identification of novel serum biomarkers is extremely important for
the diagnosis of HCC, particularly noninvasive and inexpensive
protein markers that require the collection of >100 µl serum.
Therefore, the present study recruited healthy controls and
patients with HCC, cirrhosis and chronic HBV infection in order to
evaluate the role of serum survivin protein in the diagnosis of
HCC.
In the current study, the levels of serum survivin
were detected using two different commercially-available ELISA kits
(R&D and Abnova kits). However, the expression of survivin in
the serum was undetectable in the majority of the patients and
normal controls. The positive ratios were only 8.75% (7/80) for the
R&D kit and 21.25% (18/80) for the Abnova kit. In HCC patients,
the patients with a positive expression of survivin were only 5%
(1/20) using the R&D kit and 25% (5/20) using the Abnova kit.
Furthermore, no statistically significant differences were observed
in the results of the two kits (r=0.178; P=0.114), which indicated
that survivin was not a promising serum maker for the diagnosis of
HCC. It may be reasonable to suggest that the levels of survivin
protein in the serum of patients with HCC were low and close to the
detection limits of the two different commercially-available ELISA
kits. By contrast, survivin protein may not have a secreting type,
although there has been conflicting evidence concerning the
significance of the nuclear or cytoplasmic expression of survivin
in HCC (17–20). El-Attar et al (13) reported that the median level of serum
survivin in HCC patients was 13.9 pg/ml, which was close to the
detection limits of the R&D and Abnova kits. In addition,
Matteucci et al (14)
demonstrated that serum survivin was undetectable in all 62 cases
of HCC serum samples that were analyzed. Therefore, the present
study may have failed to detect the expression of serum survivin
with the use these two kits.
Several previous studies have investigated the
levels of the autoantibody of survivin in the serum of patients
with HCC. Zhang et al (21)
reported that the positive ratio of the survivin antibody was 11.3%
(16/142) in patients with HCC and 2.4% (2/82) in heathy controls
(capture antibody, goat anti-human immunoglobulin G (IgG); Caltag
Laboratories, Burlingame, CA, USA). However, the study failed to
identify the autoantibody in the serum of the patients with
cirrhosis and chronic hepatitis using the ELISA assays (21). Using the same type of assay,
Megliorino et al (22)
established that the positive ratio was only 9.4% (15/160) in HCC
patients (capture antibody, goat anti-human IgG; Caltag
Laboratories, San Francisco, CA, USA), whilst Yagihashi et
al (23) reported that the
positive ratio was 24.1% (7/29). These studies revealed that the
positive ratios and the levels of survivin autoantibody were low in
the serum of patients with HCC, which may be in accordance with the
results of survivin protein in the serum of HCC patients in the
present study.
The present study identified significant differences
between the results of the two kits used. This may be due to the
difference in the capture antibody used by each kit. The capture
antibody for the R&D kit was a rat anti-human survivin
monoclonal antibody (Met1-Asp142; accession number (AN), O15392),
whereas that for the Abnova kit was a rabbit anti-human survivin
polyclonal antibody (M1-D142; AN, O15392), which may have a
stronger affinity. Therefore, the sensitivity of the Abnova kit may
be higher compared with that of R&D kit.
In conclusion, the levels of serum survivin protein
were close to the detection limits of the two ELISA kits used in
the present study. In addition, the positive ratios of the serum
survivin protein in the patients with HCC were significantly low
and no statistically significant difference was observed between
the levels of serum survivin in the HCC patients and controls, as
detected by the R&D and Abnova ELISA kits. Therefore, survivin
protein was not found to be a promising serological maker for the
diagnosis of HCC.
Acknowledgements
The present study was supported by a grant from the
Key Research Project of Tianjin Health Bureau (grant no.
11KG112).
References
|
1
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: a global and regional perspective. Oncologist. 15:(Suppl
4). 5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou L, Liu J and Luo F: Serum tumor
markers for detection of hepatocellular carcinoma. World J
Gastroenterol. 12:1175–1181. 2006.PubMed/NCBI
|
|
3
|
Spangenberg HC, Thimme R and Blum HE:
Serum markers of hepatocellular carcinoma. Semin Liver Dis.
26:385–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikolovska-Coleska Z, Meagher JL, Jiang S,
et al: Interaction of a cyclic, bivalent smac mimetic with the
x-linked inhibitor of apoptosis protein. Biochemistry.
47:9811–9824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lens SM, Rodriguez JA, Vader G, et al:
Uncoupling the central spindle-associated function of the
chromosomal passenger complex from its role at centromeres. Mol
Biol Cell. 17:1897–1909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vischioni B, van der Valk P, Span SW, et
al: Expression and localization of inhibitor of apoptosis proteins
in normal human tissues. Hum Pathol. 37:78–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito T, Shiraki K, Sugimoto K, et al:
Survivin promotes cell proliferation in human hepatocellular
carcinoma. Hepatology. 31:1080–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Zhu J, Gou H, et al: Clinical
significance of Cox-2, Survivin and Bcl-2 expression in
hepatocellular carcinoma (HCC). Med Oncol. 28:796–803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu H, Chen XP, Zhang WG, et al:
Expression and significance of new inhibitor of apoptosis protein
survivin in hepatocellular carcinoma. World J Gastroenterol.
11:3855–3859. 2005.PubMed/NCBI
|
|
10
|
Hui WT, Zan Y, Wang XJ, et al: Expression
of survivin, p53 and its relationship with apoptosis, proliferation
in hepatocellular carcinoma (HCC). JNMU. 22:255–259. 2008.
|
|
11
|
Chau GY, Lee AF, Tsay SH, et al:
Clinicopathological significance of survivin expression in patients
with hepatocellular carcinoma. Histopathology. 51:204–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Augello C, Caruso L, Maggioni M, et al:
Inhibitors of apoptosis proteins (IAPs) expression and their
prognostic significance in hepatocellular carcinoma. BMC Cancer.
9:1252009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Attar HA, Kandil MH, El-Kerm YM and
El-Ghandour MK: Comparison of serum survivin and alpha fetoprotein
in Egyptian patients with hepatocellular carcinoma associated with
hepatitis C viral infection. Asian Pac J Cancer Prev. 11:897–903.
2010.PubMed/NCBI
|
|
14
|
Matteucci C, Sorrentino R, Bellis L, et
al: Detection of high levels of Survivin-immunoglobulin M immune
complex in sera from hepatitis C virus infected patients with
cirrhosis. Hepatol Res. 44:1008–1018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: the BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon WS and Tarnawski AS: Nuclear
translocation of survivin in hepatocellular carcinoma: a key to
cancer cell growth? Hum Pathol. 34:1119–1126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takashima H, Nakajima T, Moriguchi M, et
al: In vivo expression patterns of survivin and its splicing
variants in chronic liver disease and hepatocellular carcinoma.
Liver Int. 25:77–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morinaga S, Nakamura Y, Ishiwa N, et al:
Expression of survivin mRNA associates with apoptosis,
proliferation and histologically aggressive features in
hepatocellular carcinoma. Oncol Rep. 12:1189–1194. 2004.PubMed/NCBI
|
|
20
|
Montorsi M, Maggioni M, Falleni M, et al:
Survivin gene expression in chronic liver disease and
hepatocellular carcinoma. Hepatogastroenterology. 54:2040–2044.
2007.PubMed/NCBI
|
|
21
|
Zhang JY, Megliorino R, Peng XX, et al:
Antibody detection using tumor-associated antigen mini-array in
immunodiagnosing human hepatocellular carcinoma. J Hepatol.
46:107–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Megliorino R, Shi FD, Peng XX, et al:
Autoimmune response to anti-apoptotic protein survivin and its
association with antibodies to p53 and c-myc in cancer detection.
Cancer Detect Prev. 29:241–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yagihashi A, Asanuma K, Kobayashi D, et
al: Autoantibodies to survivin in patients with chronic hepatitis
and hepatocellular carcinoma. Autoimmunity. 38:445–448. 2005.
View Article : Google Scholar : PubMed/NCBI
|