Introduction
Neuroblastoma accounts for ≤10% of childhood
malignancies and ∼5% of all pediatric oncology-associated mortality
(1). It is the most common
extra-cranial solid tumor in patients ≤15 years of age and the most
frequently diagnosed type of cancer during infancy (2). Neuroblastoma is a disease that develops
in the sympathetic nervous system, and the majority of cases arise
in the paravertebral sympathetic ganglia and the adrenal medulla
(2), which are derived from trunk
neural crest cells (3,4). During embryonic development, neural
crest cells originate from the ectodermal part of the embryo that
arises from the dorsal region of the neural tube, migrate along a
ventral route and aggregate at the dorsal aorta to form the primary
sympathetic chain (3–5). A group of cells in the primary
sympathetic chain migrates in a dorsal direction to give rise to
the paravertebral sympathetic ganglia, which constitutes the
secondary (definitive) sympathetic chains, while another group of
cells migrate into the adrenal gland to develop into the adrenal
medulla (4,6).
Histologically, neuroblastomas form a heterogeneous
group of tumors, ranging from tumors containing undifferentiated or
poorly differentiated neuroblasts to those with fully
differentiated sympathetic neurons (2,7,8). This observation indicates that
neuroblastoma occurrence may be the result of deregulation of
sympathetic neurogenesis. The molecular mechanism of sympathetic
neurogenesis regulation has been relatively well-characterized
(9). A number of bone morphogenetic
proteins (BMPs) are released by the dorsal aorta during early
neural crest development. This BMP signaling results in upregulated
expression of various transcription factors that are essential for
neuronal differentiation (10,11).
Initially, the sympathetic neural crest cells express the
pro-neural molecules mammalian achaete-scute homolog 1 (Mash-1) and
paired-like homeobox 2b (Phox2B), which is one of the paired
homeodomain factors. The activation of Mash1 and Phox2B results in
upregulated expression of other transcription factors, including
Hand-2, Phox2A, and the zinc-finger proteins GATA2 and GATA3, to
promote further neuronal differentiation (12–18). These
transcription factors collaborate in a complex regulatory network
to induce tyrosine hydroxylase (TH) and dopamine β-hydroxylase
(DBH) expression and result in pan-neuronal and subsequent
catecholaminergic differentiation into sympathetic ganglia
(19). A critical question is at what
point neuroblastomas arise during sympathetic neurogenesis. It is
generally considered that neuroblastomas develop from primitive
sympathetic progenitor cells (2,20), but
knowledge of their identity remains limited. Identification of the
cells that develop into neuroblastomas will increase the
understanding of the cellular mechanism of tumorigenesis and
provide a potential therapeutic target.
The genomic amplification of v-myc avian
myelocytomatosis viral oncogene neuroblastoma derived homolog
(MYCN) is the most common genetic aberration that is associated
with poor outcome in neuroblastoma (21,22). MYCN
amplification occurs in ∼20% of primary neuroblastoma cases and is
strongly correlated with advanced-stage disease and failed
treatment (23). Crucially,
transgenic mice carrying human MYCN under control of the TH
promoter develop neuroblastomas with high resemblance to human
tumors (24), suggesting that
aberrant activation of MYCN can drive neuroblastoma development. It
has been reported that the TH-MYCN mice develop tumors that closely
resemble the human disease, including tumor site, morphology and
further genomic alterations (25). In
the present study, a detailed examination of hyperplastic lesions
and primary tumors in TH-MYCN mice was performed and the
correlation between the pro-neural gene Phox2B and neuroblastoma
progression was investigated. The results of the present study
using TH-MYCN mice may elucidate the cellular basis of
neuroblastoma initiation, progression and pathogenesis.
Materials and methods
Mice
Forty male MYCN-transgenic mice [129X1/SvJ-Tg
(TH-MYCN) 41Waw] and forty of their wildtype 129X1/SvJ littermates
(age, 2 weeks; weight, 7–9 g) were purchased from the National
Cancer Institute Mouse Repository (Frederick, MD, USA). All
pathology experiments were performed in the MYCN-transgenic mice
(TH-MYCN) and their wild-type littermates. The eighteen female
NOD/SCID (age, 4 weeks; weight, 15–18 g) mice used in xenograft
assay were obtained from the Jackson Laboratory (Ben Harbor, ME,
USA). Animals had access to commercial pellet feed and water ad
libitum, and were kept in a temperature-controlled room (22°C)
and maintained under specific pathogen-free conditions in the
animal facility of Southwest University (Chongqing, China). All
animal experiments were pre-approved by the Institutional Animal
Care and Use Committee of Southwest University.
Cell culture
Five human neuroblastoma cell lines were obtained
from the American Type Culture Collection (Manassas, VA, USA).
SK-N-DZ, SK-N-AS and SH-EP1 were maintained in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), plus 1% penicillin and streptomycin (P/S). BE(2)-C and
IMR-32 were cultured in a 1:1 mixture of DMEM:F12 (F12/DMEM)
supplemented with 10% FBS and 0.1 mM non-essential amino acids plus
1% P/S. The lentiviral packaging cell line 293FT was cultured in
DMEM containing 10% FBS, 1% P/S, 0.5 mg/ml G418, 4 mM L-glutamine,
0.1 mM MEM non-essential amino acids and 1 mM MEM sodium pyruvate.
The 293FT cell line, growth media, FBS and supplemental reagents
were obtained from Invitrogen Life Technologies (Carlsbad, CA,
USA). All cells were cultured at 37°C in a 5% CO2
humidified incubator.
Lentiviral constructs and
infection
The lentiviral constructs pLK0.1-puro-GFP and
pLK0.1-puro-MYCN were used in overexpression studies. For
downregulation of MYCN and Phox2B, the lentiviral constructs
pLK0.1-puro-GFPsi, pLK0.1-puro-MYCNsi and
pLK0.1-puro-Phox2Bsi(#1/#2) were obtained from Sigma-Aldrich (St.
Louis, MO, USA). The lentiviral constructs were transfected into
293FT packaging cells using Lipofectamine 2000 reagent (Invitrogen
Life Technologies). Virus-containing supernatants were harvested
and titered, and then were used to infect target cells with 4 µg/ml
polybrene (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). One
day subsequent to the final round of infection, the cells were
cultured in the presence of 2 µg/ml puromycin (Invitrogen Life
Technologies) for 3 days and the drug-resistant cells were
pooled.
Histology
For each genotype, 20 mice were sacrificed during
the first 3 weeks after birth. The sympathetic ganglia were
collected, fixed in 10% formalin (Sigma-Aldrich), then embedded in
paraffin blocks (Sigma-Aldrich), together with the superior
cervical ganglia (SCG) and the celiac ganglia. The
paraffin-embedded samples were sectioned at 4 µm and stained with
hematoxylin and eosin (H&E; Sigma-Aldrich; hematoxylin, 8 min;
eosin, 5 min). Each ganglion was examined for the presence of
hyperplastic lesions, which were defined as clusters of ≥30 small
blue round cells. TH-MYCN (n=20) samples and wild-type control
(n=20) were examined for the presence of tumors. For histological
examination, the tumors were removed and stained with H&E.
Immunohistochemistry
The sections were deparaffinized and rehydrated,
then treated with 10 mmol/l citrate buffer (pH 6.0; Sigma-Aldrich)
at 95°C to retrieve antigens, and subsequently washed in
phosphate-buffered saline (PBS). For immunohistochemical analysis,
the endogenous peroxidase activity was quenched with 0.6%
H2O2 in methanol, and the sections were
blocked with 10% normal goat serum (Beyotime Institute of
Biotechnology, Haimen, China) and incubated sequentially with the
following primary antibodies: Monoclonal mouse anti-human MYCN
(1:100, clone AB-1; #OP10; Oncogene Research Products, La Jolla,
CA, USA); monoclonal mouse anti-human Ki67 (1:100, #558615; BD
Pharmingen, San Diego, CA, USA); monoclonal rat anti-human BrdU
(1:200; #ab6326; Abcam, Cambridge, UK) and monoclonal rabbit
anti-human Phox2B (1:1,000; #ab183741; Abcam); monoclonal mouse
anti-rat TH (1:5,000; #T1299; Sigma-Aldrich); polyclonal rabbit
anti-rat TH (1:1,000; #AB152; Chemicon International, Temecula, CA,
USA); and polyclonal rabbit anti-human Tuj1 (1:2,000; #ab18207;
Abcam). They were then incubated with secondary antibodies,
biotinylated goat anti-rabbit (#BA-1000), goat anti-rat (#BA-9400)
or anti-mouse (#BA-9200) IgG (1:200; Vector Laboratories, Inc.,
Burlingame, CA, USA) and Vectastain ABC reagent (Vector
Laboratories, Inc.). The immunostaining was visualized with
3,3′-diaminobenzidine (Sigma-Aldrich). The sections were then
counterstained with hematoxylin before being examined using a light
microscope (Eclipse 80i, Nikon, Tokyo, Japan).
Western blot analysis
Five human neuroblastoma cells were harvested and
washed once with ice-cold PBS. Cell pellets were resuspended in SDS
sample buffer (Thermo Fisher Scientific, Life Technologies, Grand
Island, NY, USA) and boiled for 10 min, then centrifuged at 1500 ×
g for 10 min. A total of 60 µg of each protein sample was separated
on a 12% SDS-polyacrylamide gel electrophoresis (stacking gel, 80
V; acrylamide gel, 100 V; polyacrylamide solution obtained from
Sigma-Aldrich), and transferred to a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA), which was then probed
with antibodies and visualized by enhanced chemiluminescence with a
BeyoECL Plus kit (Beyotime Institute of Biotechnology, Haimen,
China). The following primary antibodies were used: Monoclonal
mouse anti-human MYCN (1:100; clone AB-1; #OP10; Oncogene Research
Products), monoclonal mouse anti-human Mash1 (1:100; clone
24B72D11.1; #556604; BD Pharmingen), monoclonal rabbit anti-human
Phox2B (1:1,000; #ab183741; Abcam), and monoclonal mouse anti-human
α-tubulin (1:2,000; B-5-1-2; #T6074; Sigma-Aldrich). Horseradish
peroxidase-conjugated goat anti-mouse (#074-1806) and goat
anti-rabbit (#074-1506) IgG (1:20,000; KPL, Inc., Gaithersburg, MD,
USA) were used as secondary antibodies.
Cell proliferation assays
Human neuroblastoma cells [BE(2)-C, SK-N-DZ and
IMR-32] were seeded in 96-well culture plates. Cell proliferation
was analyzed using a Cell Counting kit 8 (CCK-8; Beyotime Institute
of Biotechnology). Briefly, 20 µl CCK-8 was added into 200 µl
medium in each well and the cells were incubated at 37°C for 2 h.
The absorbance was measured at a wavelength of 450 nm using a Model
550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Soft agar clonogenic assay
A total of 1×103 cells [BE(2)-C-GFPsi,
BE(2)-C-Phox2Bsi#1 and BE(2)-C-Phox2Bsi#2] were mixed in 0.3% Noble
agar (Sigma-Aldrich) in DMEM supplemented with 10% FBS, and plated
into 6-well plates containing a solidified bottom layer (0.6% Noble
agar in the same growth medium). Colonies were stained with 5 mg/ml
MTT (Sigma-Aldrich) after 21 days, and images were captured using a
microscope (Olympus IX71; Olympus, Tokyo, Japan) and a scanner
(Epson Perfection V850 Pro; Epson, Nagano, Japan). The results were
recorded and analyzed statistically.
Xenograft assay
Female NOD/SCID mice of 4 weeks of age were
maintained under specific pathogen-free conditions. For tumorigenic
assays, 1×106 cells [BE(2)-C, SK-N-DZ and IMR-32] were
suspended in 200 µl serum-free DMEM and were injected
subcutaneously into the flanks. The tumor growth was measured by
caliper and the tumor volume was calculated with the formula
4/3πr3, in which ‘r’ is the radius of the tumor. Three
weeks following injection, the mice were sacrificed by cervical
dislocation, and the xenograft tumors were immediately removed,
weighed and paraffin-embedded.
Quantification and statistical
analysis
All observations were confirmed by at least three
independent experiments. Quantitative data are expressed as the
mean ± standard deviation. The two-tailed Student's t-test was
performed using GraphPad Prism (version 6.0) for paired samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Phox2B+ neuronal
progenitors are the major cellular component of hyperplastic
lesions of TH-MYCN mice
To identify the cellular components during the early
stage of neuroblastoma development, the SCG and celiac ganglia
isolated from 3-week-old TH-MYCN mice were examined. Sympathetic
ganglia were isolated from 3-week-old wild-type littermates as a
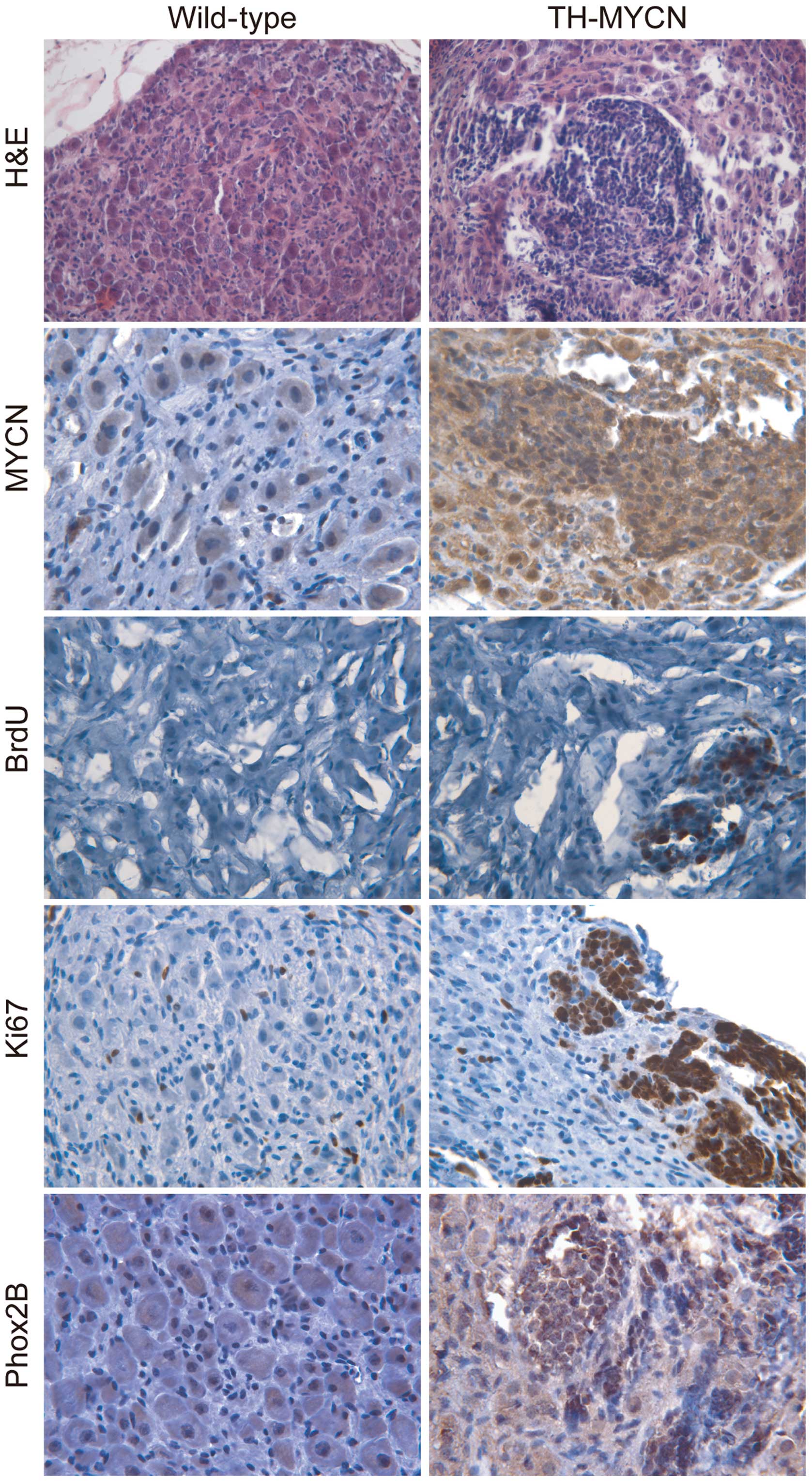
control. The H&E staining (Fig.
1) demonstrated that the sympathetic ganglia of wild-type
littermates were composed of large sympathetic neurons and small
glial cells. By contrast, multiple clusters of small blue round
cells were present in TH-MYCN sympathetic ganglia.
Immunohistochemical staining demonstrated that the hyperplastic
lesions in sympathetic ganglia of TH-MYCN mice exhibited increased
levels of MYCN compared with their wild-type littermates (Fig. 1).
Next, cell proliferation of hyperplastic lesions in
sympathetic ganglia and ganglionic cells of control wild-type
littermates were compared. Immunohistochemical staining was
performed for two independent proliferation markers, BrdU (26) and Ki67 (27). As Fig. 1
demonstrates, the sympathetic ganglia of TH-MYCN mice contained a
high level of BrdU+ cells in hyperplastic clusters,
while there was no detectable level of BrdU in the wild-type
sympathetic ganglia. Although Ki67+ cells were evenly
distributed in the wild-type sympathetic ganglia, no specific areas
or zones of proliferation were observed. Ki67+ cells
were distributed in the non-hyperplastic region of TH-MYCN
sympathetic ganglia. Notably, the majority of the hyperplastic
cells in TH-MYCN sympathetic ganglia were Ki67+
cells.
Immunohistochemical staining was then performed to
detect Phox2B, a marker of sympathetic neuronal progenitors
(28). As demonstrated in Fig. 1, the majority of hyperplastic cells in
the sympathetic ganglia of the TH-MYCN mice expressed Phox2B
compared with samples from their wild-type littermates, which
demonstrated low expression levels of Phox2B. Together, these
results indicate that MYCN expression promotes the proliferation of
Phox2B+ neuronal progenitors to form hyperplastic
lesions in sympathetic ganglia, which may lead to the initiation of
neuroblastoma development.
Primary neuroblastoma tumors are
composed predominantly of Phox2B+ progenitor cells
To investigate whether MYCN-induced proliferation of
Phox2B+ neuronal progenitors promotes neuroblastoma
progression, histological examination of primary neuroblastoma
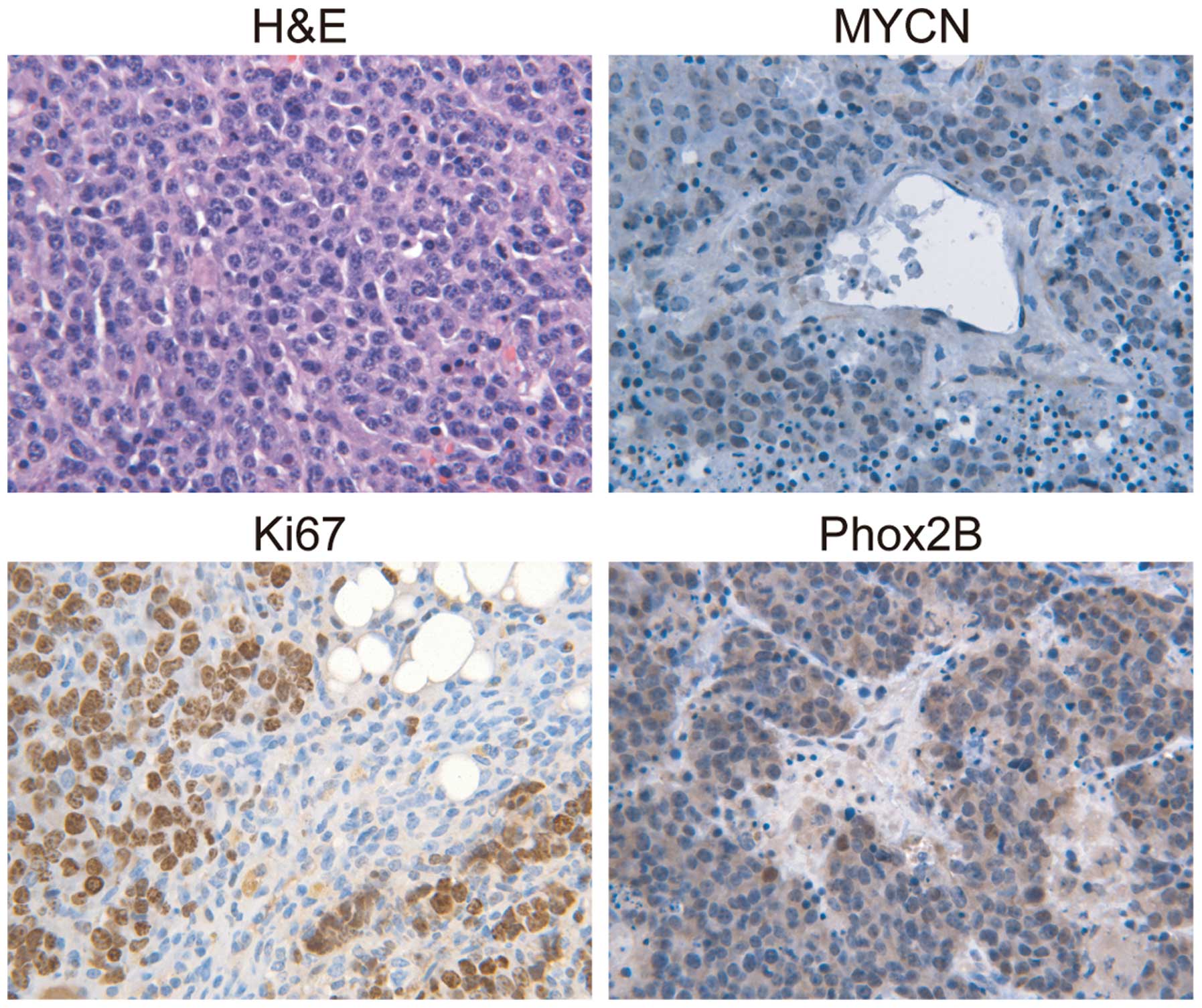
tumors from TH-MYCN mice was performed. The H&E results
demonstrated that the primary neuroblastoma tumors from the mice
were almost entirely composed of small round blue cells (Fig. 2), which were histologically similar to
the hyperplastic lesions in TH-MYCN sympathetic ganglia. The
majority of the primary tumor cells stained positively for MYCN
(Fig. 2) and the formation of nests
or lobules surrounded by thin fibrovascular septa was observed,
which are characteristics of human neuroblastomas with MYCN
amplification (29). Next, the
proliferation status of primary neuroblastoma tumors from TH-MYCN
mice was investigated. The vast majority of primary tumor cells
expressed Ki67, indicating that they were an actively proliferating
population. Notably, the majority of the primary tumor cells
expressed Phox2B, which was morphologically similar to the
hyperplastic lesions observed in the sympathetic ganglia of the
TH-MYCN mice, indicating that the primary tumor cells were in an
undifferentiated state. These data demonstrated that proliferative
Phox2B+ neuronal progenitors were the major cellular
component of primary neuroblastoma tumors from TH-MYCN mice. The
results also indicated that the proliferation of undifferentiated
Phox2B+ neuronal progenitors may be a major cellular
mechanism that promotes neuroblastoma progression.
Hyperplasia in TH-MYCN mice is mainly
composed of undifferentiated progenitor cells
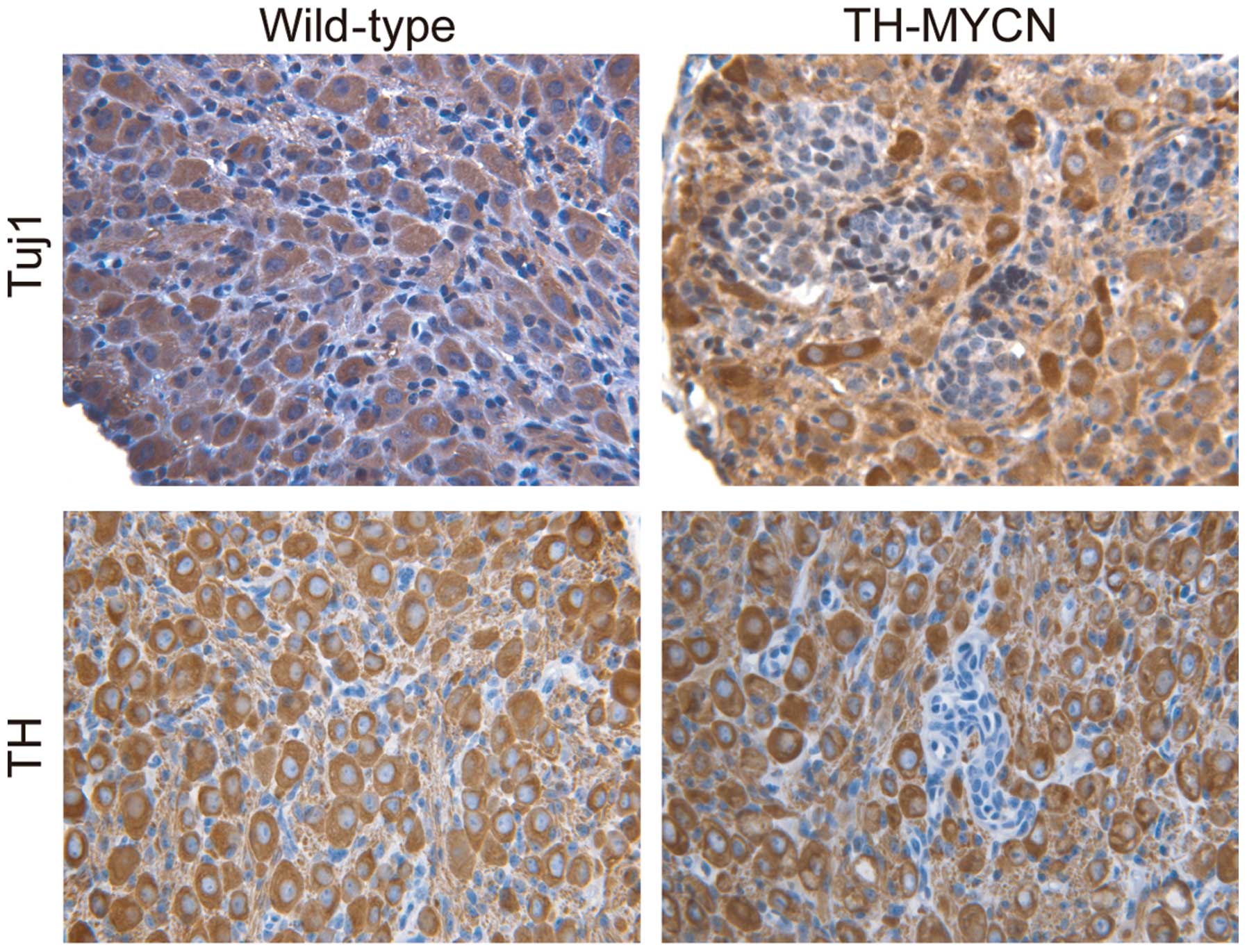
To confirm that the Phox2B+ neuronal
progenitors were in an undifferentiated state, the tissue sections
were immunostained for markers of mature sympathetic neurons.
Fig. 3 demonstrates the morphology of
mature sympathetic neurons from wild-type vs. TH-MYCN mice. In
early postnatal sympathetic ganglia of wild-type mice, the majority
of large sympathetic neurons expressed Tuj1, a marker for neuronal
cells (30). A large number of
sympathetic neurons in wild-type mice also expressed TH, a late
marker for sympathetic noradrenergic neurons (30). Similarly, cells in the
non-hyperplastic region of TH-MYCN sympathetic ganglia expressed
Tuj1 and TH. In contrast, in hyperplastic lesions of TH-MYCN
sympathetic ganglia, almost all cells were small blue round cells,
displayed an undifferentiated morphology and expressed no
detectable levels of Tuj1 and TH. These results indicated an
absence of neuronal differentiation in hyperplasia of TH-MYCN
sympathetic ganglia. Thus, these data indicated that
undifferentiated neuronal progenitors were the major cellular
component of hyperplastic cells in TH-MYCN sympathetic ganglia. In
addition, these findings indicate that expression of MYCN blocks
neuronal progenitor differentiation into mature neurons and
promotes the proliferation of Phox2B+ neuronal
progenitors, leading to the formation of hyperplastic lesions and
development of neuroblastoma.
High expression levels of Phox2B drive
neuroblastoma cell proliferation and xenograft tumor growth
The data presented thus far in the present study
indicate that MYCN-induced proliferation of undifferentiated
Phox2B+ neuronal progenitors may be an important
cellular mechanism that promotes the formation of hyperplasia in
TH-MYCN mice, resulting in neuroblastoma progression. In order to
determine whether these findings in TH-MYCN mice are also present
in the pathogenesis of human neuroblastoma, the role of Phox2B in
the regulation of human neuroblastoma cell proliferation and
xenograft tumor growth was investigated. The association between
MYCN amplification and Phox2B expression in human neuroblastoma
cells was determined. Immunoblotting demonstrated that the human
neuroblastoma cell lines BE(2)-C, SK-N-DZ and IMR-32 are
MYCN-amplified and express high levels of MYCN, however, they also
express high levels of Phox2B (Fig.
4A). This result suggested that the expression level of Phox2B
correlated with the MYCN expression level. In contrast, the human
neuroblastoma SH-EP1 cell line expressed little or undetectable
expression levels of MYCN and no detectable expression levels of
Phox2B (Fig. 4A). Notably, cell
proliferation analysis revealed that cells with higher expression
levels of Phox2B [IMR-32 and BE(2)-C] demonstrated significantly
greater levels of active proliferation compared with the SK-N-DZ
cells (P≤0.05; Fig. 4B), which were
indicated to have lower levels of Phox2B expression.
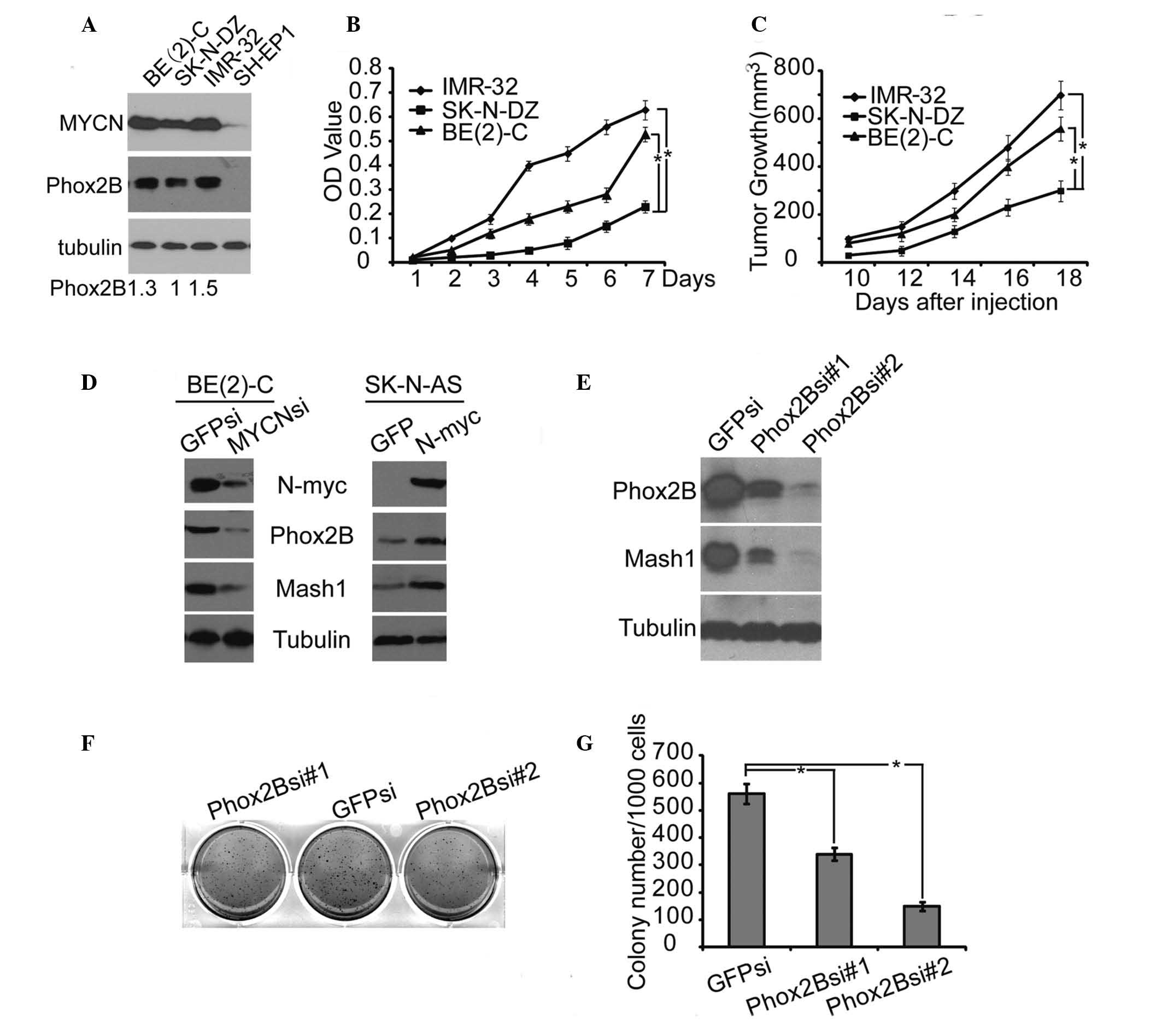 | Figure 4.High level of Phox2B expression
promoted human neuroblastoma cell proliferation and xenograft tumor
growth. (A) Western blot analysis of MYCN and Phox2B expression in
4 neuroblastoma cell lines. α-Tubulin levels are presented as the
loading control; the quantification of the expression level of
Phox2B is normalized to that of the tubulin control. (B) Cell
proliferation assay of 3 neuroblastoma cell lines, IMR-32, SK-N-DZ,
and BE(2)-C, was performed using CCK-8. (C) Tumor growth in
NOD/SCID mice injected with the 3 neuroblastoma cell lines, IMR-32,
SK-N-DZ or BE(2)-C. (D) Western blot analysis of MYCN, Phox2B and
Mash1 expression in BE(2)-C cells with MYCN knockdown by shRNA, or
in SK-N-AS cells with MYCN overexpression. (E) Western blot
analysis of Phox2B and Mash1 expression in BE(2)-C cells with
Phox2B knockdown by shRNA. (F) Soft agar colony formation assay of
BE(2)-C cells with Phox2B knockdown by shRNA. (G) Colonies ≥0.5 mm
or comprising ≥50 cells were recorded. The data in B, C and G are
presented as the mean ± standard deviation, obtained from 3
independent experiments. Statistical analysis was performed using
two-tailed Student's t-test, *P≤0.05. Phox2B, paired-like homeobox
2b; MYCN, v-myc avian myelocytomatosis viral oncogene
neuroblastoma derived homolog; CCK-8, cell counting kit 8; Mash1,
mammalian achaete-scute homolog 1; shRNA, short hairpin RNA. |
Human neuroblastoma cells were injected into
NOD/SCID mice to form xenograft tumors. Xenograft tumors derived
from the neuroblastoma cells that expressed higher levels of Phox2B
[IMR-32 and BE(2)-C] grew significantly faster than those derived
from cells that expressed lower levels of Phox2B (SK-N-DZ) (P≤0.05;
Fig. 4C). These findings indicate
that Phox2B expression may drive neuroblastoma growth.
The association between high expression levels of
Phox2B and MYCN, and the stemness of neuroblastoma cells was
investigated. The BE(2)-C cell line expressed the highest level of
MYCN and was therefore selected to investigate the effects of the
downregulation of MYCN expression. This was achieved by using a
short hairpin RNA specific to MYCN. Immunoblotting demonstrated
that the inhibition of MYCN expression in BE(2)-C cells led to a
significant downregulation of Phox2B and Mash1, another marker for
neuronal progenitors or stem cells (31) (Fig. 4D).
The human neuroblastoma cell line SK-N-AS carries a single copy of
MYCN and expresses little or undetectable levels of MYCN, therefore
overexpression of MYCN was performed in the SK-N-AS cells. This
analysis demonstrated that the overexpression of MYCN in SK-N-AS
cells promoted a marked upregulation of Phox2B and Mash1 (Fig. 4D). These findings indicate that high
expression levels of Phox2B, accompanied with MYCN amplification,
are important to the stemness of neuroblastoma cells. To confirm
the critical function of Phox2B in neuroblastoma stemness, the
expression of Phox2B was knocked down in BE(2)-C cells. This
demonstrated that knockdown of Phox2B markedly induced
downregulation of Mash1 expression (Fig.
4E), demonstrating that Phox2B was efficient in the regulation
of neuroblastoma stemness. A clonogenic assay in soft agar was
performed in order to examine the self-renewal capacity of the
neuroblastoma cells. The knockdown of Phox2B in neuroblastoma
BE(2)-C cells significantly inhibited the formation of colonies in
soft agar (P≤0.05; Fig. 4F and G),
indicating that the downregulation of Phox2B results in suppression
of the neuroblastoma cell self-renewal. This observation indicates
that Phox2B is a cellular regulator for the maintenance of
neuroblastoma stemness and promotion of neuroblastoma tumor
growth.
In conclusion, the present study demonstrated that
Phox2B is a major cellular regulator for maintaining neuroblastoma
stemness that promoted neuroblastoma tumor growth, and indicated
that the proliferation of undifferentiated Phox2B+
neuronal progenitors is a cellular mechanism that promoted
neuroblastoma progression in TH-MYCN mice.
Discussion
Neuroblastoma is a type of cancer that arises in
early neural progenitors of the sympathetic nervous system.
Approximately a third of neuroblastomas originate in the adrenal
glands; neuroblastomas also originate in the sympathetic ganglia in
the abdomen, adjacent to the spine in the neck or chest, or in the
pelvis (32). However, the causes of
the majority of neuroblastomas remain unknown. It appears that,
during embryonic development, neuroblastoma develops when normal
neural crest cells fail to become adrenal medulla cells or mature
nerve cells (2). In normal
sympathetic neurogenesis, BMP signaling released by the dorsal
aorta induces the neural crest cells to express high levels of
Mash1 and Phox2B; this activates the expression of Hand-2, Phox2A,
GATA2 and GATA3, which consequently promotes further neuronal
differentiation (17,22). We hypothesized that disorderly
regulation of these transcription factors expressed in neural crest
cells may be a potential incentive for neuroblastoma formation. The
present study demonstrates that Phox2B+ neuronal
progenitors are the major cellular component of hyperplasia and
primary tumors of TH-MYCN mice, therefore providing evidence in
support of the hypothesis above.
The oncogene MYCN is critical in the pathogenesis of
neuroblastoma (33) and aids in the
regulation of cell growth (34).
Similarly to other oncogenes, alterations in MYCN expression levels
can result in accelerated cell growth and division (35). Previous studies have reported that the
genomic amplification of MYCN is the most consistent genetic
aberration associated with poor outcome in neuroblastoma (21,36) and
the MYCN transgenic mouse develops neuroblastoma with high
resemblance to human tumors (24).
Therefore, the TH-MYCN transgenic mouse was used in the present
study as an animal model of neuroblastoma development to
investigate whether Phox2B is a critical factor in neuroblastoma
development. The present study demonstrates that neuroblastoma
development in TH-MYCN mice starts with hyperplastic lesion
formation in the sympathetic ganglia during early sympathetic
neurogenesis. The hyperplastic lesions are mainly composed of
actively proliferative Phox2B+ neuronal progenitors, as
demonstrated by their high immunoreactivity for the proliferation
markers BrdU and Ki67. In addition, in TH-MYCN hyperplastic
lesions, the majority of cells exhibited an undifferentiated
morphology and did not express detectable levels of Tuj1 and TH,
two markers of mature neurons, indicating that the majority of
cells in TH-MYCN hyperplastic lesions are maintained in a
progenitor state. Thus, the proliferation of undifferentiated
Phox2B+ neuronal progenitors may lead to hyperplasia
formation in TH-MYCN mice. The present study also demonstrated that
Phox2B+ and Ki67+ proliferative neuronal
progenitors are the major component of primary tumors from TH-MYCN
mice. This data indicates that Phox2B+ neuronal
progenitors may contribute to neuroblastoma development through the
maintenance of its proliferative activity.
The present study may also provide insight into the
pathogenesis of human neuroblastoma. A previous study demonstrated
that Phox2B is a predisposing gene to hereditary neuroblastic
tumors (37). In accordance with this
finding, the present study demonstrated that high expression levels
of Phox2B promoted the growth of xenograft tumors derived from
human neuroblastoma cells. High expression levels of Phox2B also
promoted neuroblastoma cell proliferation and was essential to
maintain neuroblastoma stemness. The evidence presented in the
current study indicated that Phox2B may be critical for the
pathogenesis of neuroblastoma.
Acknowledgements
The present study was supported by the National
Basic Research Program of China (grant no. 2012cb114603), the
National Natural Science Foundation of China (grant no. 81201551),
the Research Fund for the Doctoral Program of Higher Education of
China (grant no. 20130182110003) and the Fundamental Research Funds
for the Central Universities (grant nos. SWU111014 and
XDJK2011C017).
References
|
1
|
Hsieh MH, Meng MV, Walsh TJ, Matthay KK
and Baskin LS: Increasing incidence of neuroblastoma and
potentially higher associated mortality of children from
nonmetropolitan areas: analysis of the surveillance, epidemiology,
and end results database. J Pediatr Hematol Oncol. 31:942–946.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baker CV and Bronner-Fraser M: The origins
of the neural crest. Part I: embryonic induction. Mech Dev.
69:3–11. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mayor R and Theveneau E: The neural crest.
Development. 140:2247–2251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson DJ: Molecular control of cell
fate in the neural crest: the sympathoadrenal lineage. Annu Rev
Neurosci. 16:129–158. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Unsicker K: The chromaffin cell: paradigm
in cell, developmental and growth factor biology. J Anat.
183:207–221. 1993.PubMed/NCBI
|
|
7
|
Sano H, Bonadio J, Gerbing RB, et al:
International neuroblastoma pathology classification adds
independent prognostic information beyond the prognostic
contribution of age. Eur J Cancer. 42:1113–1119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimada H, Ambros IM, Dehner LP, et al:
The International Neuroblastoma Pathology Classification (the
Shimada system). Cancer. 86:364–372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsarovina K, Schellenberger J, Schneider C
and Rohrer H: Progenitor cell maintenance and neurogenesis in
sympathetic ganglia involves Notch signaling. Mol Cell Neurosci.
37:20–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah NM, Groves AK and Anderson DJ:
Alternative neural crest cell fates are instructively promoted by
TGFbeta superfamily members. Cell. 85:331–343. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reissmann E, Ernsberger U, Francis-West
PH, Rueger D, Brickell PM and Rohrer H: Involvement of bone
morphogenetic protein-4 and bone morphogenetic protein-7 in the
differentiation of the adrenergic phenotype in developing
sympathetic neurons. Development. 122:2079–2088. 1996.PubMed/NCBI
|
|
12
|
Vincentz JW, VanDusen NJ, Fleming AB, et
al: A Phox2- and Hand2-dependent Hand1 cis-regulatory element
reveals a unique gene dosage requirement for Hand2 during
sympathetic neurogenesis. J Neurosci. 32:2110–2120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirsch MR, Tiveron MC, Guillemot F, Brunet
JF and Goridis C: Control of noradrenergic differentiation and
Phox2a expression by MASH1 in the central and peripheral nervous
system. Development. 125:599–608. 1998.PubMed/NCBI
|
|
14
|
Lo L, Tiveron MC and Anderson DJ: MASH1
activates expression of the paired homeodomain transcription factor
Phox2a, and couples pan-neuronal and subtype-specific components of
autonomic neuronal identity. Development. 125:609–620.
1998.PubMed/NCBI
|
|
15
|
Stanke M, Junghans D, Geissen M, Goridis
C, Ernsberger U and Rohrer H: The Phox2 homeodomain proteins are
sufficient to promote the development of sympathetic neurons.
Development. 126:4087–4094. 1999.PubMed/NCBI
|
|
16
|
Tsarovina K, Pattyn A, Stubbusch J, et al:
Essential role of Gata transcription factors in sympathetic neuron
development. Development. 131:4775–4786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pattyn A, Morin X, Cremer H, Goridis C and
Brunet JF: The homeobox gene Phox2b is essential for the
development of autonomic neural crest derivatives. Nature.
399:366–370. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim KC, Lakshmanan G, Crawford SE, Gu Y,
Grosveld F and Engel JD: Gata3 loss leads to embryonic lethality
due to noradrenaline deficiency of the sympathetic nervous system.
Nat Genet. 25:209–212. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider C, Wicht H, Enderich J, Wegner M
and Rohrer H: Bone morphogenetic proteins are required in vivo for
the generation of sympathetic neurons. Neuron. 24:861–870. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dyer MA: Mouse models of childhood cancer
of the nervous system. J Clin Pathol. 57:561–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seeger RC, Brodeur GM, Sather H, et al:
Association of multiple copies of the N-myc oncogene with rapid
progression of neuroblastomas. N Engl J Med. 313:1111–1116. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maris JM and Matthay KK: Molecular biology
of neuroblastoma. J Clin Oncol. 17:2264–2279. 1999.PubMed/NCBI
|
|
23
|
Cohn SL, Pearson AD, London WB, et al:
INRG Task Force: The International Neuroblastoma Risk Group (INRG)
classification system: an INRG Task Force report. J Clin Oncol.
27:289–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiss WA, Aldape K, Mohapatra G,
Feuerstein BG and Bishop JM: Targeted expression of MYCN causes
neuroblastoma in transgenic mice. EMBO J. 16:2985–2995. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hansford LM, Thomas WD, Keating JM, et al:
Mechanisms of embryonal tumor initiation: distinct roles for MycN
expression and MYCN amplification. Proc Natl Acad Sci USA.
101:12664–12669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muñoz-Velasco I, Ortíz R, Echeverría OM,
Escobar ML and Vázquez-Nin GH: Characterization of the pre-meiotic
S phase through incorporation of BrdU during spermatogenesis in the
rat. J Histochem Cytochem. 61:680–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pathmanathan N and Balleine RL: Ki67 and
proliferation in breast cancer. J Clin Pathol. 66:512–516. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dubreuil V, Hirsch MR, Jouve C, Brunet JF
and Goridis C: The role of Phox2b in synchronizing pan-neuronal and
type-specific aspects of neurogenesis. Development. 129:5241–5253.
2002.PubMed/NCBI
|
|
29
|
Shimada H, Stram DO, Chatten J, et al:
Identification of subsets of neuroblastomas by combined
histopathologic and N-myc analysis. J Natl Cancer Inst.
87:1470–1476. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Egawa C and Kameda Y: Innervation of the
chicken parathyroid glands: immunohistochemical study with the
TuJ1, galanin, VIP, substance P, CGRP and tyrosine hydroxylase
antibodies. Anat Embryol (Berl). 191:445–450. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sommer L, Shah N, Rao M and Anderson DJ:
The cellular function of MASH1 in autonomic neurogenesis. Neuron.
15:1245–1258. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang M and Weiss WA: Neuroblastoma and
MYCN. Cold Spring Harb Perspect Med. 3:a0144152013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Biol. 6:635–645. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hipp NI, Christner L, Wirth T, et al: MYCN
and survivin cooperatively contribute to malignant transformation
of fibroblasts. Carcinogenesis. 35:479–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brodeur GM and Seeger RC: Gene
amplification in human neuroblastomas: basic mechanisms and
clinical implications. Cancer Genet Cytogenet. 19:101–111. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bourdeaut F, Trochet D, Janoueix-Lerosey
I, et al: Germline mutations of the paired-like homeobox 2B
(PHOX2B) gene in neuroblastoma. Cancer Lett. 228:51–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|