Introduction
Ovarian cancer has one of the highest mortality
rates among all gynecological malignancies (1). Paclitaxel plus platinum therapy is
rapidly gaining acceptance as the standard clinical chemotherapy
regimen for ovarian cancer, and is highly effective as a first-line
therapy for patients with advanced ovarian malignancies. However,
the majority of patients still experience relapse within a short
time following chemotherapeutic intervention (2,3).
The failure of chemotherapy may be associated with
the heterogeneity of tumor tissues, one of the primary
characteristics of malignant tumors. Previous studies have
demonstrated that tumors originate from cancer stem cells (4). However, the continuous differentiation
of stem cells during the process of tumor growth results in
daughter cells with variations in their genetic and molecular
characteristics following several rounds of proliferation, leading
to differences in tumor growth, invasiveness, drug susceptibility
and prognosis (5). Variation in the
genotype or subtype of cells among patients results in ovarian
cancer heterogeneity, which affects the efficacy of chemotherapy
agents (6,7).
Evaluating the heterogeneity of tumor tissues is
essential for predicting the chemosensitivity of tumors prior to
beginning a chemotherapy regimen. The adenosine triphosphate-tumor
chemosensitivity assay (ATP-TCA) is an in vitro drug
sensitivity testing method that has become widely used for
determining the drug sensitivity rates of solid tumors in recent
years (8–10). This method has notable advantages for
guiding individual treatments and chemotherapy protocol design, and
evaluating novel chemotherapy drugs. In particular, ATP-TCA has
been used for >20 years in research on ovarian cancers. Sevin
et al (11) first used ATP-TCA
to evaluate the chemosensitivity of ovarian cancer cell lines and
tissues in 1988, obtaining high correlations between in
vitro drug sensitivity and in vivo patient response.
Since then, a number of studies have analyzed the high sensitivity,
specificity and clinical relevance of ATP-TCA in ovarian cancers
(12–14). In recurrent ovarian cancer, ATP-TCA
could be used to guide the selection of chemotherapy drugs and
potentially improve the clinical response rate and survival of
patients (15,16).
When using the ATP-TCA protocol, it is necessary to
ensure an optimized method of calculation for interpreting the
results. In previous studies, the following parameters were
determined by analyzing the correlation between doses of various
drugs and the rate of inhibition: Calculation of the area under the
chemotherapy drug dose-inhibition curve (AUC) using the trapezoidal
rule, comparison of drug concentrations that achieve either 50 or
90% growth inhibition in vitro (IC50 and
IC90, respectively), and the sensitivity index (SI),
which is calculated by adding the percentage of tumor growth
inhibition (TGI) at each concentration tested (17). Konecny et al (12) analyzed three ATP-TCA parameters and
observed that the SI was superior to the AUC or IC50 for
the interpretation of test results. Several studies suggested that
an SI of >250 was the best standard to predict chemoresistance
(13,14). The present study therefore selected
250 as the cut-off point of SI and further proved this
hypothesis.
In the current study, the ATP-TCA method was used to
assess the heterogeneity of chemosensitivity in ovarian epithelial
cancer (OEC), and the correlation between the clinical features of
tumors and chemosensitivity was analyzed to gain further insight
into the heterogeneity of OECs and provide an additional rationale
for the use of ATP-TCA technology in guiding clinical treatment to
potentially improve patient outcomes.
Materials and methods
Ethics statement
The study was approved by the Ethics Committee of
the Beijing Shijitan Hospital of Capital Medical University
(Beijing, China). Written informed consent was obtained from all
the patients and families prior to surgery. All procedures were
conducted in accordance with the Code of Ethics of the World
Medical Association (Declaration of Helsinki, 1964, as revised in
2004).
Tumor specimens
A total of 80 fresh tumor specimens were obtained
from patients who had OEC and underwent surgery at the Beijing
Shijitan Hospital, Beijing University People's Hospital and
People's Liberation Army General Hospital, China, between April
2012 and February 2013. Routine histopathological analysis was
performed for samples obtained from the same tissues to determine
the stage and histological features of the tumor samples
simultaneously with ATP-TCA testing. ATP-TCA was performed as a
routine procedure immediately following surgery. Viable ovarian
cancer cells obtained from malignant tissues were tested for their
sensitivity to paclitaxel (PTX; Corden Pharma Latina S.p.A.,
Sermoneta, Italy), carboplatin (CBP; Corden Pharma Latina S.p.A.),
topotecan (TPT; Takara Biotechnology Co., Ltd., Dalian, China),
gemcitabine (GEM; Eli Lilly and Company, Indianapolis, IN, USA),
docetaxel (TXT; Aventis Pharma Ltd., Dagenham, UK), bleomycin (BLM;
Nippon Kayaku Co. Ltd., Tokyo, Japan), etoposide (VP-16; Jiangsu
Hengrui Medicine Co., Ltd., Jiangsu, China) and
4-hydroperoxycyclophosphamide (4-HC; Toronto Research Chemicals,
Toronto, ON, Canada) using an in vitro ATP-TCA
procedure.
In vitro ATP-TCA
Chemosensitivity was assessed in OEC tumor tissue
samples using an ATP-TCA kit (Huzhou Haichuang Biotech Co., Ltd.,
Huzhou, Zhejiang, China), containing serum-free complete assay
medium (CAM), digestive enzymes and luciferin-luciferase reagent.
ATP-TCA tests were performed according to previously described
methods (18,19). Specimens (1 cm3) from solid
tumors were obtained during surgery and cut into smaller fragments
(1 mm3), which were then dissociated to prepare
suspensions of single cells by incubation in 5–10 ml sterile
digestive enzyme reagent for 2–3 h at 37°C in a 5% CO2
incubator. Subsequent to adjusting the concentration of the cell
suspension to 2–4×105/ml, 100-µl cell suspensions were
added to each well of a 96-well polypropylene microplate. Single
agents were tested at five different doses (12.5, 25, 50, 100 and
200%) of a standard test drug concentration (TDC). The TDC values
were 13.8 µg/ml for PTX, 25 µg/ml for CBP, 0.14 µg/ml for TPT, 25
µg/ml for GEM, 10 µg/ml for TXT, 3 µg/ml for 4-HC, 0.6 µg/ml for
BLM and 20 µg/ml for VP-16. For each concentration, two wells were
used as controls, one containing 100 µl ATP inhibitor for maximum
inhibition (positive control) and the other containing CAM only (no
drug, negative control). Plates were incubated for 5–6 days at 37°C
with 95% humidity in a 5% CO2 incubator. Following
incubation, the cells were lysed by the addition of 50 µl ATP
extraction reagent, and 50 µl luciferin-luciferase reagent was
added to each well. Measurements of luminescence were recorded
using a microplate luminometer (Orion II; Berthold Diagnostic
Systems, Bad Wildbad, Germany), and inhibition curves were
established.
Data analysis
Data were exported to an Excel spreadsheet (2010;
Microsoft, Redmond, WA, USA), and the results were interpreted and
compared using the parameters IC50, IC90 and
SI (SI = 500 - sum of % TGI at 200, 100, 50, 25 and 12.5% TDC).
Three categories of in vitro sensitivity were defined as
follows: Sensitivity (S), IC90≤100% TDC and
IC50<25% TDC; weak sensitivity (WS),
IC90≤100% TDC and IC50<25% TDC or SI≤250;
and resistance (R), SI>250. According to the current clinical
criteria with regard to platinum resistance and sensitivity,
clinical CBP-sensitive patients indicates those patients who
achieved complete remission and experienced relapse six months or
later following initial platinum-containing chemotherapy, whereas
CBP-resistant patients are those who demonstrated recurrence within
<6 months (20).
Quality controls for each assay were conducted as
follows: The variability of individual ATP values was controlled by
measuring each drug-treated sample twice. Samples with a
coefficient of variation (CV)>0.15 were rejected and retested.
In the present study, the average CV was 0.065 (range,
0.024–0.127).
Statistical analysis was performed using SPSS
software (version 17.0 for Windows; SPSS, Inc., Chicago, IL, USA).
Data are presented as the mean ± standard deviation, and
comparisons were made using Student's t-test, the χ2
test and an analysis of variance. The correlation analysis was
performed using Spearman's rank correlation test. P<0.05 was
considered to indicate a statistically significant difference.
Results
In vitro results
The median age of the patients was 56.15 years
(range, 23–79 years). All the specimens collected produced
evaluable results (100%), and the tumor characteristics of the
samples were analyzed (Table I). The
results revealed considerable heterogeneity in chemosensitivity
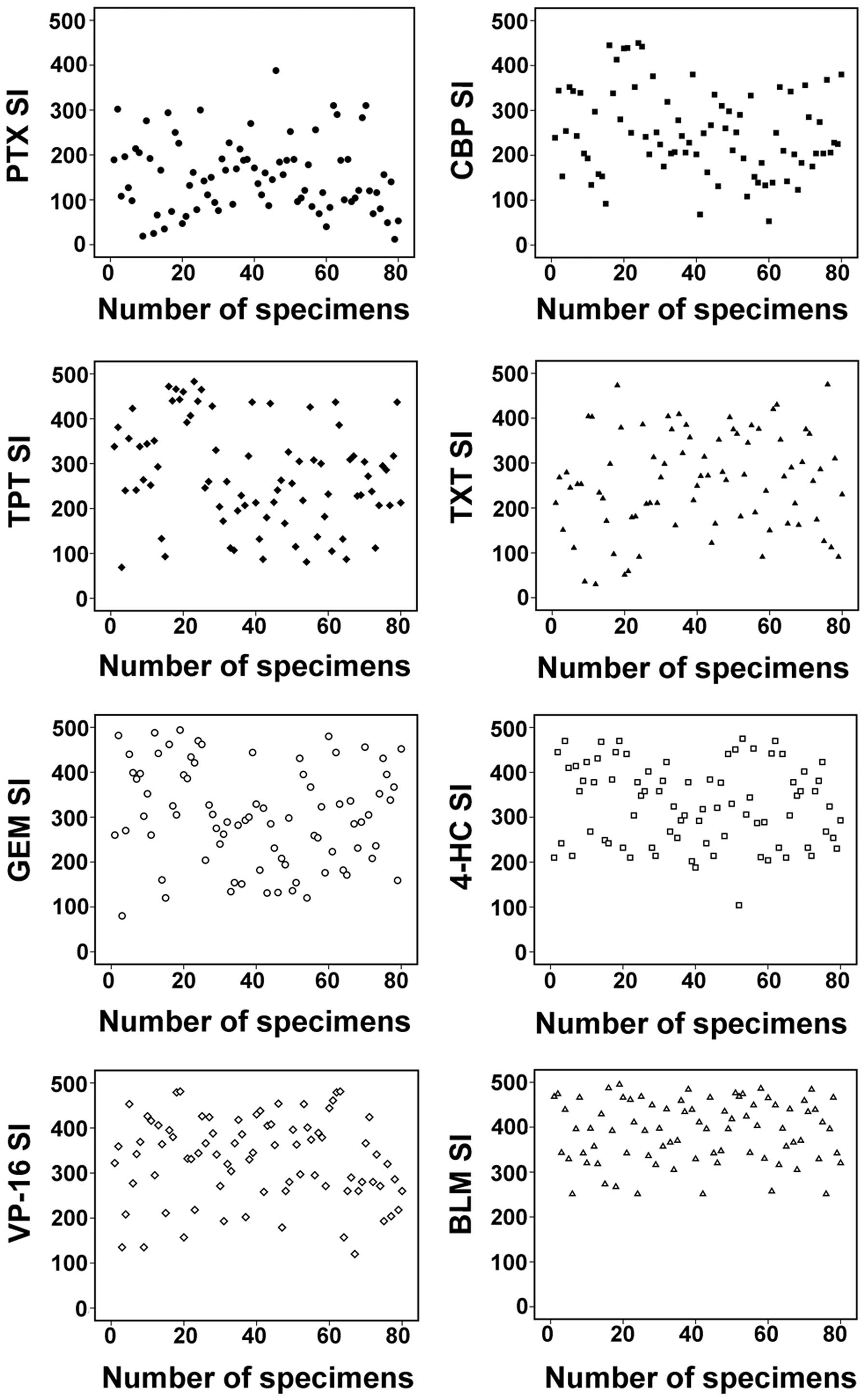
among the tumor samples tested. There were significant differences
between the mean SIs obtained using various agents (Fig. 1A). The agent with the lowest SI values
was PTX (mean SI, 152.79), followed by CBP (mean SI, 250.69), TXT
(mean SI, 258.34) and TPT (mean SI, 275.29). The agent with the
highest SIs was BLM (mean SI, 390.04).
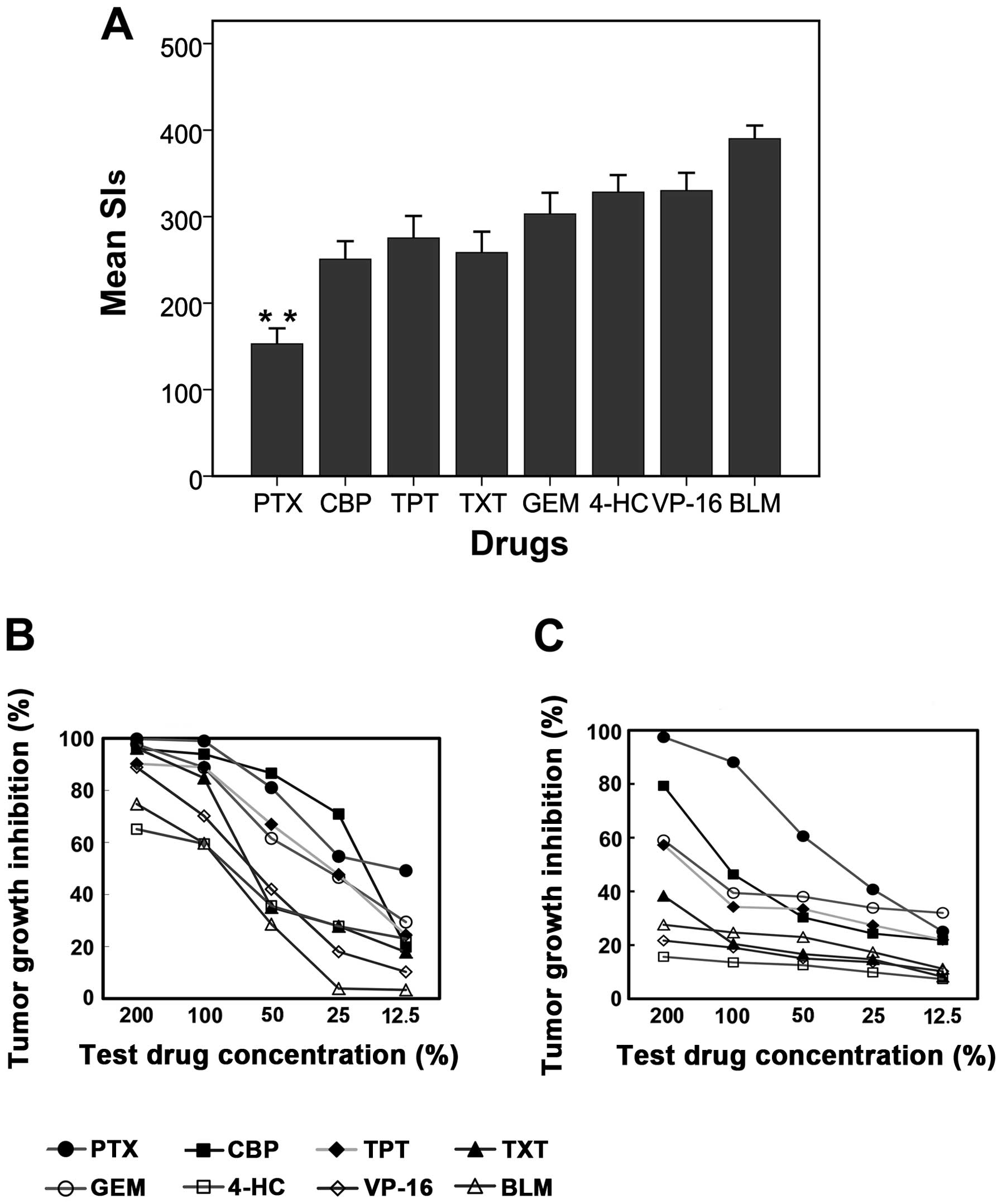 | Figure 1.Heterogeneity in chemosensitivity
among the ovarian epithelial cancer specimens tested. (A) Frequency
histograms (mean+95% confidence interval) demonstrating substantial
heterogeneity of the sensitivity indices (SIs) among the
chemotherapy agents tested; (B and C) tumor growth inhibition
curves from two ovarian tumor specimens of the same stage, and with
the same histological features and grade of differentiation,
demonstrating considerable heterogeneity in sensitivity to the same
chemotherapy agents. The X-axis is the test drug concentration
percentage, and the Y-axis is the tumor growth inhibition
percentage. (B) The results of a specimen with higher
chemosensitivity and (C) the results of a specimen with lower
chemosensitivity. PTX, paclitaxel; CBP, carboplatin; TPT,
topotecan; TXT, docetaxel; GEM, gemcitabine; 4-HC,
4-hydroperoxycyclophosphamide; VP-16, etoposide; BLM, bleomycin.
**P<0.01 vs. CBP, TXT, TPT, GEM, 4-HC, VP-16 or BLM. |
 | Table I.Characteristics of tumor samples
(n=80). |
Table I.
Characteristics of tumor samples
(n=80).
| Characteristic | n (%) |
|---|
| Histology |
|
|
Serous | 51 (63.8) |
|
Mucinous | 4 (5.0) |
| Clear
cell | 13 (16.2) |
|
Endometrioid | 8 (10.0) |
|
Transitional cell | 4 (5.0) |
| FIGO stage |
|
| I | 8 (10.0) |
| II | 9 (11.2) |
| III | 63 (78.8) |
| Grade of
differentiation |
|
| High | 6 (7.5) |
| Mild | 10 (12.5) |
| Low | 64 (80.0) |
| Primary | 53 (66.2) |
| Recurrent | 27 (33.8) |
The differences in SIs were observed between
specimens of the same stage, and with the same histological
features and grade of differentiation (Fig. 1B and C). There were also distinct
differences among the SIs of the 80 OEC specimens obtained using
the same drugs (Fig. 2). Overall, the
sensitivity of the tumors to the agents tested was in the following
order: PTX > CBP > TPT > TXT > GEM > 4-HC > VP-16
> BLM (Table II).
 | Table II.Results of chemosensitivity assays in
ovarian epithelial cancer samples. |
Table II.
Results of chemosensitivity assays in
ovarian epithelial cancer samples.
|
| No. (%) |
|
|---|
|
|
|
|
|---|
| Drug | S | WS | R | Sensitivity
assessed, % |
|---|
| PTX | 44 (55.0) | 22 (27.5) | 14 (17.5) | 82.5 |
| CBP | 21 (26.4) | 26 (32.4) | 33 (41.2) | 58.8 |
| TPT | 14 (17.5) | 23 (28.7) | 43 (53.8) | 46.2 |
| TXT | 18 (22.5) | 18 (22.5) | 44 (55.0) | 45.0 |
| GEM | 13 (16.3) | 13 (16.3) | 54 (67.5) | 32.5 |
| 4-HC | 3 (3.7) | 18 (22.5) | 59 (73.8) | 26.2 |
| VP-16 | 4 (6.3) | 10 (11.2) | 66 (82.5) | 17.5 |
| BLM | 0 (0.0) | 0 (0.0) | 80 (100.0) | 0.0 |
In samples from recurrent ovarian cancer, the
sensitivity rates for PTX and CBP were 85.7% (24/28) and 60.7%
(17/28), respectively. Analysis of the clinical data revealed that
all 17 recurrent specimens sensitive to CBP in vitro
experienced a relapse at six months or later following initial
platinum-containing chemotherapy and achieved complete remission,
whereas the 11 recurrent specimens resistant to CBP in vitro
experienced recurrence within the first six months following
initial treatment.
Correlation of ATP-TCA with clinical
sensitivity/resistance results
All cases were followed up for at least six months
following initial chemotherapy. A total of 44 patients (55%) were
classified as clinical CBP-sensitive and 36 patients (45%) as
clinical CBP-resistant. The assay demonstrated a sensitivity,
specificity, positive predictive value (PPV) and negative
predictive value (NPV) of 88.6, 77.8, 83 and 84.8%, respectively,
at SI 250. The sensitivity, specificity, PPV and NPV at SI 200 and
SI 300 were also tested, and the results revealed a distinct
advantage when choosing 250 as the cut-off point of SI (Table III).
 | Table III.Sensitivity, specificity, PPV and NPV
at different SI cut-off points. |
Table III.
Sensitivity, specificity, PPV and NPV
at different SI cut-off points.
| SI cut-off
point | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|
| 250 | 88.6 | 77.8 | 83.0 | 84.8 |
| 300 | 88.6 | 50.0 | 68.0 | 78.0 |
| 200 | 43.0 | 91.6 | 86.0 | 56.9 |
Correlation analysis between pairs of
chemotherapy drugs tested
The correlation analysis among the SIs of all eight
drugs was performed using Spearman's rank correlation test. The
results revealed positive correlations between several drugs. There
were significant correlations between PTX and TXT (P<0.001) and
among CBP, TPT and GEM (P<0.001) (Table IV).
 | Table IV.Results of correlation analysis
between pairs of chemotherapy drugs tested. |
Table IV.
Results of correlation analysis
between pairs of chemotherapy drugs tested.
| Drug | Correlation
coefficient, r | P-value |
|---|
| PTX and TXT | 0.701 | 0.000a |
| CBP and TPT | 0.686 | 0.000a |
| TPT and GEM | 0.660 | 0.000a |
| CBP and GEM | 0.563 | 0.000a |
| TXT and VP16 | 0.422 | 0.000a |
Correlation between the ATP-TCA
results and clinical indicators of tumor samples
An association analysis between the ATP-TCA results
and the stage or differentiation grade of samples was performed
using the χ2 test. The results revealed that early-stage
(I/II) or high- to mildly-differentiated OEC specimens had lower
chemosensitivity to PTX or CBP than advanced-stage (III) or
low-differentiated specimens, respectively (Table V). The SIs with different stages or
differentiation grades of samples also demonstrated distinct
differences (Fig. 3).
 | Table V.Correlation between ATP-TCA results
for PTX or CBP and the stage or grade of differentiation of tumor
samples. |
Table V.
Correlation between ATP-TCA results
for PTX or CBP and the stage or grade of differentiation of tumor
samples.
|
| FIGO stage | Grade of
differentiation |
|---|
|
|
|
|
|---|
| Drug | I/II, n | III, n | P-value | High-mild, n | Low, n | P-value |
|---|
| PTX |
|
|
S+WS | 9 | 57 | 0.001a | 8 | 58 | 0.001a |
| R | 8 | 6 |
| 8 | 6 |
|
| CBP |
|
|
S+WS | 5 | 42 | 0.007a | 6 | 41 | 0.05b |
| R | 12 | 21 |
| 10 | 23 |
|
Discussion
In the present study, the heterogeneity in OECs was
evaluated using an in vitro ATP-TCA, which has been widely
used for determining the drug sensitivity rates of solid tumors,
and reliable results were obtained. To ensure the accuracy of the
results, the reasonable SI cut-off point was determined by
comparing the in vitro drug sensitivity and clinical
sensitivity at various SI values. It was observed that the
sensitivity, specificity, PPV and NPV of ATP-TCA were better at SI
250, which was consistent with previous studies (12–14). The
results indicated that ATP-TCA is a reliable in vitro drug
sensitivity testing method and that the suitable cut-off point of
SI is 250.
The present results demonstrated that there was
considerable heterogeneity in chemosensitivity between the OEC
samples tested, even between specimens of the same pathological
type, clinical stage and tumor classification, which may be the
basis for examining the mechanism of chemotherapy resistance in
ovarian cancer. The present study investigated the differentiated
tumor response to eight chemotherapeutic drugs when dividing the
OEC specimens into recurrent or primary, early-stage (I/II) or
advanced-stage (III), high- to mildly-differentiated or
low-differentiated cases. PTX demonstrated the highest sensitivity
of all agents tested (82.5% in all specimens, 85.7% in recurrent
specimens), followed by CBP (58.8 and 60.7%, respectively), then
TPT, TXT, GEM, 4-HC and VP-16. All specimens were resistant to
BLM.
Analysis of the clinical data of recurrent OEC
specimens revealed the correlation of chemotherapy resistance and
recurrence interval following initial chemotherapy. All the
clinical platinum-sensitive recurrent specimens were also sensitive
to CBP in vitro, whereas the clinical platinum-resistant
recurrent specimens were resistant to CBP in vitro, and the
majority of the recurrent specimens were sensitive to PTX in
vitro, which is consistent with current clinical criteria with
regard to recurrent ovarian cancers. The 2014 National
Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines
for ovarian cancer state that combination platinum-based
chemotherapy is the preferred treatment following the first
recurrence in platinum-sensitive patients (20–22). The
guidelines also indicate that altering the schedule of PTX
administration may produce secondary responses to treat
platinum-resistant ovarian cancer, such as weekly single-agent PTX
administration (80 mg/m2/week) or combined treatment
with CBP (AUC 3/week) and PTX (70 mg/m2/week) (23,24). In
2006, Kyrgiou et al (25) used
multiple-treatment meta-analysis methodologies to analyze 198
clinical trials from the previous 40 years, and observed that CBP
and PTX are not only the best first-line chemotherapy agents, but
that they are also the best option as second-line drugs. The
present results revealed the relatively high efficiency of PTX and
CBP in recurrent ovarian cancers, particularly PTX, indicating the
potential effectiveness of PTX retreatment in recurrent ovarian
cancer. A highly significant correlation was also identified
between PTX and TXT sensitivity, in addition to correlations
between CBP, TPT and GEM treatments. These results highlight the
significance of considering potential correlations among drug
sensitivities to avoid potentially ineffective therapy and
additional adverse effects when selecting appropriate drug
regimens.
Prognoses of early-stage ovarian cancer are
generally good, but responses to chemotherapy vary considerably in
advanced-stage disease. According to NCCN guidelines,
post-operative chemotherapy is not recommended for ovarian cancer
patients with stage IA or IB, grade 1 disease. These patients
demonstrate good survival rates with surgical treatment alone,
whereas adjuvant chemotherapy is recommended for all other
patients. Several studies have identified age, capsular rupture,
histological subtype, stage, grade and positive cytology results as
prognostic factors for recurrence and survival in early-stage
ovarian cancers (26–30), and other studies have reported
improved survival for adjuvant treatment in early-stage patients
with high-risk factors (31). Swart
et al (32) analyzed long-term
follow-up data of patients with early-stage ovarian cancer and
reported a significant benefit of adjuvant chemotherapy. In the
adjuvant chemotherapy group, the 10-year absolute recurrence-free
and overall survival rates demonstrated improvement compared with
those for patients receiving no post-surgical chemotherapy (from 57
to 67% and from 64 to 72%, respectively). However, Swart et
al (32) also indicated that
adjuvant chemotherapy reduced the risk of recurrence/mortality or
mortality alone in high-risk patients, but not in the low-risk
group. Chan et al (33)
analyzed 74 patients with recurrent early-stage, high-risk ovarian
cancer (stages IA-IB, grade 3; stages IC and II, all grades; and
clear cell carcinomas stages I-II), and observed that survival
following recurrence was poor and comparable with that of patients
with recurrent advanced-stage disease; the study recommended
developing novel therapeutic modalities for these high-risk
patients.
In the present study, it was observed that
sensitivity to PTX or CBP in early-stage, high- to
mildly-differentiated ovarian cancer specimens was remarkably lower
than that of advanced stage or low-differentiated specimens. This
finding was consistent with previous studies and suggests the poor
effectiveness of chemotherapy following early-stage ovarian cancer
recurrence. However, due to the limitations of the number of cases,
further research to determine the characteristics associated with
the response of early-stage ovarian cancer to chemotherapy is
required. These results highlight the significance of developing
more comprehensive initial treatment programs for early-stage
ovarian cancer, particularly in patients with novel high-risk
factors associated with early-onset disease, including the presence
of BRCA1 and BRCA2 mutations or in families affected
by Lynch syndrome (34,35). The use of in vitro sensitivity
testing methods may be an effective way to identify suitable agents
for treating high-risk, early-stage ovarian cancer patients.
Thus, the results of the present study demonstrated
the notable heterogeneity in chemosensitivity among specimens from
ovarian cancer patients. This heterogeneity was to be associated
with differing gene expression among individuals (6). Further studies are required to analyze
differences in gene expression in ovarian cancer specimens and
explore the mechanisms underlying chemotherapy resistance. ATP-TCA
should be considered as an effective method for guiding the choice
of chemotherapy drugs, avoiding ineffective treatment regimens, and
investigating novel chemotherapy agents to improve patient
prognosis and increase ovarian cancer survival.
Acknowledgements
This study was supported by the Capital Health
Research and Development Projects of China (grant no.
2011-2008-05).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armstrong DK and Brady MF: Intraperitoneal
therapy for ovarian cancer: a treatment ready for prime time. J
Clin Oncol. 24:4531–4533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gore M, du Bois A and Vergote I:
Intraperitoneal chemotherapy in ovarian cancer remains
experimental. J Clin Oncol. 24:4528–4530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bao B, Ahmad A, Azmi AS, Ali S and Sarkar
FH: Overview of cancer stem cells (CSCs) and mechanisms of their
regulation: implications for cancer therapy. Curr Protoc Pharmacol.
14:Unit. 14–25. 2013.
|
|
5
|
Marusyk A and Polyak K: Tumor
heterogeneity: causes and consequences. Biochim Biophys Acta.
1805:105–117. 2010.PubMed/NCBI
|
|
6
|
Glaysher S, Gabriel FG, Johnson P, et al:
Molecular basis of chemosensitivity of platinum pre-treated ovarian
cancer to chemotherapy. Br J Cancer. 103:656–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meinhold-Heerlein I and Hauptmann S: The
heterogeneity of ovarian cancer. Arch Gynecol Obstet. 289:237–239.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glaysher S and Cree IA: Cell sensitivity
assays: the ATP-based tumor chemosensitivity assay. Methods Mol
Biol. 731:247–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurbacher CM and Cree IA: Chemosensitivity
testing using microplate adenosine triphosphate-based luminescence
measurements. Methods Mol Med. 110:101–120. 2005.PubMed/NCBI
|
|
10
|
Qi CJ, Ning YL, Zhu YL, et al: In vitro
chemosensitivity in breast cancer using ATP-tumor chemosensitivity
assay. Arch Pharm Res. 32:1737–1742. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sevin BU, Peng ZL, Perras JP, et al:
Application of an ATP-bioluminescence assay in human tumor
chemosensitivity testing. Gynecol Oncol. 31:191–204. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Konecny G, Crohns C, Pegram M, et al:
Correlation of drug response with the ATP tumor chemosensitivity
assay in primary FIGO stage III ovarian cancer. Gynecol Oncol.
77:258–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neubauer H, Stefanova M, Solomayer E, et
al: Predicting resistance to platinum-containing chemotherapy with
the ATP tumor chemosensitivity assay in primary ovarian cancer.
Anticancer Res. 28:949–955. 2008.PubMed/NCBI
|
|
14
|
Zhao D, Zhang W, Li XG, et al: Predicting
clinical chemo-sensitivity of primary ovarian cancer using
adenosine triphosphate-tumor chemosensitivity assay combined with
detection of drug resistance genes. Zhonghua Fu Chan Ke Za Zhi.
46:193–198. 2011.[(In Chinese)]. PubMed/NCBI
|
|
15
|
Cree IA, Kurbacher CM, Lamont A, et al: A
prospective randomized controlled trial of tumour chemosensitivity
assay directed chemotherapy versus physician's choice in patients
with recurrent platinum-resistant ovarian cancer. Anticancer Drugs.
18:1093–1101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma S, Neale MH, Di Nicolantonio F, et
al: Outcome of ATP-based tumor chemosensitivity assay directed
chemotherapy in heavily pre-treated recurrent ovarian carcinoma.
BMC Cancer. 3:192003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cree IA, Kurbacher CM, Untch M, et al:
Correlation of clinical response to chemotherapy in breast cancer
with ex vivo chemosensitivity. Anticancer Drugs. 7:630–635. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andreotti PE, Cree IA, Kurbacher CM, et
al: Chemosensitivity testing of human tumors using a microplate
adenosine triphosphate luminescence assay: clinical correlation for
cisplatin resistance of ovarian carcinoma. Cancer Res.
55:5276–5282. 1995.PubMed/NCBI
|
|
19
|
Ling ZQ, Qi CJ, Lu XX, et al:
Heterogeneity of chemosensitivity in esophageal cancer using
ATP-tumor chemosensitivity assay. Acta Pharmacol Sin. 33:401–406.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Comprehensive Cancer Network:
Ovarian cancer including fallopian tube cancer and primary
peritoneal cancer. Version 1. 2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#ovarianAccessed.
February 13–2015.
|
|
21
|
Fung-Kee-Fung M, Oliver T, Elit L, et al:
Optimal chemotherapy treatment for women with recurrent ovarian
cancer. Curr Oncol. 14:195–208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parmar MK, Ledermann JA, Colombo N, et al:
Paclitaxel plus platinum-based chemotherapy versus conventional
platinum-based chemotherapy in women with relapsed ovarian cancer:
the ICON4/AGO-OVAR-2.2 trial. Lancet. 361:2099–2106. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gynecologic Oncology Group; Markman M,
Blessing J, Rubin SC, Connor J, Hanjani P and Waggoner S: Phase II
trial of weekly paclitaxel (80 mg/m2) in platinum and
paclitaxel-resistant ovarian and primary peritoneal cancers: a
Gynecologic Oncology Group study. Gynecol Oncol. 101:436–440. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharma R, Graham J, Mitchell H, Brooks A,
Blagden S and Gabra H: Extended weekly dose-dense
paclitaxel/carboplatin is feasible and active in heavily
pre-treated platinum-resistant recurrent ovarian cancer. Br J
Cancer. 100:707–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kyrgiou M, Salanti G, Pavlidis N,
Paraskevaidis E and Ioannidis JP: Survival benefits with diverse
chemotherapy regimens for ovarian cancer: meta-analysis of multiple
treatments. J Natl Cancer Inst. 98:1655–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vergote I: Prognostic factors in stage I
ovarian carcinoma. Verh K Acad Geneeskd Belg. 63:257–271.
2001.PubMed/NCBI
|
|
27
|
Gadducci A, Cosio S, Zola P, Sostegni B,
Fuso L and Sartori E: Prognostic factors and clinical outcome of
patients with recurrent early-stage epithelial ovarian cancer: an
Italian multicenter retrospective study. Int J Gynecol Cancer.
23:461–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seidman JD, Yemelyanova AV, Khedmati F, et
al: Prognostic factors for stage I ovarian carcinoma. Int J Gynecol
Pathol. 29:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan JK, Tian C, Monk BJ, et al:
Prognostic factors for high-risk early-stage epithelial ovarian
cancer: a Gynecologic Oncology Group study. Cancer. 112:2202–2210.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tropé C, Kaern J, Hogberg T, et al:
Randomized study on adjuvant chemotherapy in stage I high-risk
ovarian cancer with evaluation of DNA-ploidy as prognostic
instrument. Ann Oncol. 11:281–288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bertelsen K, Hølund B, Andersen JE,
Nielsen K, Strøyer I and Ladehoff P: Prognostic factors and
adjuvant treatment in early epithelial ovarian cancer. Int J
Gynecol Cancer. 3:211–218. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Swart AC: on behalf of ICON collaborators:
Long-term follow-up of women enrolled in a randomized trial of
adjuvant chemotherapy for early stage ovarian cancer (ICON1). J
Clin Oncol, ASCO Annual Meeting Proceedings Part I 25 (June 20
Supplement). 55092007.
|
|
33
|
Chan JK, Tian C, Teoh D, et al: Survival
after recurrence is poor for women with early-stage high-risk
ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol.
116:307–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sinilnikova OM, Mazoyer S, Bonnardel C, et
al: BRCA1 and BRCA2 mutations in breast and ovarian cancer
syndrome: reflection on the Creighton University historical series
of high risk families. Fam Cancer. 5:15–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Daly MB, Axilbund JE, Buys S, et al:
Genetic/familial high-risk assessment: breast and ovarian. J Natl
Compr Canc Netw. 8:562–594. 2010.PubMed/NCBI
|