Introduction
Pancreatic cancer is one of the most common
malignancies and the fourth leading cause of cancer-associated
mortality in the USA (1). During
previous decades, the diagnosis and treatment of pancreatic
adenocarcinoma has been notably improved. However, pancreatic
cancer continues to possess the poorest prognosis of any human
cancer. Overall, >85% of patients have lost the opportunity for
surgical intervention at the time of diagnosis, and therefore,
chemotherapy is important in the treatment of advanced pancreatic
cancer (2). Gemcitabine (GEM) is the
conventional chemotherapeutic drug used in the treatment of
pancreatic cancer. Unfortunately, the therapeutic effect of GEM is
poor (3,4). Therefore, a novel treatment strategy
that is able to enhance the anti-tumor effect of GEM is urgently
required.
α-N-phthalimidoglutarimide, also known as
thalidomide (THD), was introduced in the 1950s as an antiemetic and
sedative in Europe. However, the drug was rapidly banned due to
teratogenic effects (5). In 1994, THD
was found to be an antiangiogenic agent (6). Subsequently, it was revealed in 1999
that THD can be used in the treatment of multiple myeloma (7). In the following years, a large number of
studies revealed that THD exerted an antitumor effect in several
types of cancer, including prostate, colorectal, non-small-cell
lung and breast cancer, and renal cell carcinoma (8–12). The
invasion and metastasis of tumors depends on the process of
angiogenesis, and THD may be able to inhibit the growth of tumor
tissue by preventing the development of the necessary blood
vessels. In addition, THD may promote early-stage apoptosis and
inhibit the proliferation of carcinoma cells (13,14).
However, the antitumor mechanism of THD has yet to be
elucidated.
B-cell lymphoma 2 (Bcl-2), is an anti-apoptotic
protein, which protects against cell death; by contrast,
Bcl-2-associated X protein (Bax) exhibits the opposite effect,
promoting cell death. Vascular endothelial growth factor (VEGF) has
also been demonstrated to be a significant indicator of tumor
angiogenesis.
The aim of the present study was to investigate the
effect and mechanism of THD in combination with GEM on the human
pancreatic carcinoma SW-1990 cell line in vitro and in
vivo by monitoring levels of Bcl-2, Bax and VEGF.
Materials and methods
Cell lines and cell culture
The human pancreatic carcinoma SW-1990 cell line was
provided by the Department of Gastroenterology at the Changhai
Hospital of The Second Military Medical University (Shanghai,
China). The cells were grown in 75 cm2 cell culture
flasks and maintained in RPMI-1640 medium (Gibco Life Technologies,
Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal
bovine serum (Gibco Life Technologies), 100 units/ml penicillin and
100 µg/ml streptomycin at 37°C in a humidified atmosphere of 95%
air and 5% CO2.
Cell counting kit (CCK)-8 assay. The SW-1990 cells
were trypsinized with 0.05% trypsin when 80% confluency was
achieved. The cells were then plated into 96-well plates, with 100
µl medium per well, and cultured overnight in RPMI-1640 medium. THD
(0–200 µg/ml; Calbiochem, San Diego, CA, USA) was added to the
cells, with six replicates being performed for each concentration.
After 24, 48 or 72 h incubation, the cell viability was determined
using a CCK-8 assay (Peptide Institute, Inc., Osaka, Osaka, Japan),
and the survival and inhibition rates of the cells were calculated.
In the combined treatment condition, 50 µg/ml THD and 20 µmol/l GEM
were tested alone or in combination for their ability to inhibit
the proliferation of the SW-1990 cell line using the aforementioned
method.
Annexin V/propidium iodide (PI)
assay
The SW-1990 cells were seeded into six-well plates,
and treated with normal saline and THD at various concentrations
(0, 25, 50, 100, 150, and 200 µg/ml). The cells were collected 48 h
later and washed twice using cold phosphate-buffered saline (PBS).
The cells were then trypsinized and stained using an Annexin V/PI
double staining solution (Sigma-Aldrich, St. Louis, MO, USA) at
room temperature. After 15 min, the Annexin V/PI stained cells were
analyzed by flow cytometry using the ModFitLT software (Verity
Software House, Topsham, ME, USA), and the percentage of apoptotic
and necrotic cells was calculated. In the combined treatment
investigation, the cells were treated with 50 µg/ml THD, 20 µmol/l
GEM or 50 µg/ml THD and 20 µmol/l GEM in combination. The analysis
of cell death was performed by flow cytometry, as previously
described (15).
Animals
Female athymic Balb/c nu/nu mice aged 4–6 weeks and
weighing 15–16 g were obtained from Shanghai Laboratory Animal
Center (Chinese Academy of Sciences, Shanghai, China). The mice
were housed in a laminar airflow cabinet under specific
pathogen-free conditions and were allowed free access to sterilized
water and standard pellet food. The protocol for the in vivo
study was in accordance with the guidelines of animal care and was
approved by the Soochow University Animal Experiments Committee
(Suzhou, Jiangsu, China) (16).
Nude mouse xenograft assay
For the nude mice xenograft assay, the SW-1990 cells
were trypsinized and resuspended in serum-free RPMI-1640 at a
concentration of 1×107 cells/ml. The cell suspension was
then subcutaneously injected into the right anterior armpit of nude
mice to generate a primary transplanted tumor. The mice were
divided into four groups: Normal saline (NS)-treated control group;
melatonin-treated group; GEM-treated group; and the combined
treatment group. There were five mice per group. When the size of
the tumor xenograft reached ∼5 mm in diameter, the mice were
administered with 200 mg/kg THD, 50 mg/kg gemcitabine or the
combined treatment, comprising 200 mg/kg THD and 50 mg/kg GEM,
through injections provided every other day. Four weeks later, the
nude mice were sacrificed and the tumors were measured and weighed.
The tumor volume (cm3) was calculated as follows: 4π/3 ×
(width/2)2 × (length/2)
Semi-quantitative RT-PCR assay
Total RNA were extracted from the SW-1990 cells and
tumor tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
and quantitated by absorbance analysis performed at 260 nm,
according to the manufacturer's instructions. The first-strand cDNA
was synthesized in 20 µl reaction reagent with 2,000 ng total RNA,
using the Omniscript RT kit (Qiagen, Hilden, Germany). The PCR
reactions were performed over 45 cycles. Each cycle was performed
using the following cycling conditions: Denaturation for 40 sec at
95°C; annealing for 40 sec at 59°C; and polymerization for 38 sec
at 72°C. The primers used for the detection of Bcl-2, Bax and VEGF
mRNA were as follows: Bcl-2 forward, 5′-CAGCTGCACCTGACGCCCTT-3′ and
reverse, 5′-GCCTCCGTTATCCTG GATCC-3′; Bax forward,
5′-GCGTCCACCAAGAAGCTGA-3′ and reverse, 5′-ACCACCCTGGTCTTGGATCC-3′;
VEGF forward, 5′-GGACAGACAGACAGACACCG-3′ and reverse,
5′-GCACCCAAGACAGCAGAAAG-3′; and β-actin forward,
5′-AGCGGGAAATCGTGCGTG-3′ and reverse,
5′-CAGGGTACATGGTGGTGCC-3′.
Western blot assay
The sample proteins were separated on 8–12% SDS-PAGE
and then electroblotted onto nitrocellulose membranes (GenScript
USA Inc., Piscataway, NJ, USA). The membranes were blocked with
0.1% Tween-20 (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) in PBS (PBST) containing 5% fresh milk at room temperature
for 60 min. Firstly, the membranes were incubated at 4°C overnight
with the primary mouse monoclonal antibodies against VEGF, Bcl-2
and Bax (catalog nos. sc-152, sc-56018 and sc-7480, respectively;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The membranes
were then washed with PBST three times and incubated with the
appropriate horseradish peroxidase-conjugated secondary antibody at
room temperature for 45 min. Finally, the membranes were washed
with PBST three times and visualized using an enhanced
chemiluminescence detection system (Beyotime, Jiangsu, China).
Immunohistochemistry assay
The tumor tissues were fixed in formalin and
embedded in paraffin prior to being sectioned into 4-µm thick
slices for immunohistochemical staining. Subsequent to
deparaffinization, the sections were incubated with the antibodies
against VEGF, Bcl-2 and Bax (Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The primary antibody was then removed and the slices
were washed with PBST three times. Subsequently, the appropriate
biotinylated goat polyclonal secondary antibody was added and
incubated at room temperature for 60 min. The slides were then
washed with PBST three times, incubated in diaminobenzidine
solution for 10 min and counterstained with hematoxylin for 1 min.
Finally, the images were captured using a light microscope
(magnification, ×200; Olympus CKX41-A32RC; Olympus, Tokyo, Japan).
Immunohistochemical analysis of CD34 was used to calculate the
microvessel density (MVD) of the tumor xenograft.
Statistical analysis
The data were expressed as the mean ± standard error
and analyzed using SPSS software, version 18.0 (SPSS Inc., Chicago,
IL, USA). Statistical analysis was performed using one-way analysis
of variance (ANOVA), and the Student-Newman-Keuls test was
performed as a post-hoc test subsequent to ANOVA. The
Kruskal-Wallis test was used to evaluate the differences of
categorical values followed by the Mann-Whitney U test,
which was performed as a post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
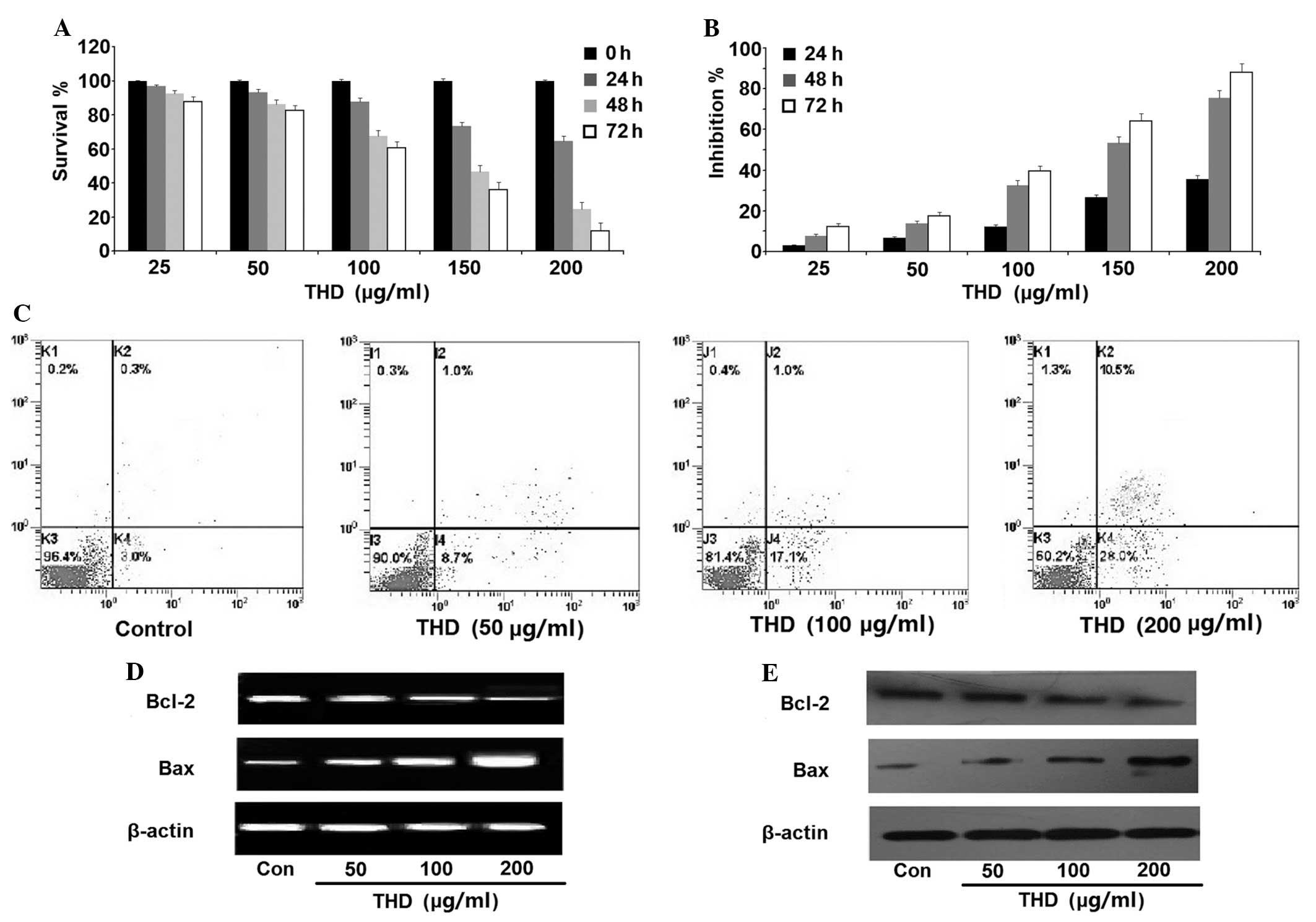
Effect of THD on the growth of SW-1990
cells
The SW-1990 cells were treated with various
concentrations (0–200 µg/ml) of THD for 24 h, 48 h and 72 h. The
survival and growth inhibition rates of SW-1990 cells were
determined using the CCK-8 kit assay. It was found that the
proliferation and survival of the cells was inhibited in a dose-
and time-dependent manner (Fig. 1A and
B). SW-1990 cell death was measured using an Annexin V/PI
assay. The levels of apoptosis and necrosis in the cells cultured
with THD for 48 h were found to be increased in a dose-dependent
manner compared with the control group (Fig. 1C; Table
I). In addition, 48 h after THD incubation, RT-PCR and western
blot analysis revealed that the expression of Bcl-2 was
downregulated, while the expression of Bax was upregulated
(Fig. 1D and E).
 | Table I.Effect of thalidomide on viability of
SW-1990 cells in vitro (Annexin V/PI assay). |
Table I.
Effect of thalidomide on viability of
SW-1990 cells in vitro (Annexin V/PI assay).
|
| Thalidomide
concentration, µg/ml |
|---|
|
|
|
|---|
| Form of cell
death | 0 | 50 | 100 | 200 |
|---|
| Apoptosis | 2.57±0.73 |
8.77±1.30a |
18.53±1.95a |
29.43±3.75a |
| Necrosis | 0.36±0.29 | 1.05±0.46 |
1.70±0.73a |
13.04±1.52a |
Effect of THD and GEM combined
treatment on the growth of SW-1990 cells
The SW-1990 cells were treated with THD, GEM or a
combination of the two. Firstly, the CCK-8 assay revealed that THD
and GEM were each able to inhibit the proliferation and survival of
SW-1990 cells. Furthermore, combined inhibition of THD and GEM
demonstrated a marked increase in the suppression of cell
proliferation compared with the inhibition demonstrated by THD or
GEM alone (Fig. 2A and B). In
addition, the Annexin V/PI assay detected an increased number of
apoptotic and necrotic cells in the THD- or GEM-treated groups
compared with the control group, and treatment with a combination
of THD and GEM demonstrated an increased ability to promote
apoptosis and necrosis compared with the administration of either
of the two drugs alone (Fig. 2C;
Table II). In addition, although the
mRNA and protein expression of Bcl-2 were each downregulated in the
cells treated with THD or GEM alone, a significantly greater
decrease of Bcl-2 expression was observed in the cells treated with
combined treatment. Additionally, it was found that the level of
Bax expression was significantly upregulated in the combined
treatment group compared with the groups treated with THD or GEM
alone (Fig. 2D and E).
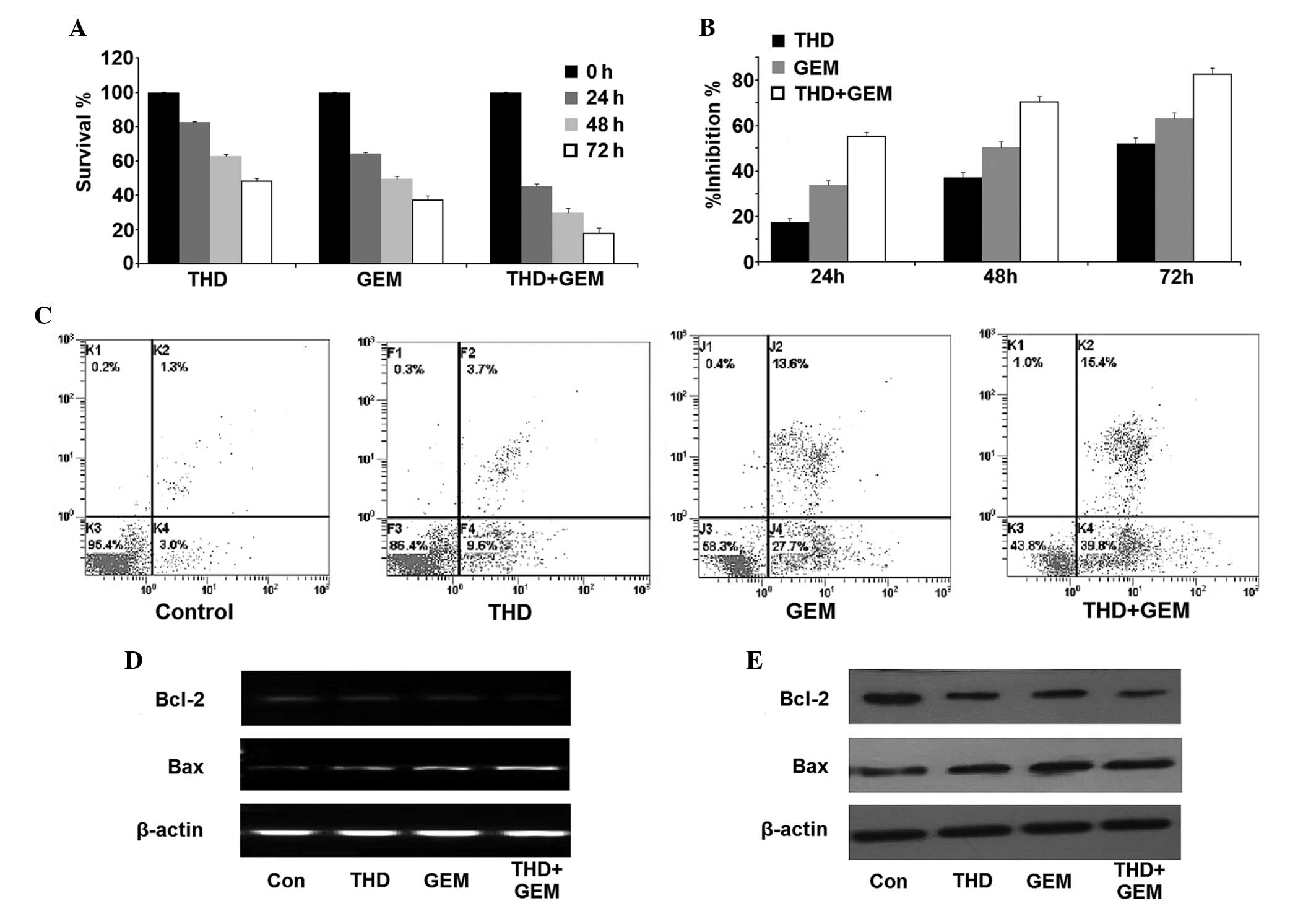 | Figure 2.Inhibitory effect of THD combined with
GEM on the growth of the pancreatic cancer SW-1990 cell line in
vitro. (A) The SW-1990 cells were incubated with 50 µg/ml THD,
20 µmol/l GEM or a combination of the two for 0, 24, 48 and 72 h. A
counting cell kit 8 assay was then used to analyze the viability of
the cells. (B) The growth inhibition exerted on SW-1990 cells was
calculated and the data are presented as the mean ± standard error.
(C) The percentage of apoptotic and necrotic SW-1990 cells was
analyzed using an Annexin V/propidium iodide assay. (D) Subsequent
to treatment with the indicated concentrations of 50 µg/ml THD, 20
µmol/l GEM or a combination of the two, the SW-1990 cells were
harvested and the levels of Bcl-2 and Bax mRNA were analyzed by
reverse transcription-polymerase chain reaction. (E) The expression
of the Bcl-2 and Bax proteins in the SW-1990 cells was determined
by western blotting. THD, thalidomide; GEM, gemcitabine; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; Con,
control. |
 | Table II.Effect of thalidomide combined with
gemcitabine on the viability of SW1990 cells in vitro. |
Table II.
Effect of thalidomide combined with
gemcitabine on the viability of SW1990 cells in vitro.
|
| Treatment group |
|---|
|
|
|
|---|
| Form of cell
death | Control | 50 µg/ml
thalidomide | 20 µmol/l
gemcitabine | 50 µg/ml thalidomide
+ 20 µmol/l gemcitabine |
|---|
| Apoptosis | 2.75±1.32 |
10.88±2.97a |
29.79±4.10a |
40.02±4.65a |
| Necrosis | 1.44±0.74 |
2.51±1.32a |
15.98±3.21a |
17.69±3.37a |
Effect of THD on the growth of tumor
xenograft in nude mice
All nude mice survived for the duration of the
treatment, and the doses of THD and GEM administered resulted in no
detectable toxic side-effects on the nude mice, including changes
in body weight. The volumes and weights of tumor tissue were
measured subsequent to the mice being sacrificed. Compared with the
NS-treated control, the tumor xenograft treated with THD, GEM or
combined treatment was significantly decreased in size. In
addition, there was a significant decrease in the tumor volume and
weight in the combined treatment group throughout the whole
observation period, compared with the other groups (Fig. 3A–C). The change in Bcl-2 and Bax
expression was similar to the results of the in vitro study.
The combined treatment group, in particular, demonstrated a
significant difference in the expression of Bcl-2 and Bax compared
with the other groups (Fig. 3D and
E). Additionally, the level of VEGF mRNA and protein was
downregulated, in addition to the reduction in the MVD in the tumor
tissues (Fig. 4).
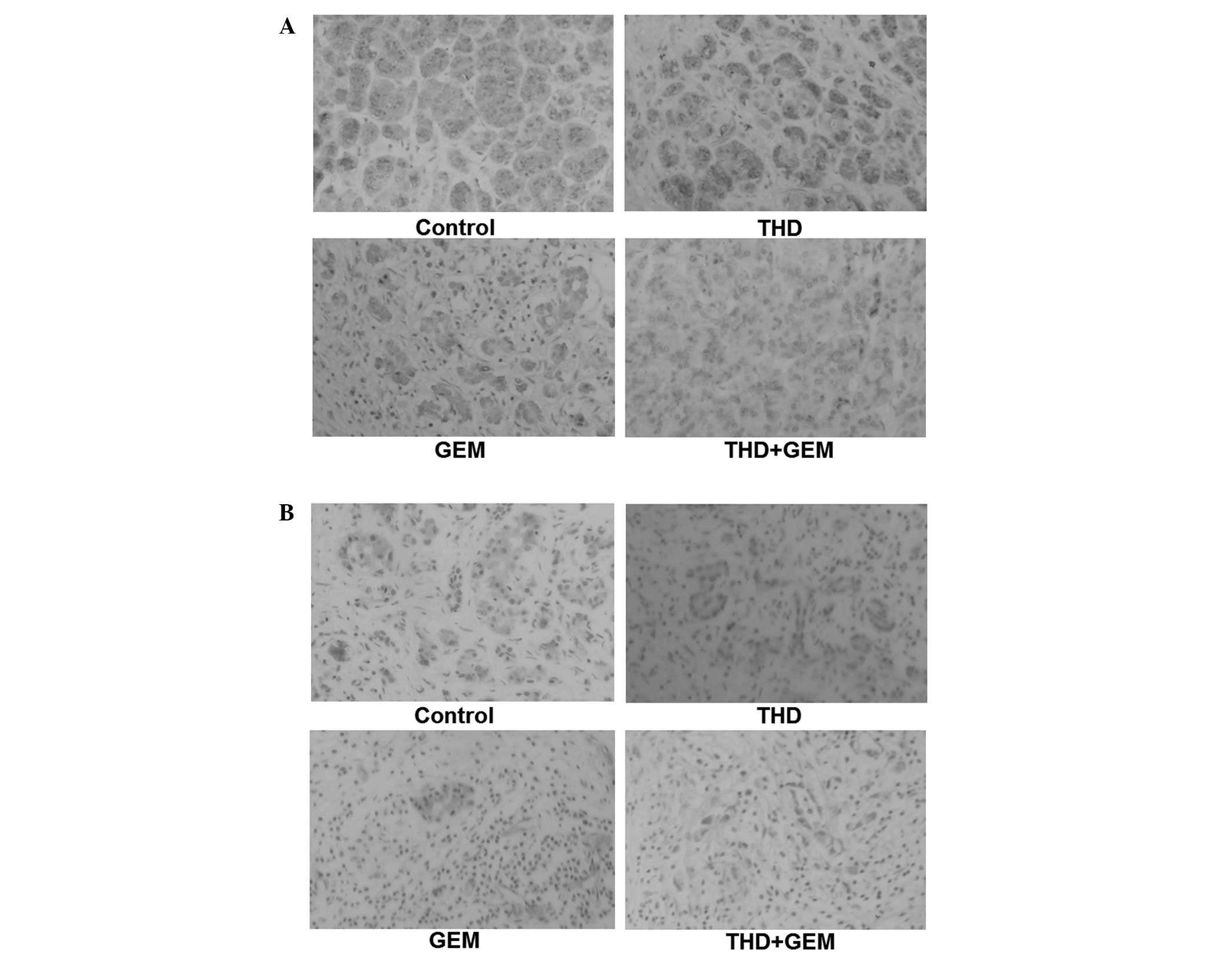
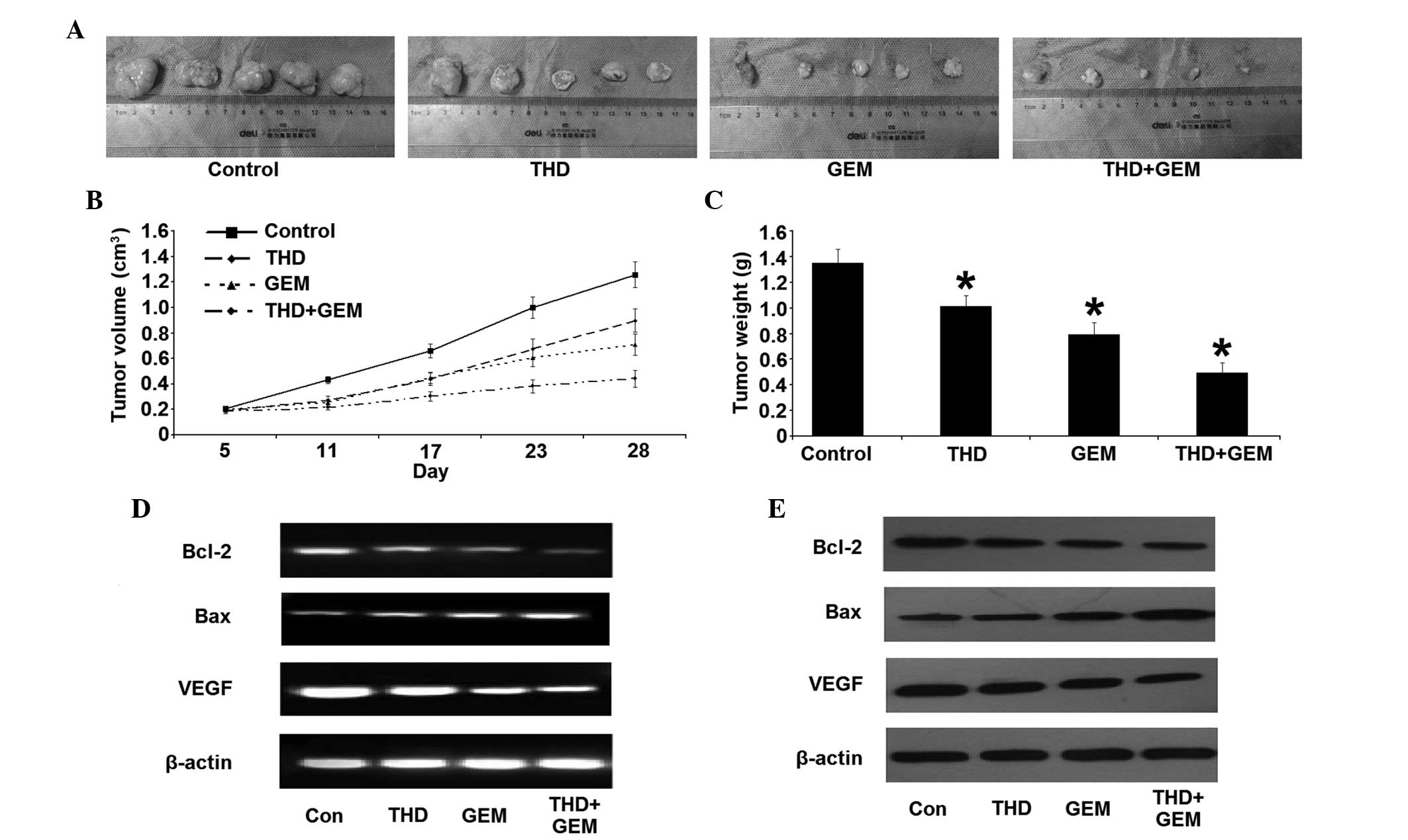 | Figure 3.Inhibitory effect of combined
treatment with THD and GEM on the growth of the pancreatic cancer
SW-1990 cell line in vivo. (A) Gross morphology of
xenografts in nude mice on the 28th day of treatment. (B) Changes
in the volume of the xenograft in nude mice subsequent to treatment
with THD and GEM. Compared with the NS-treated control, treatment
with THD, GEM and a combination of the two demonstrated a
significantly increased inhibition of tumor growth. (C) Four weeks
after treatment with THD, GEM and the combination of the two, the
tumor weights were evidently decreased compared with the NS-treated
control treatment. The data are expressed as the mean ± standard
error. (D) Subsequent to treatment with the indicated
concentrations of 200 mg/kg THD, 50 mg/kg gemcitabine and combined
treatment, the mRNA levels of Bcl-2, Bax and VEGF in nude mice
xenografts were analyzed by reverse transcription-polymerase chain
reaction. (E) The expression of the Bcl-2, Bax and VEGF proteins in
nude mice xenografts was determined by western blotting. *P<0.05
vs. NS-treated mice. THD. thalidomide; GEM, gemcitabine; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; VEGF, vascular
endothelial growth factor. |
Discussion
Previous studies have reported that THD is able to
inhibit the growth of several cancer cell lines and xenograft
tumors (17,18). However, the precise anti-tumor
mechanism of THD is not fully understood. At present, a number of
studies have demonstrated that the main source of the therapeutic
effect of THD on tumors is associated with the following two
properties of THD. THD is able to not only suppress the
proliferation and migration of tumor cells, but also induces an
increase in the number of apoptotic and necrotic cells. In
addition, THD may possess anti-angiogenic function that allows for
the inhibition of vascular formation and growth of tumor tissues
(19,20). The present study aimed to examine the
effect of THD on the apoptosis and necrosis of the pancreatic
carcinoma SW-1990 cell line, in addition to the anti-angiogenic
effect exerted on a tumor xenograft in a nude mouse model.
According to the results of the experiment, the mechanism behind
the THD-mediated induction of apoptosis and necrosis in tumor cells
may be associated with changes in the expression of Bcl-2 and Bax.
Additionally, THD may block the neovascularization process in tumor
xenograft tumors through a decrease in the expression of VEGF mRNA
and protein, in addition to reducing the MVD.
Several studies have suggested that pro-apoptosis
proteins play a major role in tumor formation and treatment
response (21). The members of the
Bcl-2 family, including Bcl-2 and Bax, are important regulators of
apoptosis and anti-apoptotic processes. Bcl-2 protects against cell
death and possesses anti-apoptotic characteristics. By contrast,
Bax demonstrates the opposite effect and promotes cell death.
Therefore, the Bax/Bcl-2 ratio is crucial to the apoptosis
signaling pathway (22). In the
present study, THD has been revealed to induce apoptosis and
necrosis in SW-1990 cells through a decrease in the level of Bcl-2.
However, the associated signaling pathways of necrosis require
additional elucidation. Notably, Sung et al found that the
upregulation of Bcl-2 was inversely correlated with necrosis in
pancreatic acinar cells in experimental pancreatitis (23). Also, Bcl-2 has been reported to
demonstrate anti-necrotic functions in other cell types. Barbu
et al revealed that the increased expression of Bcl-2 is
able to prevent cytokine-induced apoptosis and necrosis of β-cells
in the pancreas by counteracting mitochondrial permeability
transition (24). These achievements
indicated that Bcl-2 may be an important factor in the process of
necrosis in cells and tissues. Additionally, the present findings
are similar to the aforementioned results and suggest that the
pro-necrotic effect of THD may be another mechanism for the
inhibition of the growth of pancreatic cancer cells, in addition to
the induction of apoptosis.
Angiogenesis is essential to the growth of any solid
tumor. Antiangiogenic therapy can inhibit tumor progression
indirectly through the suppressive functions of vascular formation,
and result in tumor necrosis and shrinkage. THD was considered to
be an angiogenesis inhibitor and used in the treatment of multiple
myeloma in 1999. Subsequently, numerous studies demonstrated that
THD possessed the ability to downregulate the VEGF concentration in
several types of cancer (6,25). VEGF is one of the most important
indicators of tumor angiogenesis and may become a major target in
the treatment strategy for pancreatic cancer. MVD is another
important regulator of tumor angiogenesis in histological specimens
and tumor models. Certain studies hypothesize that MVD may be an
independent parameter for identifying the response to
antiangiogenic treatment and is inversely associated with cancer
survival (26,27). The results of the present study, which
revealed significantly lower VEGF expression and MVD in the
carcinoma xenograft tumors treated with THD, support these
aforementioned theories.
Only 14–20% of all patients with pancreatic cancer
can be treated with radical surgical intervention at the time of
diagnosis. Therefore, chemotherapy is important for the treatment
of advanced cancer. GEM, a cytotoxic nucleoside analog, is the most
commonly used antitumor drug for pancreatic cancer (3). Nevertheless, a previous study has
revealed that the curative efficacy of GEM monotherapy is poor,
resulting in the overall response rate of 5.0–11.0% and the median
survival duration of 5.7–6.3 months (28). In order to improve therapeutic
efficacy of unresectable pancreatic cancer, numerous clinical
studies have investigated GEM-based combination regimens, but the
outcomes are poor. A variety of randomized phase III trials have
revealed that combination therapies, including the administration
of gemcitabine with other agents, such as cisplatin, capecitabine
or exatecan, failed to demonstrate any improvement at a
statistically significant level (29–31).
Furthermore, certain authors have indicated that combination
treatments may be more toxic and less well tolerated compared with
the administration of GEM alone (32). As demonstrated in the present study,
THD and GEM each exert an antitumor effect on pancreatic cancer
cells in vitro and in vivo. In addition, the
inhibitive effect is dramatically increased subsequent to a
four-week treatment with GEM and THD compared with monotherapy.
Considering that there is no marked difference between the body
weights of nude mice in the NS-treated, THD-treated, GEM-treated
and combination treatment groups, the dose applied demonstrated no
detectable toxic side-effects in the mice. Thus, THD may be used in
the treatment of advanced pancreatic cancer as an adjuvant
agent.
In summary, THD was able inhibit the growth of
pancreatic cancer and was associated with the induction of tumor
cell apoptosis and necrosis, as well as inhibition of tumor
angiogenesis. By contrast, combined administration of THD and GEM
demonstrated significantly greater therapeutic efficacy that
treatment with each of the agents alone. These findings may provide
an alternative therapeutic option for the treatment of pancreatic
cancer.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (No. 81300357).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrone CR, Brennan MF, Gonen M, et al:
Pancreatic adenocarcinoma: The actual 5-year survivors. J
Gastrointest Surg. 12:701–706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first-line therapy for patients with advanced pancreas cancer: A
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
4
|
Hagmann W, Jesnowski R and Löhr JM:
Interdependence of gemcitabine treatment, transporter expression,
and resistance in human pancreatic carcinoma cells. Neoplasia.
12:740–747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McBride WG: Thalidomide embryopathy.
Teratology. 16:79–82. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci USA. 91:4082–4085. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singhal S, Mehta J, Desikan R, et al:
Antitumor activity of thalidomide in refractory multiple myeloma. N
Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rezvani H, Haghighi S, Ghadyani M and
Attarian H: Efficacy of taxotere, thalidomide, and prednisolone in
patients with hormone-resistant metastatic prostate cancer. Urol J.
9:673–677. 2012.PubMed/NCBI
|
|
9
|
Lv J, Liu N, Liu KW, et al: A Randomised
Controlled Phase II Trial of the Combination of XELOX with
Thalidomide for the First-line Treatment of Metastatic Colorectal
Cancer. Cancer Biol Med. 9:111–114. 2012.PubMed/NCBI
|
|
10
|
Lee SM and Hackshaw A: A potential new
enriching trial design for selecting non-small-cell lung cancer
patients with no predictive biomarker for trials based on both
histology and early tumor response: Further analysis of a
thalidomide trial. Cancer Med. 2:360–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Souza CM, Araújo e Silva AC, de Jesus
Ferraciolli C, et al: Combination therapy with carboplatin and
thalidomide suppresses tumor growth and metastasis in 4T1 murine
breast cancer model. Biomed Pharmacother. 68:51–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tunio MA, Hashmi A, Qayyum A, Naimatullah
N and Masood R: Low-dose thalidomide in patients with metastatic
renal cell carcinoma. J Pak Med Assoc. 62:876–879. 2012.PubMed/NCBI
|
|
13
|
Dmoszynska A, Podhorecka M, Manko J, et
al: The influence of thalidomide therapy on cytokine secretion,
immunophenotype, BCL-2 expression and microvessel density in
patients with resistant or relapsed multiple myeloma. Neoplasma.
52:175–181. 2005.PubMed/NCBI
|
|
14
|
Marriott JB, Clarke IA, Czajka A, et al: A
novel subclass of thalidomide analogue with anti-solid tumor
activity in which caspase-dependent apoptosis is associated with
altered expression of bcl-2 family proteins. Cancer Res.
63:593–599. 2003.PubMed/NCBI
|
|
15
|
Georgakoudi I, Solban N, Novak J, et al:
In vivo flow cytometry: a new method for enumerating circulation
cancer cells. Cancer Res. 64:5044–5047. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forni M: Laboratory animals science: a
resource to improve the quality of science. Vet Res Commun.
31:Suppl 1. 43–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yabu T, Tomimoto H, Taguchi Y, et al:
Thalidomide-induced antiangiogenic action is mediated by ceramide
through depletion of VEGF receptors, and is antagonized by
sphingosine-1-phosphate. Blood. 106:125–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang ZL, Liu ZS and Sun Q: Effects of
thalidomide on angiogenesis and tumor growth and metastasis of
human hepatocellular carcinoma in nude mice. World J Gastroenterol.
11:216–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steins MB, Padró T, Bieker R, et al:
Efficacy and safety of thalidomide in patients with acute myeloid
leukemia. Blood. 99:834–839. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu WM, Strauss SJ, Chaplin T, et al:
s-thalidomide has a greater effect on apoptosis than angiogenesis
in a multiple myeloma cell line. Hematol J. 5:247–254. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen J, Wan R, Hu G, et al: miR-15b and
miR-16 induce the apoptosis of rat activated pancreatic stellate
cells by targeting Bcl-2 in vitro. Pancreatology. 12:91–99. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung KF, Odinokova IV, Mareninova OA, et
al: Prosurvival Bcl-2 proteins stabilize pancreatic mitochondria
and protect against necrosis in experimental pancreatitis. Exp Cell
Res. 315:1975–1989. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barbu A, Welsh N and Saldeen J:
Cytokine-induced apoptosis and necrosis are preceded by disruption
of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic
RINm5F cells: Prevention by Bcl-2. Mol Cell Endocrinol. 190:75–82.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aydoğan S, Celiker U, Türkçüoğlu P, Ilhan
N and Akpolat N: The effect of thalidomide on vascular endothelial
growth factor and tumor necrosis factor-alpha levels in retinal
ischemia/reperfusion injury. Graefes Arch Clin Exp Ophthalmol.
246:363–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coultas L, Chawengsaksophak K and Rossant
J: Endothelial cells and VEGF in vascular development. Nature.
438:937–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giatromanolaki A, Koukourakis MI,
Stathopoulos GP, et al: Angiogenic interactions of vascular
endothelial growth factor, of thymidine phosphorylase, and of p53
protein expression in locally advanced gastric cancer. Oncol Res.
12:33–41. 2000.PubMed/NCBI
|
|
28
|
Xu C, Wu A, Zhu H, et al: Melatonin is
involved in the apoptosis and necrosis of pancreatic cancer cell
line SW-1990 via modulating of Bcl-2/Bax balance. Biomed
Pharmacother. 67:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colucci G, Labianca R, Di Costanzo F, et
al: Gruppo Oncologico Italia Meridionale (GOIM); Gruppo Italiano
per lo Studio dei Carcinomi dell'Apparato Digerente (GISCAD);
Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC): Randomized
phase III trial of gemcitabine plus cisplatin compared with
single-agent gemcitabine as first-line treatment of patients with
advanced pancreatic cancer: The GIP-1 study. J Clin Oncol.
28:1645–1651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Herrmann R, Bodoky G, Ruhstaller T, et al:
Swiss Group for Clinical Cancer Research; Central European
Cooperative Oncology Group: Gemcitabine plus capecitabine compared
with gemcitabine alone in advanced pancreatic cancer: A randomized,
multicenter, phase III trial of the Swiss Group for Clinical Cancer
Research and the Central European Cooperative Oncology Group. J
Clin Oncol. 25:2212–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abou-Alfa GK, Letourneau R, Harker G, et
al: Randomized phase III study of exatecan and gemcitabine compared
with gemcitabine alone in untreated advanced pancreatic cancer. J
Clin Oncol. 24:4441–4447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chauffert B, Mornex F, Bonnetain F, et al:
Phase III trial comparing intensive induction chemoradiotherapy (60
Gy, infusional 5-FU and intermittent cisplatin) followed by
maintenance gemcitabine with gemcitabine alone for locally advanced
unresectable pancreatic cancer. Definitive results of the 2000-01
FFCD/SFRO study. Ann Oncol. 19:1592–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|