Introduction
More than 800,000 mortalities occur annually as a
result of gastric cancer (GC), which is the second leading cause of
cancer-associated mortality worldwide (1). Although the surgical techniques,
chemoradiation therapy regimens, and pre- and postoperative
medications used to treat GC have improved, the median overall
survival time of patients with advanced GC remains at ∼13 months
(2). Tumor heterogeneity has been
identified as a factor that negatively affects patient survival and
numerous studies have indicated that the involvement of small
populations of cancer stem cells (CSCs) within tumor tissues may
contribute to this heterogeneity and thus to chemoradiation therapy
resistance, local invasion and metastasis to other organs (3–14).
The CSC hypothesis was initially proposed following
the prospective identification of leukemia-initiating cells in
patients with acute myeloid leukemia (AML) (3,4). The AML
studies, which were conducted in immunocompromised mice, revealed a
significantly higher leukemic potential in cluster of
differentiation (CD)34+CD38− leukemic cells
than in CD34+CD38+ or CD34− cells
(3,4).
Following the identification of these leukemic stem cells, CSCs or
cancer-initiating cells have been identified in various types of
solid tumors, including breast, brain, colon, pancreatic, liver and
gastric tumors (5–11). Xenotransplantation assays have
demonstrated the high capacity of CSCs for self-renewal and,
thereby, the likelihood that these cells may regenerate the
heterogeneous types of cancer cells that complicate therapeutic
approaches (12,13). When characterizing CSCs, the use of
human samples, which have usually been resected via surgical
procedures, is important to elucidate the mechanisms of
chemoresistance, radioresistance, metastasis and recurrence, and to
identify novel drug targets against neoplastic diseases with poor
prognoses (12,14).
Regarding gastric CSC surface markers, several
molecules or combinations of molecules have been reported to
indicate cell subsets, including CD44+,
CD44+EpCAM+,
CD44+CD24+, and CD90+ cells, as
well as cells with high aldehyde dehydrogenase activity (11,15–20).
However, the hyaluronic acid receptor, CD44, cannot be used to
specifically detect gastric CSCs, even when combined with CD133
(21). In the present study, the cell
surface markers expressed on surgically resected GC cells were
comprehensively profiled in combination with a xenograft study in
immunodeficient mice to identify novel markers for the detection of
gastric CSCs. This approach facilitated the identification of the
combination of CD44 and CD26 as a useful marker of cancer stem cell
properties.
Materials and methods
Cancer cells
Fresh surgically resected well-differentiated GC
specimens were obtained at Osaka University Medical Hospital
(Osaka, Japan) between 2011 and 2012 and written informed consent
was obtained from all patients. This study was approved by the
ethics committee of Osaka University (Osaka, Japan). The GC cell
lines (MKN7, MKN28, MKN45 and AZ521, were provided by the
Department of Gastroenterological Surgery, Osaka University) were
cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific Inc., Waltham, MA, USA) and an antibiotic/antimycotic
solution (Sigma-Aldrich). To facilitate sphere formation, the cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)/F12
medium supplemented with 20 ng/ml of recombinant human epidermal
growth factor (EGF; Promega Corporation, Madison, WI, USA) and 10
ng/ml of basic fibroblast growth factor (FGF; PeproTech Inc., Rocky
Hill, NJ, USA) in 96-well plates, six-well plates, or 100-mm dishes
with Ultra-Low Attachment surfaces (Corning Inc., Corning, NY,
USA).
Antibody arrays and flow cytometric
studies
A Lyoplate® assay (BD Biosciences, San Jose, CA,
USA) was used to screen surface markers, and was equipped with
antibodies against 242 human cell surface markers, (mouse
anti-human monoclonal antibodies: CD1a, CD1b, CD1d, CD2, CD3, CD4,
CD4v4, CD5, CD6, CD7, CD8a, CD8b, CD9, CD10, CD11a, CD11b, CD11c,
CD13, CD14, CD15, CD15s, CD16, CD18, CD19, CD20, CD21, CD22, CD23,
CD24, CD25, CD26, CD27, CD28, CD29, CD30, CD31, CD32, CD33, CD34,
CD35, CD36, CD37, CD38, CD39, CD40, CD41a, CD41b, CD42a, CD42b,
CD43, CD44, CD45, CD45RA, CD45RB, CD45RO, CD46, CD47, CD48, CD49a,
CD49b, CD49c, CD49d, CD49e, CD50, CD51/61, CD53, CD54, CD55, CD56,
CD57, CD58, CD59, CD61, CD62E, CD62L, CD62P, CD63, CD64, CD66a, -c,
-d, -e), CD66b, CD66f, CD69, CD70, CD71, CD72, CD73, CD74, CD75,
CD77, CD79b, CD80, CD81, CD83, CD84, CD85, CD86, CD87, CD88, CD89,
CD90, CD91, CDw93, CD94, CD95, CD97, CD98, CD99, CD99R, CD100,
CD102, CD103, CD105, CD106, CD107a, CD107b, CD108, CD109, CD112,
CD114, CD116, CD117, CD118, CD119, CD120a, CD121a, CD121b, CD122,
CD123, CD124, CD126, CD127, CD128b, CD130, CD134, CD135, CD137,
CD137 ligand, CD138, CD140a, CD140b, CD141, CD142, CD144, CD146,
CD147, CD150, CD151, CD152, CD153, CD154, CD158a, CD158b, CD161,
CD162, CD163, CD164, CD165, CD166, CD171, CD279, CD282, CD305,
CD309, CD314, CD321, CDw327, CDw328, CDw329, CD335, CD336, CD337,
CD338, CD304, αβT CR, β2-microglobulin, human leukotriene B4
receptor-1, CLIP, CMRF-44, CMRF-56, epidermal growth factor
receptor, fMLP receptor, γδTCR, hybrid protein complex, human
leukocyte antigen (HLA)-A, -B, -C, HLA-A2, HLA- DQ, HLA- DR,
HLA-DR, -DP, -DQ, Invariant NK T, Disialoganglioside GD2, MIC A/B,
NKB1, stage-specific embryonic antigen (SSEA)-1, SSEA- 4, TRA-1-60,
TRA-1-81, Vβ 23, Vβ 8 and CD326; rat anti-human monoclonal
antibodies: CD49f, CD104, CD120b, CD132, CD201, CD210, CD212,
CD267, CD294, SSEA4, cutaneous lymph antigen and Integrin β7),
according to the manufacturer's instructions. Briefly, to evaluate
the cell lines, the cells were detached from the culture dishes
(Iwaki & Co., Ltd. Tokyo, Japan) using the Accutase™ Cell
Detachment Solution (BD Biosciences) and were washed with
Dulbecco's phosphate-buffered saline (Wako Pure Chemical
Industries, Ltd., Osaka, Japan). To evaluate fresh surgical
specimens or xenografts, the tumor tissues were dissected into
small sections, and digested with collagenase H (Roche Diagnostics,
Basel, Switzerland) and DNase I (Worthington Biochemical
Corporation, Lakewood, NJ, USA) for 1 h at 37°C. Next, the digested
tissues were filtered through a 100-µm filter, layered over Ficoll
1077 (GE Healthcare Life Sciences, Pittsburgh, PA, USA) and
centrifuged to remove dead cells and debris. The resuspended cells
were treated with TruStain fcX™ monoclonal rat anti-mouse CD16/32
antibody (BioLegend, Inc., San Diego, CA, USA) and a human FcR
blocking reagent (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
to block the Fc receptors, followed by staining with antibodies
against CD26-phycoerythrin (PE), CD24, CD44, CD47 and CD147 (BD
Biosciences); CD26-PE-Cyanin 5 (BioLegend); and CD15
(Beckman-Coulter, Indianapolis, IN, USA) for 30 min to evaluate the
surface expression levels. To exclude mouse cells, the cells were
first incubated with a biotinylated anti-mouse Lineage Panel
(monoclonal rat TER-119, CD11b, Gr-1, CD45R/B220 and hamster CD3e
antibodies), monoclonal rat anti-mouse H-2Kd and CD31 antibodies
(all purchased from BioLegend), followed by streptavidin-conjugated
Pacific Blue (Invitrogen Life Technologies, Carlsbad, CA, USA).
Human GC cell lines were cultured in RPMI-1640 medium supplemented
with 10% FBS with or without 5FU. For sphere formation, the cell
lines were cultured for 7 d in Ultra-low Attachment dishes (Corning
Inc.) at a density of ≤5×106 cells/dish. Briefly, the
cells were collected and washed to remove serum and then suspended
in serum-free DMEM/F12 supplemented with 20 ng/ml of human
recombinant EGF, 10 ng/ml human recombinant basic FGF, 2% B27
supplement without vitamin A and 1% N2 supplement (Invitrogen Life
Technologies). The spheres with a diameter of >50 µm were
counted and collected via gentle centrifugation with a pipette.
Tumorigenicity
A total of 24 four- to six-week-old female non-obese
diabetic/severe combined immunodeficiency (NOD/SCID) mice (CLEA
Japan Inc., Tokyo, Japan) were used as subcutaneous xenograft
models under specific pathogen-free conditions. The tumor volumes
were calculated using the following formula: Tumor volume =
(longest diameter) × (shortest diameter)2 × 0.5. This
study was approved by the Animal Experiments Committee of Osaka
University.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Continuous variables were compared using the Student's t-test.
Statistical analyses were performed using JMP statistical software,
version 9.0 (SAS Institute Inc., Cary, NC, USA).
Results
Antibody-based array and flow
cytometry
Two surgically resected GC xenografts were assessed
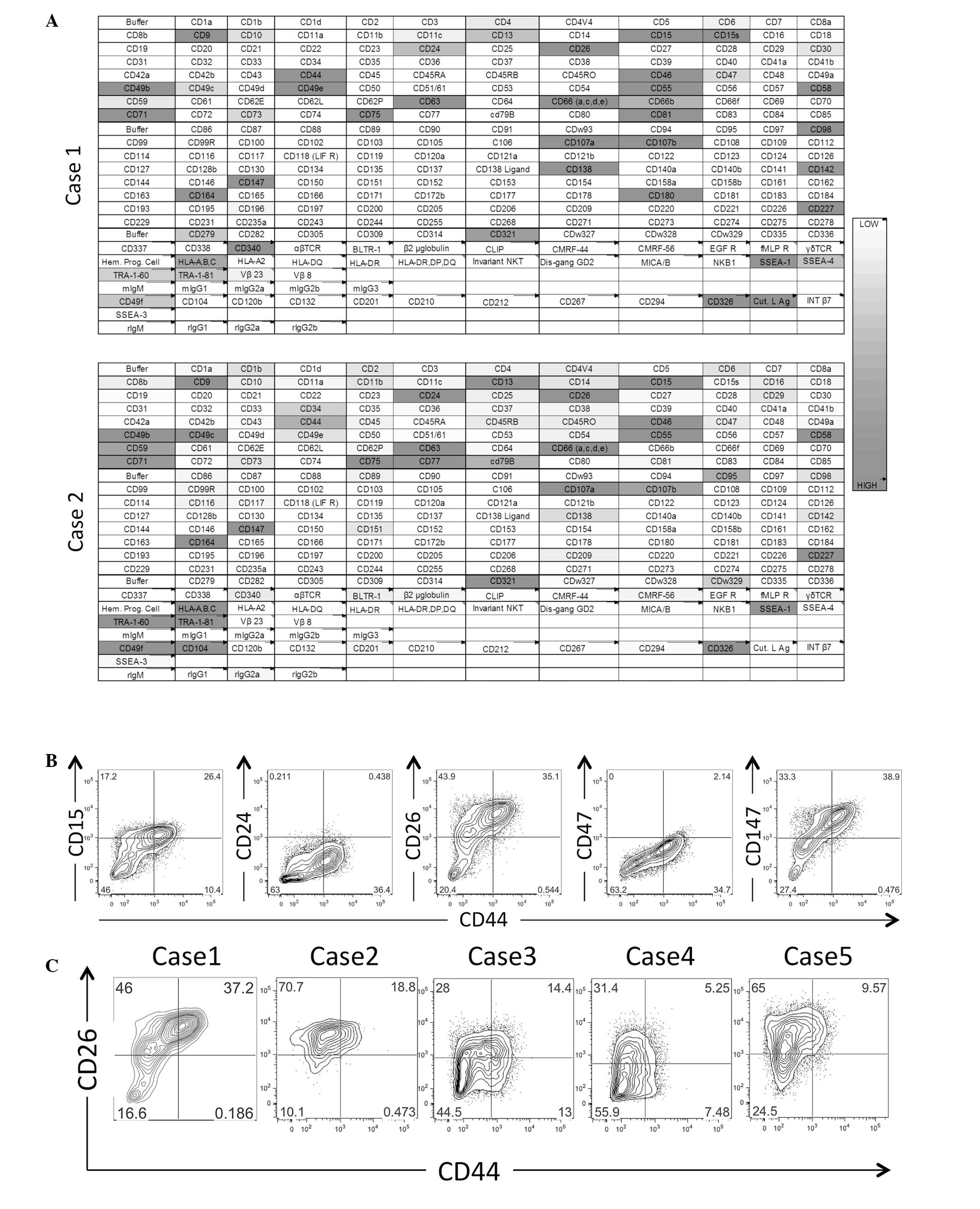
for surface marker expression via flow cytometry (Fig. 1A). The antibody-based array study
indicated that 27 of the 242 evaluated surface markers were
expressed in the two xenografts. The subsequent multicolor flow
cytometric analysis confirmed that among these markers, CD15, CD24,
CD26, CD44, CD47 and CD147 extended the expression profiling from
the negative to the positive range and further revealed that the
combination of CD26 and CD44 may be used to fractionate the GC
xenograft cells into three subsets (Fig.
1B). The confirmation study indicated that five of the flow
cytometrically analyzed cases were consistently fractionated into
at least three subsets according to the combined CD26 and CD44
expression statuses (Fig. 1C).
Sphere formation induces
CD26+ cells
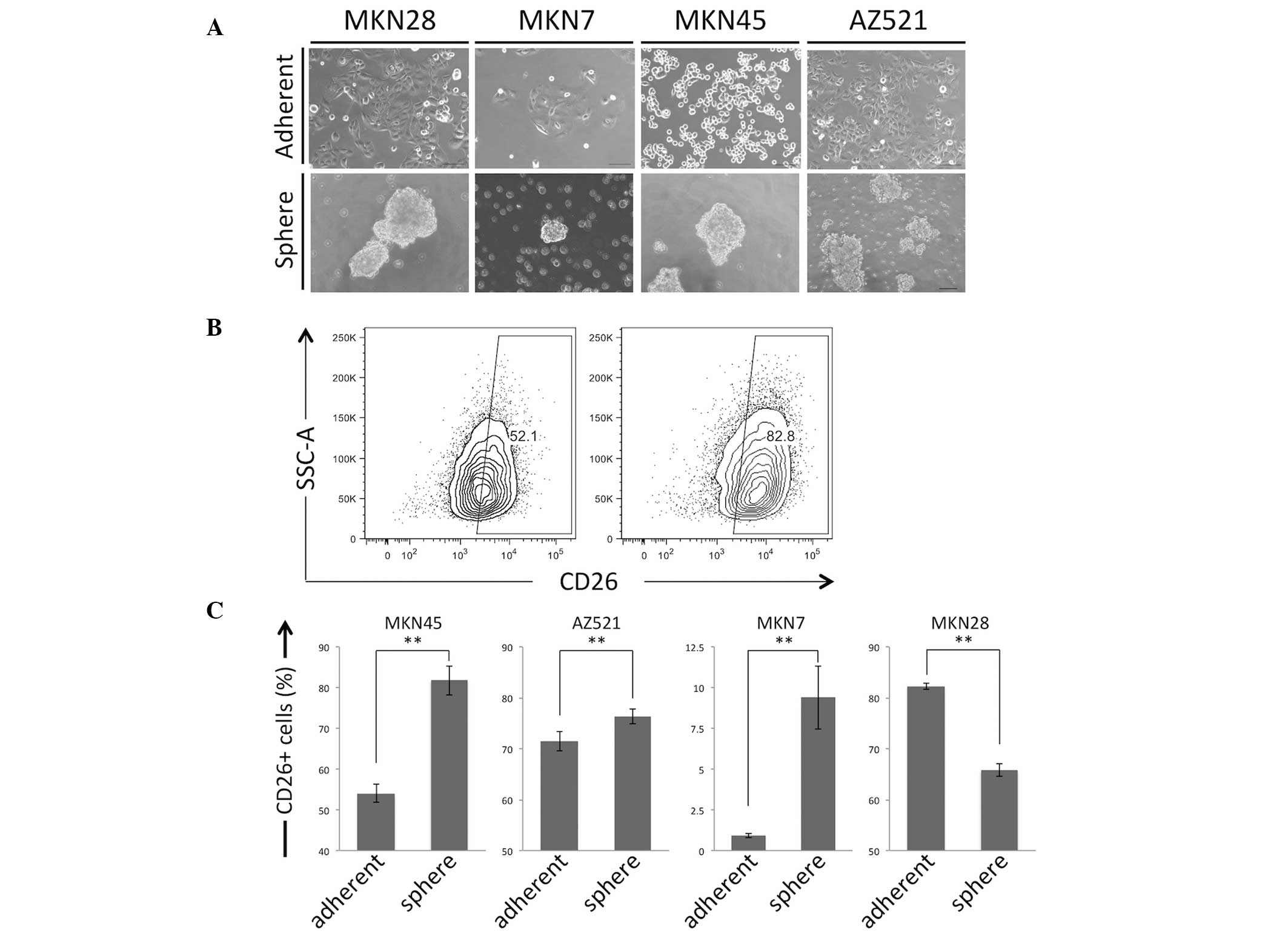
Cell culture conditions suitable for sphere
formation have been reported to support stem-like cell maintenance
and, presumably, to induce at least partial CSC induction, which
consequently enriches CSC cell populations (6,22). Four GC
cell lines (MKN7, MKN28, MKN45 and AZ521) were used in a sphere
formation assay. Distinct spheres were formed after culturing the
cell lines in serum-free and unattached conditions (Fig. 2A). Flow cytometric analysis of the
adherent cells and spheres revealed that sphere formation enriched
the CD26+ cell subset in three out of four of the
examined cell lines (Fig. 2B and
C).
CD26+CD44+ cells
exhibit high tumorigenic potential
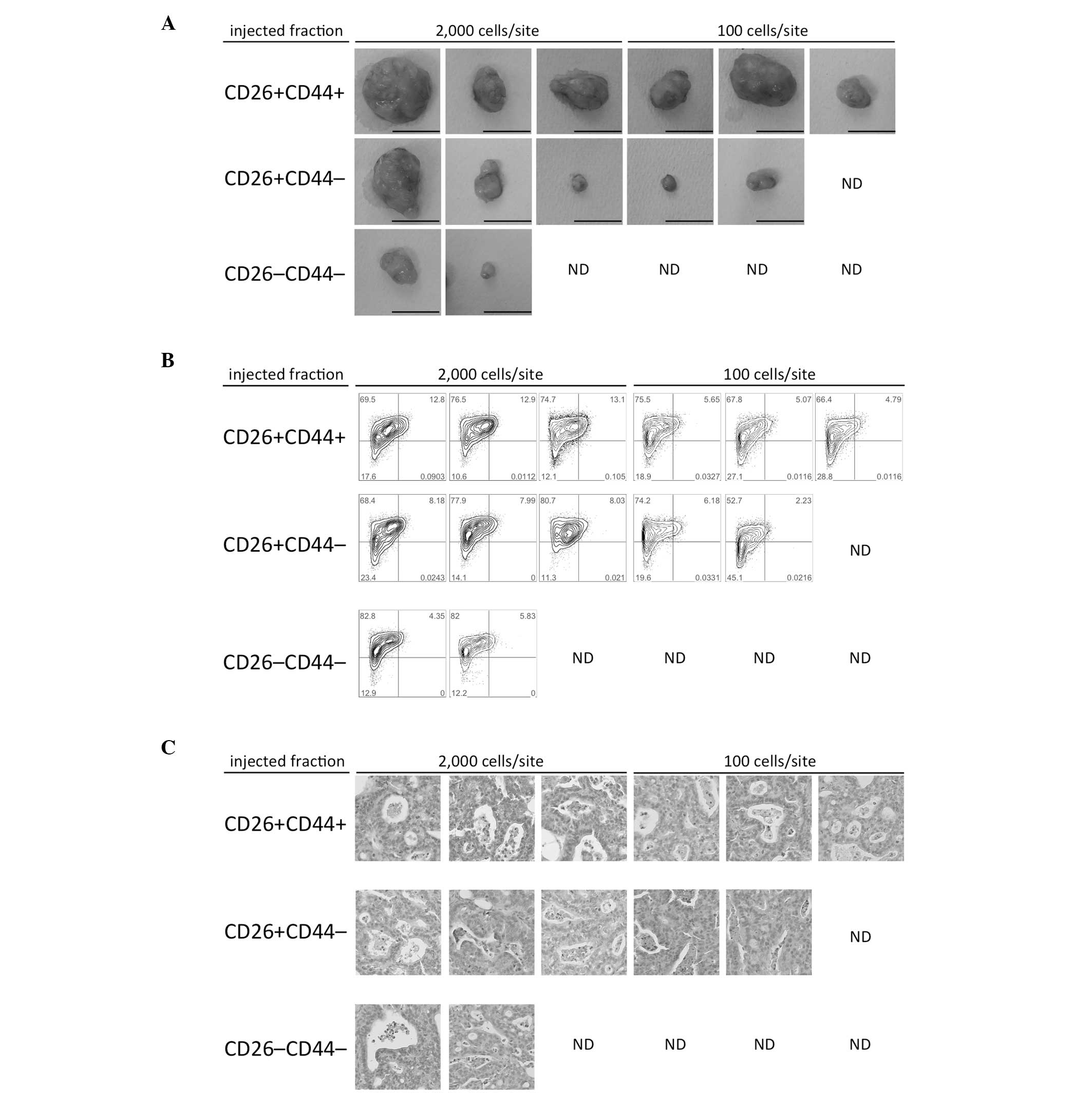
The tumorigenicity of each CD26- and CD44-defined
subset was assessed. Cells from a surgically obtained
specimen-derived GC xenograft (Case 1) were sorted via
fluorescence-activated cell sorting into three subsets,
CD26+CD44+, CD26+CD44−,
and CD26−CD44−, and were then subcutaneously
injected into NOD/SCID mice. The largest and most frequent tumor
formations were obtained with the CD26+CD44+
subset. The subset formed relatively small tumors; however, the
frequency of tumor formation was higher with
CD26+CD44− subset than with the
CD26−CD44− subset (Fig. 3A). Flow cytometric analysis of the
formed tumors revealed the recapitulation of three subsets similar
to those in the original xenograft (Figs.
1C and 3B). The histological
characteristics of the formed tumors were similar to those of the
xenograft prior to sorting and of the original patient sample
(Fig. 3C).
Roles of CD26 and CD44 in
chemoresistance
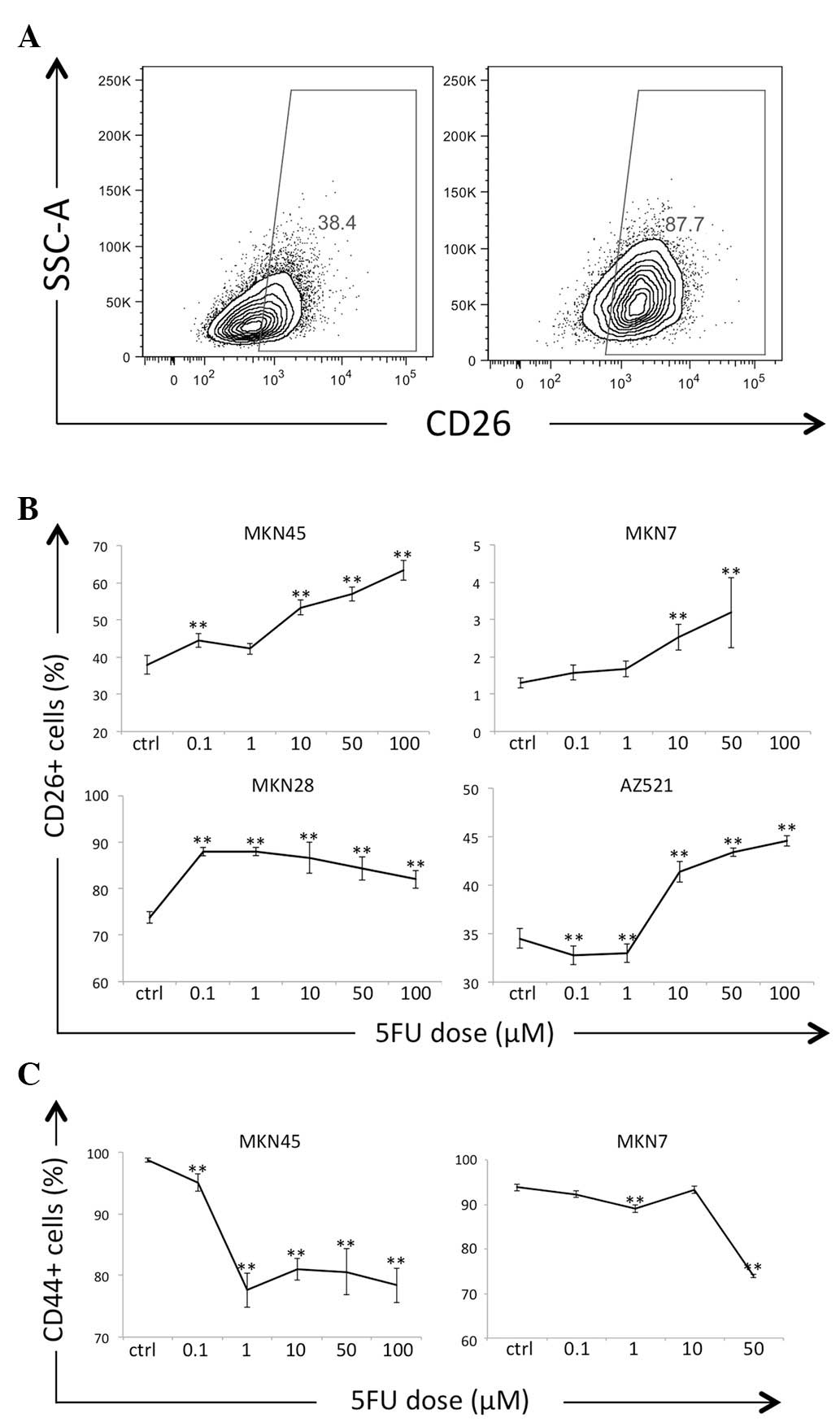
Chemoresistance is a clinically important feature of
CSCs. GC cells cultured in the presence of 5FU exhibited increased
frequencies (%) of CD26+ cells in a 5FU dose-dependent
manner (Fig. 4A and B). In contrast
to CD26, the frequencies of CD44+ cells decreased
following 5FU exposure (Fig. 4C). The
MKN28 and AZ521 cells were completely CD44− prior to and
following 5FU exposure, as determined via flow cytometry (data not
shown).
Discussion
Monoclonal antibodies have been used to identify and
characterize cell surface molecules, and similar techniques have
been utilized in various research fields, including immunology,
hematopoietic stem cell biology and cancer stem cell biology
(23). To date, >350 cell surface
molecules are numbered according to CD nomenclature; however, a
recent bioinformatics study reported that 3,702 transmembrane
proteins are expressed on the surfaces of human cells (24).
To the best of our knowledge, there have been no
comprehensive reports of cell surface molecule expression
evaluations in surgically resected primary GC using >242
molecules, as evaluated in the current study. Among the screened
surface markers in this study, the combination of CD26 and CD44 was
shown to be capable of dividing GC xenograft cells into three to
four subsets via flow cytometry (Fig. 1B
and C). In a xenotransplantation experiment, similar
tumorigenic efficiencies were demonstrated in the
CD26+CD44+ and
CD26+CD44− cells, in contrast to the low
tumorigenicity of the CD26−CD44− subset
(Fig. 3A and B). These data indicate
that the insufficiency of CD44 as a CSC marker may be improved by
the complementary use of CD26 as a co-marker.
As a result of the CSC hypothesis, cancer cells
within whole tumor tissues have been classified into two major
groups, the tumorigenic and non-tumorigenic CSCs (non-CSCs).
However, recently, tumorigenic cell heterogeneity with respect to
clonal dominancy and chemoresistance was identified using of clonal
tracking techniques based on lentiviral insertion sites (25,26). Kreso
et al (25) reported five
types of clones within colorectal cancer xenografts. In contrast to
actively proliferating clones, the slow-growing cell types
supposedly became dominant following chemotherapy (25). In the present study,
CD26+CD44+ cells formed relatively larger
tumors when compared with CD26+CD44− cells
(Fig. 3A). This observation indicates
that CD26+CD44+ cells underwent rapid
proliferation, unlike the CD26+CD44− cells.
However, following 5FU exposure, the frequencies of
CD44+ cells decreased significantly in vitro,
whereas the frequencies of CD26+ cells increased
(Fig. 4). These results indicate that
although CD26+CD44+ cells and
CD26+CD44− cells may initiate tumor growth
and regenerate histologically and phenotypically similar tumors
(Fig. 3B and C), the cell subsets may
differ with respect to proliferative activity and chemotherapeutic
sensitivity. Consequently, the role of CSC in therapeutic
resistance remains clinically important. The identification of a
therapy-resistant CSC subset may accelerate the process of CSC
hypothesis-based novel therapy development.
Acknowledgements
The authors would like to thank Miyuki Ozaki and
Yuko Noguchi for their technical support. The current study was
partly supported by a Core Research Grant-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science
and Technology in Japan (to M.K., H.I. and M.M.; grant nos.;
26670604, 23390199 and 21229015); a Grant-in-Aid from the Third
Term Comprehensive 10-year Strategy for Cancer Control of the
Ministry of Health, Labor, and Welfare in Japan (to H.I. and M.M.;
grant no. H26-018); a grant from the Kobayashi Cancer Research
Foundation (to H.I.; grant no. H24); a grant from the Princess
Takamatsu Cancer Research Fund, Japan (to H.I.; grant no. H22); and
a grant from the SENSHIN Medical Research Foundation (to H.I.;
grant no. H25).
The authors received partial support from Chugai
Pharmaceutical Co., Ltd. (Tokyo, Japan), Yakult Honsha Co., Ltd.
(Tokyo, Japan), and Takeda Pharmaceutical Co., Ltd (Osaka,
Japan).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): a phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumor initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumor
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haraguchi N, Ishii H, Mimori K, et al:
CD13 is a therapeutic target in human liver cancer stem cells. J
Clin Invest. 120:3326–3339. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Brien CA, Kreso A and Jamieson CH:
Cancer stem cells and self-renewal. Clin Cancer Res. 16:3113–3120.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medema JP: Cancer stem cells: the
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukuda K, Saikawa Y, Ohashi M, et al:
Tumor initiating potential of side population cells in human
gastric cancer. Int J Oncol. 34:1201–1207. 2009.PubMed/NCBI
|
|
16
|
Han ME, Jeon TY, Hwang SH, et al: Cancer
spheres from gastric cancer patients provide an ideal model system
for cancer stem cell research. Cell Mol Life Sci. 68:3589–3605.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Li C, He F, Cai Y and Yang H:
Identification of CD44+CD24+ gastric cancer
stem cells. J Cancer Res Clin Oncol. 137:1679–1686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang J, Zhang Y, Chuai S, et al:
Trastuzumab (herceptin) targets gastric cancer stem cells
characterized by CD90 phenotype. Oncogene. 31:671–682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katsuno Y, Ehata S, Yashiro M, Yanagihara
K, Hirakawa K and Miyazono K: Coordinated expression of REG4 and
aldehyde dehydrogenase 1 regulating tumourigenic capacity of
diffuse-type gastric carcinoma-initiating cells is inhibited by
TGF-β. J Pathol. 228:391–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishikawa S, Konno M, Hamabe A, et al:
Aldehyde dehydrogenase high gastric cancer stem cells are resistant
to chemotherapy. Int J Oncol. 42:1437–1442. 2013.PubMed/NCBI
|
|
21
|
Rocco A, Liguori E, Pirozzi G, et al:
CD133 and CD44 cell surface markers do not identify cancer stem
cells in primary human gastric tumors. J Cell Physiol.
227:2686–2693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zola H: Medical applications of leukocyte
surface molecules - the CD molecules. Mol Med. 12:312–316.
2006.PubMed/NCBI
|
|
24
|
da Cunha JP, Galante PA, de Souza JE, et
al: Bioinformatics construction of the human cell surfaceome. Proc
Natl Acad Sci USA. 106:16752–16757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kreso A, O'Brien CA, van Galen P, et al:
Variable clonal repopulation dynamics influence chemotherapy
response in colorectal cancer. Science. 339:543–548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dieter SM, Ball CR, Hoffmann CM, et al:
Distinct types of tumor-initiating cells form human colon cancer
tumors and metastases. Cell Stem Cell. 9:357–365. 2011. View Article : Google Scholar : PubMed/NCBI
|