Introduction
Extraskeletal osteosarcomas (EOSs) are rare,
high-grade malignant mesenchymal tumors of the soft tissue. The
tumors are characterized by an osteoid and/or cartilaginous matrix,
which is produced by neoplastic cells with an aberrant osteoblastic
phenotype. The distinguishing feature of EOS, which may exhibit
similar histological features to primary osteogenic sarcoma of the
bone, is the localization in the soft tissues without direct
attachment to bone or periosteum. EOSs account for <5% of all
osteosarcomas and 1% of all soft-tissue tumors (1–5). Typically
EOSs arise as large, deep and painless lesions in the lower
extremities, most commonly in the thigh. Unlike skeletal
osteosarcoma, which predominantly affects patients in the second
and third decades of life, EOSs are frequently observed in patients
aged >50 years at the time of diagnosis (1–7).
Due to the rarity of EOSs and the features that
distinguish them from conventional bone sarcomas, limited reliable
data are available with regard to the incidence, clinical behavior
and therapeutic management of these tumors. Furthermore, in the era
of histology-driven therapy for the treatment of sarcoma subtypes
(8), no specific guidelines have been
established for the treatment of EOS. In retrospective case
studies, contradictory data have been reported regarding the use of
chemotherapy regimens indicated for bone (6,7) or
soft-tissue sarcoma (9,10).
The present study reports a combination treatment of
gemcitabine and docetaxel, utilized as a salvage therapy in a
typical case of EOS previously treated with chemotherapy compounds
designed either for soft-tissue or bone sarcomas. To the best of
our knowledge, no effective salvage therapies have previously been
established for patients with recurrent or refractory EOS, and this
study is the first to report the use of this combination regimen
resulting in synergistic antitumor activity in this disease.
Written informed consent was obtained from the patient's
family.
Case report
A 50-year-old male farmer presented to the Oncology
Department of the National Cancer Research Centre (Bari, Italy) in
July 2011 with a progressively enlarging mass on the medial side of
the left proximal thigh. The past medical and family history
revealed no illnesses of note. The patient smoked heavily, but did
not consume alcohol or use illicit drugs. Although no clear history
of trauma was reported, the painless enlarging mass on the thigh
was initially misdiagnosed as a hematoma upon physical examination.
However, due to the rapid increase in the size and hardness of the
palpable mass in the previous month, the patient underwent magnetic
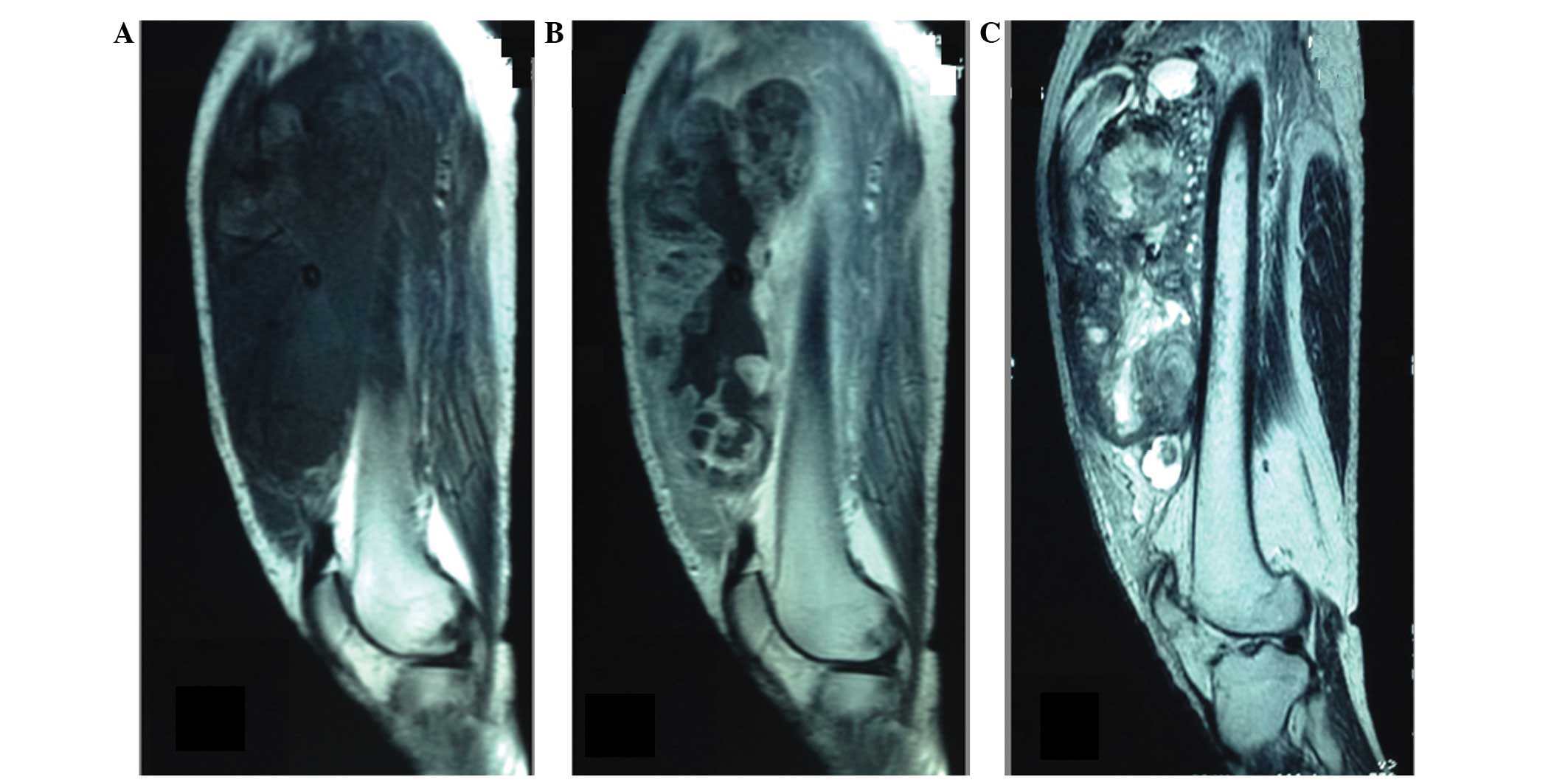
resonance imaging (MRI) in August 2011, revealing the presence of a
large, well-defined, irregularly lobulated mass in the left
quadriceps femoris muscle. The mass was marginally hypointense to
skeletal muscle on T1-weighted images and markedly heterogeneously
hyperintense on T2-weighted sequences, with patchy intratumoral
cystic areas of variable size. The mass had reached a size of
18.5×18.0 cm, and contrast-enhanced MRI and computed tomography
(CT) scans revealed irregular contrast enhancement of the tumor
with massive central necrosis (Fig.
1).
Results from routine laboratory analyses were within
normal limits. Upon physical examination, a hard, bulky mass, which
was slightly mobile and not adherent to the skin, was palpable in
the left thigh.
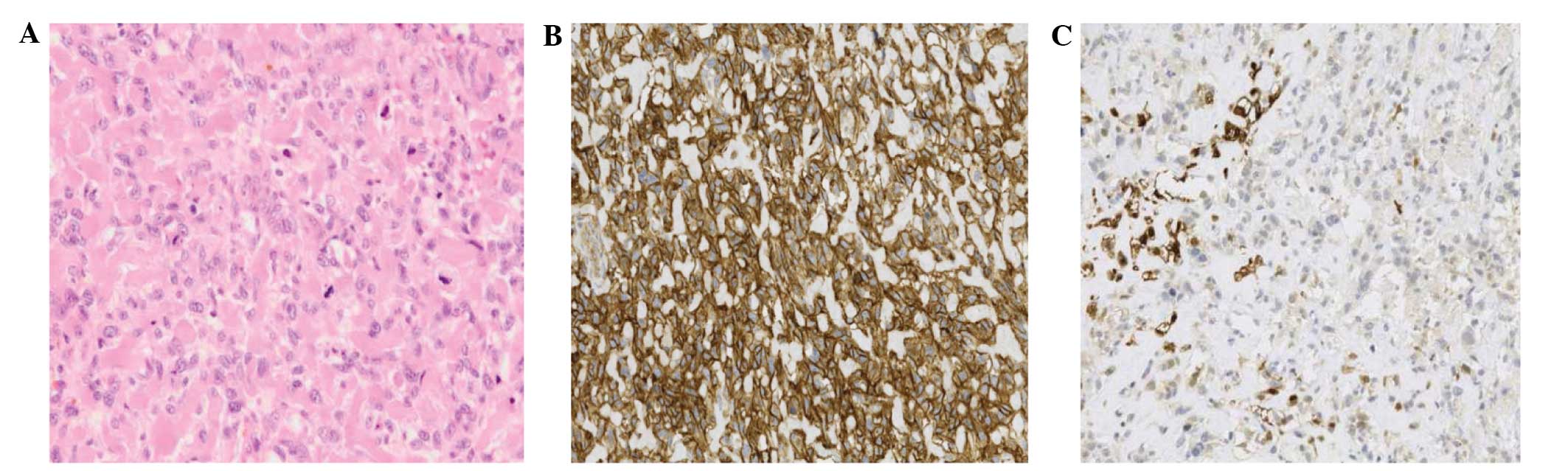
An incisional biopsy of the thigh lesion was
subsequently performed. Histopathological analysis of the specimen
indicated a highly malignant neoplasm with large areas of necrosis,
composed of pleomorphic spindle and ovoid cells with a high mitotic
rate, which were closely intermingled in a lace-like pattern;
intercellular eosinophilic, dense, homogeneous and curvilinear
material with focal mineralization was also observed. The
neoplastic cells exhibited diffuse, moderate membranous and
cytoplasmic positivity for cluster of differentiation 99, diffuse,
moderate positivity for smooth muscle actin and focal positivity
for S-100. Taken together, these features indicated a diagnosis of
EOS (Fig. 2).
As no metastasis was observed on a whole-body CT
scan, and in order to attempt subsequent limb-sparing surgery, the
patient commenced pre-operative chemotherapy with a regimen
consisting of 60 mg/m2 epirubicin on days 1–2, and 1,800
mg/m2 ifosfamide on days 1–5, with mesna (20% of
ifosfamide dose, three times) every 21 days, commencing in
September 2011. However, the therapy was discontinued after two
cycles due to the onset of local progression, characterized by
tumor-associated ulceration and intense swelling of the thigh, and
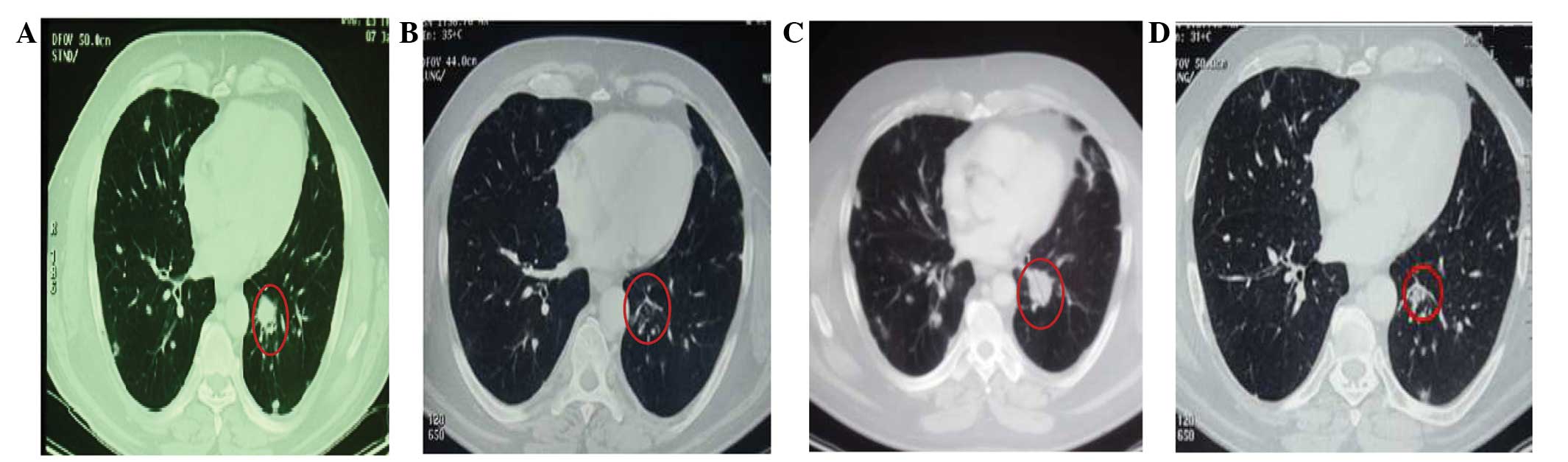
due to the emergence of lung metastases revealed by a further CT
scan (Fig. 3A).
The patient experienced progressive anemia caused by
bleeding from a lesion of the thigh; a surgical above-knee
amputation was therefore conducted to remove the primary EOS.
Subsequently, in December 2011, second-line chemotherapy was
commenced with 25 mg/m2 doxorubicin on days 1–3 and 100
mg/m2 cisplatin on day 1, every 21 days, a regimen
typically utilized for the treatment of bone sarcoma (11). Prophylactic polyethylene glycol
granulocyte-colony stimulating factor was also administered the day
after this chemotherapy. Following three cycles of treatment, a CT
scan revealed a partial response (PR) according to the Response
Evaluation Criteria In Solid Tumors (version 1.1) (12), with a reduction in the number and size
of pulmonary nodules (Fig. 3B). The
patient therefore continued this treatment for a further two cycles
until May 2012 when, due to a protracted grade 3 pancytopenia,
chemotherapy was discontinued. A subsequent CT scan performed in
July 2011 showed further PR of the lung lesions and the patient was
referred for close follow-up.
In September 2012, a new CT scan indicated disease
progression in the lung (Fig. 3C) and
the appearance of two nodular brain lesions that were 1 cm in
diameter. Despite an absence of symptoms caused by the brain
metastases, the patient underwent whole-brain irradiation. At this
time, the patient's Eastern Cooperative Oncology Group status was 0
and in the absence of an established salvage therapy, chemotherapy
with 800 mg/m2 gemcitabine and 50 mg/m2
docetaxel was recommenced, administered on day 1 of every two week
period. This regimen was selected based on data from a number of
studies on the use of this combination regimen in soft-tissue
(11,13–16) and
bone (17,14,18–22)
sarcomas.
Treatment was well-tolerated, without the occurrence
of toxicity. During therapy, a CT scan indicated a stable disease
state after four cycles, and a PR of the lung metastases after 10,
16 and 22 cycles (Fig. 3D). Therapy
was continued for 24 cycles (14 months) without relevant
side-effects.
In February 2014, 25 months after the diagnosis of
metastatic disease and 14 months after the start of the
gemcitabine-docetaxel regimen, the patient succumbed to a sudden
onset of a cerebral bleeding caused by disease progression in the
brain.
Discussion
EOSs are rare variants of primary osteosarcoma of
the bone, and are defined as sarcomas located in the soft tissues
and characterized by osteoid production, with no attachment to the
skeletal system. The condition was initially described by Wilson in
1941 (23), in the first reported
case, and fewer than 300 cases have been reported since then. These
cases suggest that aside from the histologically common features,
EOS has distinctive demographic, imaging and prognostic features
compared with osteosarcoma of bone origin. Furthermore, it is not
currently clear whether EOS may be managed in a similar way to
osteosarcoma or soft-tissue sarcoma. The rarity of EOSs, in
addition to the lack of prospective and randomized studies, has
hindered the resolution of this issue.
In all the reported studies of EOS, the importance
of a multidisciplinary approach to treatment has been clearly
emphasized; such treatment has involved surgery, radiotherapy and
chemotherapy, depending on the disease stage. In particular, it has
been demonstrated that due to its typical location in the lower
extremities, limb-sparing surgical techniques may be applied to
patients with EOS, as has been firmly established in cases of bone
sarcoma (1,7,10).
Furthermore, a wide resection, with R0 status, of surgical margins
is one of the most important clinicopathological prognostic
factors, in addition to tumor size and tumor-node-metastasis stage
(1–7,10,12). Extremely poor experiences have been
reported with regard to pre-operative chemotherapy, without a
conclusive definition of its modality (1,6,7,9,10). In the adjuvant setting, the largest
reported series was that of the Co-operative German-Austrian-Swiss
Osteosarcoma Study Group, in which 15 patients were treated
according to protocols for high-grade central osteosarcoma between
1986 and 2002 (6). In these patients,
whose median age was lower than that reported elsewhere,
polychemotherapy regimens, including doxorubicin, ifosfamide,
cisplatin and methotrexate in certain cases, led to an estimated
overall survival rate of 77% at five years, the highest reported
rate of any previous studies. The study concluded that EOS has a
favorable prognosis when treated as per conventional osteosarcoma.
In another 27 patients with measurable and assessable EOS, treated
at the M.D. Anderson Cancer Center between 1960 and 1999, the use
of doxorubicin-based chemotherapy, including additional cisplatin
in 13 of these patients and ifosfamide in a further 8, led to a low
overall response rate of 19% (10).
In this series, a median survival time of 8 months was documented
for metastatic EOS, leading to the conclusion [as previously
reported by the same authors (9)]
that EOS may be considered to be a relatively chemoresistant form
of sarcoma with a poor prognosis, which should be treated
distinctly from bone sarcoma.
Following this approach, the patient in the present
case was initially treated with a pre-operative soft-tissue
sarcoma-oriented chemotherapy regimen (24,25) of
epirubicin and ifosfamide, which was found to be ineffective.
Subsequently, a different schedule of doxorubicin and cisplatin,
which has been well-documented in bone sarcoma (26) was commenced. Although a PR was
documented, the patient experienced protracted grade 3
pancytopenia, which necessitated the suspension of the
treatment.
As a further relapse of the EOS occurred, the
previously experienced severe side-effects were considered during
the planning of a new treatment strategy, with the aim of devising
a therapy that would be effective against the disease without
leading to heavy myelotoxicity. A gemcitabine-docetaxel regimen was
therefore selected as salvage therapy. This combination schedule
had previously demonstrated synergistic activity against various
sarcoma cell lines, including osteosarcoma, in in vitro
experiments (14). However, the
translation of this evidence into the clinical setting for the
treatment of soft-tissue sarcoma was more promising than for bone
sarcoma, as phase II trials have demonstrated (11,15,16,18).
The role of gemcitabine-docetaxel combination therapy in refractory
or recurrent osteosarcoma remains poorly defined due to the
relatively small number of patients included in the trials, and due
to the retrospective nature of the reports (13,17,19–22).
To the best of our knowledge, the present study was
the first clinical experience with this combination regimen for the
treatment of EOS. The outcome included an objective PR and a
progression-free survival time of 14 months, with a well-tolerated
treatment that was easily administered in an outpatient
setting.
In conclusion, the lack of novel agents active
against these tumors prompted the investigation of a different
strategy for the treatment of this rare sarcoma, for which there
are currently few therapeutic options. The present case
demonstrated that EOS may maintain relative chemosensitivity,
indicating the potential to control the advanced disease in the
long term and plan sequential chemotherapy regimens.
The establishment of definitive guidelines with
regard to the management of EOS has been hampered by the rarity of
this sarcoma. In addition, previous studies have been biased by the
long retrospective period and poor control over pathological and
clinical information. Therefore, it is necessary to strengthen
collaborative networks in order to confirm the effectiveness of the
gemcitabine-docetaxel combination in large retrospective or
prospective studies in EOS patients, for whom established
therapeutic strategies are presently lacking.
Acknowledgements
The authors would like to thank Miss. Caroline
Oakley (National Cancer Research Centre, Bari, Italy) and Miss.
Silvana Valerio (National Cancer Research Centre, Bari, Italy) for
providing assistance in the preparation of this manuscript.
Glossary
Abbreviations
Abbreviations:
|
EOS
|
extraskeletal osteosarcoma
|
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
PR
|
partial response
|
References
|
1
|
McCarter MD, Lewis JJ, Antonescu CR and
Brennan MF: Extraskeletal osteosarcoma: Analysis of outcome of a
rare neoplasm. Sarcoma. 4:119–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lidang Jensen M, Schumacher B, Myhre
Jensen O, Steen Nielsen O and Keller J: Extraskeletal
osteosarcomas: A clinicopathologic study of 25 cases. Am J Surg
Pathol. 22:588–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sordillo PP, Hajdu SI, Magill GB and
Golbey RB: Extraosseous osteogenic sarcoma. A review of 48
patients. Cancer. 51:727–734. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bane BL, Evans HL, Ro JY, Carrasco CH,
Grignon DJ, Benjamin RS and Ayala AG: Extraskeletal osteosarcoma. A
clinicopathologic review of 26 cases. Cancer. 65:2762–2770. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allan CJ and Soule EH: Osteogenic sarcoma
of the somatic soft tissues. Clinicopathologic study of 26 cases
and review of literature. Cancer. 27:1121–1133. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldstein-Jackson SY, Gosheger G, Delling
G, Berdel WE, Exner GU, Jundt G, Machatschek JN, Zoubek A, Jurgens
H and Bielack SS: Cooperative Osteosarcoma Study Group COSS:
Extraskeletal osteosarcoma has a favourable prognosis when treated
like conventional osteosarcoma. J Cancer Res Clin Oncol.
131:520–526. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torigoe T, Yazawa Y, Takagi T, Terakado A
and Kurosawa H: Extraskeletal osteosarcoma in Japan:
Multiinstitutional study of 20 patients from the Japanese
Musculoskeletal Oncology Group. J Orthop Sci. 12:424–429. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casali PG: Histology- and
non-histology-driven therapy for treatment of soft tissue sarcomas.
Ann Oncol. 23:x167–x169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel SR and Benjamin RS: Primary
extraskeletal osteosarcoma - experience with chemotherapy. J Natl
Cancer Inst. 87:1331–1333. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmad SA, Patel SR, Ballo MT, et al:
Extraosseous osteosarcoma: Response to treatment and long-term
outcome. J Clin Oncol. 20:521–527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bay JO, Ray-Coquard I, Fayette J, et al:
Groupe Sarcome Francais: Docetaxel and gemcitabine combination in
133 advanced soft-tissue sarcomas: a retrospective analysis. Int J
Cancer. 119:706–711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maki RG, Wathen JK, Patel SR, et al:
Randomized phase II study of gemcitabine and docetaxel compared
with gemcitabine alone in patients with metastatic soft tissue
sarcomas: Results of sarcoma alliance for research through
collaboration study 002 [corrected]. J Clin Oncol. 25:2755–2763.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leu KM, Ostruszka LJ, Shewach D, et al:
Laboratory and clinical evidence of synergistic cytotoxicity of
sequential treatment with gemcitabine followed by docetaxel in the
treatment of sarcoma. J Clin Oncol. 22:1706–1712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rapkin L, Qayed M, Brill P, Martin M,
Clark D, George BA, Olson TA, Wasilewski-Masker K, Alazraki A and
Katzenstein HM: Gemcitabine and docetaxel (GEMDOX) for the
treatment of relapsed and refractory pediatric sarcomas. Pediatr
Blood Cancer. 59:854–858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pautier P, Floquet A, Penel N, et al:
Randomized multicenter and stratified phase II study of gemcitabine
alone versus gemcitabine and docetaxel in patients with metastatic
or relapsed leiomyosarcomas: A Federation Nationale des Centres de
Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM
study). Oncologist. 17:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Navid F, Willert JR, McCarville MB, Furman
W, Watkins A, Roberts W and Daw NC: Combination of gemcitabine and
docetaxel in the treatment of children and young adults with
refractory bone sarcoma. Cancer. 113:419–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frustaci S, Gherlinzoni F, De Paoli A, et
al: Adjuvant chemotherapy for adult soft tissue sarcomas of the
extremities and girdles: Results of the Italian randomized
cooperative trial. J Clin Oncol. 19:1238–1247. 2001.PubMed/NCBI
|
|
19
|
Hensley ML, Maki R, Venkatraman E, Geller
G, Lovegren M, Aghajanian C, Sabbatini P, Tong W, Barakat R and
Spriggs DR: Gemcitabine and docetaxel in patients with unresectable
leiomyosarcoma: Results of a phase II trial. J Clin Oncol.
20:2824–2831. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi WX, He AN, Tang LN, Shen Z, Lin F and
Yao Y: Efficacy and safety of gemcitabine-docetaxel combination
therapy for recurrent or refractory high-grade osteosarcoma in
China: A retrospective study of 18 patients. Jpn J Clin Oncol.
42:427–431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He A, Qi W, Huang Y, Sun Y, Shen Z, Zhao
H, Yang Y and Yao Y: Comparison of pirarubicin-based versus
gemcitabine-docetaxel chemotherapy for relapsed and refractory
osteosarcoma: A single institution experience. Int J Clin Oncol.
18:498–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fox E, Patel S, Wathen JK, Schuetze S,
Chawla S, Harmon D, Reinke D, Chugh R, Benjamin RS and Helman LJ:
Phase II study of sequential gemcitabine followed by docetaxel for
recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally
recurrent chondrosarcoma: Results of Sarcoma Alliance for Research
Through Collaboration Study 003. Oncologist. 17:3212012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilson H: Extraskeletal ossifying tumors.
Ann Surg. 113:95–112. 1941. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gosiengfiao Y, Reichek J, Woodman J,
Ben-Ami T and Walterhouse D: Gemcitabine with or without docetaxel
and resection for recurrent osteosarcoma: the experience at
Children's Memorial Hospital. J Pediatr Hematol Oncol. 34:e63–e65.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gronchi A, Verderio P, De Paoli A, et al:
Quality of surgery and neoadjuvant combined therapy in the ISG-GEIS
trial on soft tissue sarcomas of limbs and trunk wall. Ann Oncol.
24:817–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Souhami RL, Craft AW, Van der Eijken JW,
et al: Randomised trial of two regimens of chemotherapy in operable
osteosarcoma: A study of the European Osteosarcoma Intergroup.
Lancet. 350:911–917. 1997. View Article : Google Scholar : PubMed/NCBI
|