Introduction
Thyroid carcinoma (TC) is the most common endocrine
malignancy (1); according to data
presented by the National Comprehensive Cancer Network, it is
currently the sixth most common malignancy diagnosed in females
(2). The incidence of TC,
particularly papillary thyroid carcinoma (PTC), is increasing by
6.2% per year (2,3).
More than 70% of PTCs have genetic alterations
associated with the mitogen-activated protein kinase pathway
(4). BRAF mutations have been
commonly observed in all three subtypes of Raf kinase, the first
identified and well-characterized downstream cytosolic effector of
RAS. Of the greater than 45 BRAF mutations that have been
identified in human cancer types, approximately 90% are T→A
transversions in exon 15 at nucleotide 1799 (T1799A), leading to a
valine→glutamic acid replacement at position 600 (BRAF
V600E) (4). In PTC, the prevalence of
the BRAF V600E mutation varies from 29 to 83%, and occurs
mainly in classic PTC (approximately 60%). In tall-cell variant PTC
and PTC-derived anaplastic thyroid cancer, the BRAF V600E
mutation was also reported; however, it has rarely been identified
in follicular-variant PTC (FV-PTC) and never reported in follicular
thyroid carcinoma (FTC) (4).
Numerous studies have demonstrated that the
BRAF mutation is the main genetic event in PTC, and is
associated with poor prognosis. In rat thyroid PCCL3 cells
conditionally expressing oncogenic BRAF, increased invasion
into Matrigel was observed compared with cells expressing RET/PTC3
in vitro, which was consistent with the biological behavior
of human PTC (5). In addition, PTC
without the BRAF V600E mutation mainly corresponded to
FV-PTC, and maintained a thyroid differentiation expression level
close to that of normal tissue and thus had a better prognosis than
PTC with the gene alteration (6).
Furthermore, the BRAF V600E mutation may induce genetic
instability in PTC, facilitating secondary genetic alternation of
members of the PI3k/Akt pathway that may induce PTC progression to
a more aggressive thyroid cancer (7).
However, whether the BRAF V600E mutation represents an
independent risk factor for predicting thyroid cancer recurrence or
metastasis is a matter of debate. In addition, although PTC is
usually accompanied with Hashimoto thyroiditis (HT), which is
considered a precancerous lesion of PTC by certain investigators
(8), its involvement in the
pathogenesis of PTC is controversial. Therefore, this study aimed
to investigate the prevalence of the BRAF V600E mutation in
PTCs with or without current HT in patients from eastern coastal
China. Specifically, the association of the BRAF V600E
mutation with the clinicopathological features of PTC patients was
analyzed. These studies may provide the basis of developing
individual diagnostic protocols and treatments for TC patients.
Patients and methods
Patients
A total of 154 consecutive patients diagnosed with
thyroid nodules (206 affected nodules in total) were enrolled from
January 2012 to January 2013. All patients (31 males and 123
females, with a mean age of 50.3 years, range 22–76 years)
underwent thyroidectomy at the Department of Surgical Oncology at
Hangzhou First Peoples Hospital, (Hangzhou, China). Written
informed consent was obtained from all study participants, and the
study was approved by the Institutional Ethics Committee of
Hangzhou First Peoples Hospital.
Nodules were classified according to the following
diagnostic categories described by the World Health Organization
and Diagnostic Histopathology of Tumors (9): PTC (73 patients, 78 nodules), nodular
goiter (NG; 65 patients, 95 nodules), adenomatoid nodule (AN; seven
nodules), thyroid adenoma (TA; four nodules), and HT (six nodules).
HT was diagnosed according to previously described criteria
(10): the presence of goiter,
increased anti-thyroid antibodies (Tg-Ab, TPO-Ab) by >50%, or
pathological confirmation. Fourteen adjacent normal tissues that
were >1 cm distance from the PTC were also analyzed as normal
controls. Fresh thyroid tissue was quickly frozen at −80°C. Of the
206 nodules, 38 were excluded due to insufficient quality of the
nucleic acids, poor sequencing signal or improper storage.
Instruments
An Ultrasonic Doppler was purchased from Esaote
S.p.A. (Genova, Italy), a multi-image gel imaging system was
provided by Bio-Rad Laboratories Inc. (Hercules, CA, USA) and a
2720 Thermal Cycler PCR instrument was obtained from Applied
Biosystems (Life technologies Co. Ltd., Foster City, CA, USA).
DNA extraction and direct
sequencing
DNA was extracted by fresh-frozen thyroid tissue
using a kit (Takara MiniBEST Universal Genomic DNA extraction kit,
Ver. 4.0, Code D824A) provided by Bao-Biology Ltd. (Dalian, China)
following the manufacturer's recommendations. A 5-µl sample of the
DNA template amplified by polymerase chain reaction (PCR) was added
in a 50-µl reaction containing 5 µl 10X buffer, 4 µl dNTPs, 1 µl
each primer, 0.25 µl Taq DNA polymerase and 32.75 µl
dH2O (all from Bao-Biology Ltd.). PCR amplification of
the BRAF V600E mutation was carried out using the following
primers obtained from Huirui Biological Technology Ltd. (Shanghai,
China): sense, 5′-ACCTAAACTCTT CATAATGCTTGCT-3 and antisense,
5′-CTGATTTTTGTG AATACTGGGAACT-3′. PCR was carried out with an
initial denaturation step at 95°C for 5 min; then 30 cycles at 95°C
for 30 sec, 52°C for 30 sec and 72°C for 30 sec; and a final
extension cycle at 72°C for 10 min. Reaction products were
visualized on a 1.5% agarose gel with ethidium bromide, using
standard molecular weight DNA as a control.
Bi-directional sequencing was used to detect the
presence of the BRAF V600E point mutation by Inhuaiweiji
Biological Technology Ltd. (Shanghai, China). DNA sequences were
compared with those of the normal BRAF gene exon 15 in the
GenBank database using sequence assembly software (Sequencher 4.8;
Gene Codes Corporation, Ann Arbor, MI, USA).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA). The
association between categorical and continuous variables was
evaluated using the two-tailed Fisher's exact test and Student's
t-test, respectively. Binary logistic regression analysis was used
to assess the correlation between the BRAF V600E mutation
and clinicopathological features. P<0.05 was considered to
indicate a statistically significant difference.
Results
Electrophoresis and sequencing
results
Representative agarose gel electrophoresis results
of the PCR products are shown in Fig.
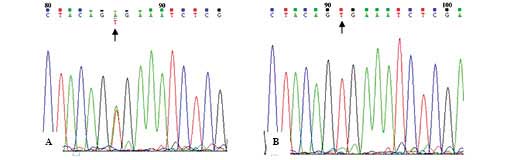
1, lanes 2–8. Sequencing results are shown in Fig. 2. In one patient, two different
BRAF mutations were noted; the BRAF V600E mutation
was identified in the PTC lesion, and the BRAF K601E
mutation was detected in the contralateral NG lesion. An A→G
transversion at exon 15 nucleotide 1801 (A1801G) of the BRAF
gene resulted in the replacement of lysine with glutamic acid at
position 601 (BRAF K601E) as shown in Fig. 3.
Detection of thyroid function and
BRAF V600E mutation
Table I shows the
levels of T3 and T4 thyroid hormone, free triiodothyronine, free
thyroxine and thyroid stimulating hormone (TSH), as well as the
prevalence of the BRAF V600E mutation for each group. No
significant difference was noted in thyroid function among the
groups (P>0.05). The prevalence of the BRAF V600E
mutation in PTCs was 61.5%, which was higher than that observed in
patients with benign lesions (χ2=75.732, P<0.001).
 | Table I.Correlation of BRAF V600E
mutation and thyroid function in thyroid nodules [n(%), (mean ±
SD)]. |
Table I.
Correlation of BRAF V600E
mutation and thyroid function in thyroid nodules [n(%), (mean ±
SD)].
|
|
| BRAF V600E
mutation |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Group | n | – | + | T3 (µg/l) | T4 (µg/l) | FT3 (pmol/l) | FT4 (pmol/l) | TSH (mIU/l) |
|---|
| NT | 14 | 13 (92.9) | 1 (7.1) | 1.04±0.26 | 97.58±22.02 | 4.66±0.43 | 16.02±2.44 | 2.15±1.21 |
| NG | 77 | 76 (98.7) | 1 (1.3) | 1.07±0.40 | 93.29±17.05 | 4.75±0.46 | 16.53±7.79 | 2.02±1.55 |
| AN | 5 | 5 (100.0) | 0 (0.0) | 1.08±0.29 | 86.45±26.00 | 4.51±0.93 | 13.29±3.79 | 2.65±2.87 |
| TA | 3 | 3 (100.0) | 0 (0.0) | 1.16±0.40 | 106.83±29.53 | 4.78±0.59 | 15.80±1.07 | 1.65±0.92 |
| TC | 65 | 25 (36.9) | 40
(61.5)a | 1.07±0.56 | 90.72±21.78 | 4.59±0.73 | 19.00±15.56 | 2.34±1.37 |
| HT | 4 | 4 (100.0) | 0 (0.0) | 1.61±1.66 | 60.87±36.17 | 4.31±0.82 | 13.19±7.64 | 3.04±1.71 |
Comparisons between 17 NGs and their contralateral
PTCs, five cases of bilateral PTCs and 15 cases of bilateral NGs
were also conducted. Among the five bilateral PTC patients, two
cases had the BRAF V600E mutations in both bilateral PTC
lesions, and two cases had wild-type BRAF in both bilateral
PTC lesions. The remaining PTC patient carried the BRAF
V600E mutation in one PTC lesion, but was negative in the
contralateral PTC lesion. Notably, the BRAF V600E mutation
was identified in two benign lesions; in normal tissue adjacent to
the PTC harboring the BRAF V600E and in NG tissue. The PTC
lesion offside to NG tissue carrying the BRAF V600E was also
observed to be mutated. In addition, immunohistochemistry analysis
revealed that both galectin-3 and HBME-1 were partially expressed
in the mutated NG tissue. Conversely, none of the other benign
lesions, including 14 bilateral NGs, 38 unilateral NGs, five ANs
and three TAs, had the BRAF V600E mutation.
Correlation of the BRAF V600E
mutation and HT
After the NG, AN and TA groups were merged into a
benign tissue (BT) group, this group was compared with the PTC
group; there was no significant difference in the prevalence of
patients with concurrent HT between the two groups (20.0 vs. 20.2%,
χ2=0.001, P=0.973; Table II). The
BRAF V600E mutation was detected mainly in PTCs (χ2=70.186,
P<0.001). Comparative analysis using layered HT from BT and PTC
indicated that the prevalence of the BRAF V600E mutation was
lower in PTCs accompanied with HT than in those without HT (38.5
vs. 67.3%), but it was not statistically significant (χ2=3.656,
P=0.056).
 | Table II.Prevalence of BRAF V600E
mutation in thyroid nodules in relation to Hashimoto thyroiditis
[n(%)]. |
Table II.
Prevalence of BRAF V600E
mutation in thyroid nodules in relation to Hashimoto thyroiditis
[n(%)].
|
|
|
| BRAF V600E
mutation |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | HT | n | – | + | χ2 | P-value |
|---|
| BT | – | 71 | 70 (98.6) | 1 (1.4) | 0.256 | 0.613 |
|
| + | 18 | 18 (100.0) | 0 (0.0) |
|
|
| PTC | – | 52 | 17 (32.7) | 35 (67.3) | 3.656 | 0.056 |
|
| + | 13 | 8 (61.5) | 5 (38.5) |
|
|
Correlation of the BRAF V600E
mutation with clinicopathological features
As shown in Table
III, no significant difference in gender (22.8 vs. 16.7%,
χ2=1.068, P=0.301) or age (49.0±11.1 vs. 51.1±10.1 years, t=1.134,
P=0.259) was noted between patients with the mutated and
non-mutated nodules. However, patients harboring the BRAF
V600E mutation had higher TSH levels compared with the patients
with wild-type BRAF (2.453±1.464 vs. 1.966±1.296 mIU/l,
respectively; t=2.019; P=0.045). Among the PTCs, 44 (67.7%) were
papillary thyroid microcarcinomas (PTMCs) with a diameter of 1 cm
or less. Compared with non-mutated PTCs, PTCs harboring the
BRAF V600E mutation were less likely to be a PTMC (55.0 vs.
88%, P<0.05), and they had a higher occurrence of lymph node
metastasis (LNM; 42.5 vs. 16.0%, P<0.05). Similarly, PTMC tumors
harboring the BRAF V600E mutation had a higher incidence of
LNM, although the difference was not significant (36.4 vs. 13.6%,
χ2=3.030, P=0.162).
 | Table III.Correlation of BRAF V600E
mutation and clinicopathological characteristics in thyroid nodules
[n(%), (mean ± SD)]. |
Table III.
Correlation of BRAF V600E
mutation and clinicopathological characteristics in thyroid nodules
[n(%), (mean ± SD)].
|
| Gender |
|
| PTMC | Lymph node
metastasis |
|---|
|
|
|
|
|
|
|
|---|
|
| Male | Female | Age (years) | TSH (mIU/l) | – | + | – | + |
|---|
| BRAF V600E
− | 21 (16.7) | 105 (83.3) | 51.1±10.1 | 1.966±1.296 | 3 (12.0) | 22 (88.0) | 21 (84.0) | 4 (16.0) |
| BRAF V600E
+ | 10 (23.8) | 32 (76.2) | 49.0±11.1 | 2.453±1.464 | 18 (45.0) | 22 (55.0) | 23 (57.5) | 17 (42.5) |
| Total | 31 (18.5) | 137 (81.5) | 50.6±10.4 | 2.198±1.514 | 21 (32.3) | 44 (67.7) | 44 (67.7) | 21 (32.3) |
|
|
χ2=1.068 | t=1.134 | t=2.019 |
χ2=7.661 |
χ2=4.940 |
| P-value | 0.301 | 0.259 | 0.045 | 0.006 | 0.026 |
Next, correlation analysis of the BRAF V600E
mutation and clinicopathological features was conducted. Binary
logistic regression analysis revealed that LNM of PTC was
associated with the BRAF V600E mutation, gender and PTMC,
but not with age, TSH or HT (Table
IV). In addition, the BRAF V600E mutation [hazard ratio
(HR), 5.051; 95% confidence interval (CI), 1.068–23.893; P=0.041)
and female gender (HR, 0.147; 95% CI, 0.031–0.697; P=0.016) were
independent factors that predicted LNM.
 | Table IV.Associations between
clinicopathological features and lymph node metastasis of papillary
thyroid carcinoma. |
Table IV.
Associations between
clinicopathological features and lymph node metastasis of papillary
thyroid carcinoma.
| Clinical
features | P-value | HR | 95% CI |
|---|
| Gender | 0.016 | 0.147 | 0.031–0.697 |
| Age, years | 0.279 |
|
|
| TSH level | 0.374 |
|
|
| PTMC | 0.181 |
|
|
| HT | 0.121 |
|
|
| BRAF
mutation | 0.041 | 5.051 | 1.068–23.893 |
Discussion
The prevalence of the BRAF V600E mutation in
PTCs ranges from 29 to 83% and is reported only in malignant
thyroid tumors (4,11,12);
similarly, the incidence of the BRAF V600E mutation was
61.5% in PTC patients from China's eastern coast in this study. All
of the BRAF V600E mutations identified in these PTCs were
heterozygous mutations. Similar to the results of previous studies,
most of the benign lesions, including 14 bilateral NGs, 38
unilateral NGs, five ANs and three TAs, carried the wild-type
BRAF gene. In contrast to other studies that failed to
identify BRAF mutations in benign thyroid disease, two
benign tissues carried the BRAF V600E mutation in the
present study. In the first case, the mutation was detected in
normal tissue adjacent to PTC that also carried the BRAF
V600E mutation, suggesting the presence of infiltrative growth. In
the second case, the BRAF V600E mutation was detected in the
NG tissue, on the contralateral PTC lesion also carrying the
mutation, suggesting that this mutation may represent an early
event in PTC progression. Moreover, immunohistochemistry analysis
of galectin-3 and HBME-1 expression in the BRAF
V600E-positive NG revealed that both were weakly expressed.
Combined immunohistochemistry for galectin-3 and HBME-1 was useful
for the differentiation of benign and malignant thyroid tumors
(13); therefore, the mutation in the
NG tissue may be indicative of a trend toward malignant biological
progression. In summary, the BRAF V600E mutation may
represent a critical step in tumor progression.
Another notable finding in the present study was
that one patient exhibited double mutations in the BRAF
gene; the BRAF V600E mutation was identified in the PTC
lesion, and the BRAF K601E mutation was identified in the
contralateral NG lesion. An A→G transversion at exon 15 nucleotide
1801 (A1801G) of the BRAF gene resulted in the replacement
of lysine with glutamic acid at position 601 (BRAF K601E),
which was also a heterozygous mutation in this patient. Trovisco
et al (14) first reported the
BRAF K601E mutation in a PTC patient in 2005, and 18
additional cases have been subsequently described (15–24).
Meta-analysis demonstrated that the BRAF K601E mutation was
predominantly identified in FV-PTC, followed by FTC and PTC, and
only one case of TA had the BRAF K601E mutation. Although it
may affect the PI3k/Akt pathway, this mutation exhibited relatively
inactive biological characteristics in the pathogenesis of TC
compared with the BRAF V600E mutation (15–24).
Moreover, in multifocal papillary thyroid carcinoma (mPTC),
individual tumor foci may be identical and are frequently composed
of various histological types, and individual tumor foci also
harbor different mutations. For example, Kim et al (23) described a case of mPTC consisting of
one FV-PTC harboring the BRAF K601E mutation and three
conventional foci harboring the BRAF V600E mutation.
Similarly, we present a patient with both the BRAF V600E and
BRAF K601E mutations although the BRAF K601E mutation
was detected in a NG. To our knowledge, this is the first study of
a BRAF K601E mutation in NG tissue. We suspect that the
BRAF K601E mutation in the NG tissue may be an indication of
oncogene activation that is believed to take place early in the
course of tumorigenesis. However, determining the exact mechanism
requires further research.
Numerous studies have demonstrated an association
between the BRAF V600E mutation with aggressive
clinicopathological characteristics of PTC, including
extrathyroidal invasion, LNM, advanced tumor-node-metastasis stage,
loss of radioiodine avidity and disease recurrence (5–7).
BRAF mutation presents a low positive predictive value (28%)
and a high negative predictive value (87%) for PTC recurrence,
suggesting that BRAF mutational status in clinical
management of PTC should be used with caution. However, it is still
controversial to use BRAF mutation as an independent
predictive risk factor for PTC due to the fact that the BRAF
V600E mutation is identified in approximately half of PTCs, among
which less than 10–15% of the tumors exhibit aggressive behavior
(25). In accordance with previous
studies, a higher rate of LNM was noted in BRAF
V600E-positive PTCs than in negative ones at the time of surgery in
this study. In addition, binary logistic regression analysis
indicated that this mutation was an independent predictive factor
of LNM in PTC patients. The proportion of PTMC was lower in PTCs
with the BRAF V600E mutation than in those with the
wild-type gene. Taken together, this mutation may promote tumor
growth and LNM; therefore, the BRAF V600E mutation was
correlated with a more aggressive behavior of PTC.
Even though tumor-related mortality is as low as
0.5% for PTMC, microcarcinoma harboring the BRAF V600E
mutation is also associated with features predictive of a high risk
of recurrence and metastasis (26–28). In
our study, 67.7% (44/65) of PTCs were PTMCs, among which 50%
(22/44) of the patients had the BRAF V600E mutation.
Consistent with previous studies, the presence of the BRAF
V600E mutation in PTMC further suggests that the mutation is an
early event in thyroid carcinogenesis. Similarly, patients with
PTMCs harboring the BRAF V600E mutation had a higher
incidence of LNM although this was not significant (P=0.162).
However, we expect that this correlation will be confirmed in
further studies with larger sample sizes.
A number of studies (29,30) have
reported a weak association between serum TSH concentration and
thyroid cancer, with certain investigators suggesting that higher
preoperative TSH serum concentration may be associated with
advanced tumor stage and poor prognosis (29); however, the correlation between TSH
and PTC remains controversial. Although TSH suppressive therapy is
a well-known adjuvant therapy to prevent recurrence in
differentiated thyroid cancer, no significant difference in TSH
level was identified among different pathological types in the
present study, which may be due to the insufficient sample size. In
the present study, compared with tumors with wild-type BRAF,
patients with PTCs harboring the BRAF V600E mutation had
higher TSH levels. Whether the mutation is involved in the
activation of TSH receptor remains unclear and requires further
analysis.
PTC with HT is frequently observed; however, the
association between HT and PTC is controversial. When compared with
benign lesions, there was no significant difference in the rate of
PTCs with concurrent HT; therefore, the correlation between HT and
TCs remains unclear. However, Kim et al (30) reported that in Korean patients with
PTC, the BRAF V600E mutation was associated with a low
frequency of background HT and a high frequency of LNM. Similarly,
PTCs accompanied with HT had a lower frequency of the BRAF
V600E mutation in our study, although this was not significant
(P=0.056). However, additional analysis in an expanded sample size
may yield significant differences.
In conclusion, in PTC patients from China's eastern
coast, the BRAF V600E mutation was prevalent (61.5%).
Similar to findings in previous studies, our results supported the
notion that the mutation was an early and phenotypically defining
molecular event in PTC, associated with features predictive of a
high risk of LNM even in microcarcinomas. In addition, one patient
in our study exhibited two mutations in the BRAF gene;
BRAF V600E and BRAF K601E, with the latter identified
in NG tissue.
Acknowledgements
This study was supported by grants from the Medical
Science Research Foundation of Zhejiang Province (2010KYA161) and
the Key Project of Scientific and Technological Innovation in
Hangzhou (20131813A08).
References
|
1
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuttle RM, Ball DW, Byrd D, et al: Thyroid
carcinoma. J Natl Compr Canc Netw. 8:1228–1274. 2010.PubMed/NCBI
|
|
3
|
Veiga LH, Neta G, Aschebrook-Kilfoy B, Ron
E and Devesa SS: Thyroid cancer incidence patterns in Sao Paulo,
Brazil and the US SEER Program. Thyroid. 23:748–757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang KT and Lee CH: BRAF mutation
in papillary thyroid carcinoma: pathogenic role and clinical
implications. J Chin Med Assoc. 73:113–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mesa C Jr, Mirza M, Mitsutake N, et al:
Conditional activation of RET/PTC3 and BRAFV600E in thyroid
cells is associated with gene expression profiles that predict a
preferential role of BRAF in extracellular matrix
remodeling. Cancer Res. 66:6521–6529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durand S, Ferraro-Peyret C, Joufre M,
Chave A, Borson-Chazot F, Selmi-Ruby S and Rousset B: Molecular
characteristics of papillary thyroid carcinomas without BRAF
mutation or RET/PTC rearrangement: relationship with
clinico-pathological features. Endocr Relat Cancer. 16:467–481.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing M: Genetic alterations in the
phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.
Thyroid. 20:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Konturek A, Barczynski M, Wierzchowski W,
Stopa M and Nowak W: Coexistence of papillary thyroid cancer with
Hashimoto thyroiditis. Langenbecks Arch Surg. 398:389–394. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou GY, Liu HQ and Zhang QH: Thyroid and
parathyroid gland tumorsDiagnostic Histopathology of Tumors.
Fletcher CD: 1st. Shandong Science and Technology Press; Jinan: pp.
5992001
|
|
10
|
Liao EY and Chao CS: Chronic lymphocytic
thyroiditisEndocrinology. Zhang H, Dai RC and Liao EY: 1st.
People's Medical Publisher; Beijing: pp. 7152001
|
|
11
|
Gouveia C, Can NT, Bostrom A, Grenert JP,
van Zante A and Orloff LA: Lack of association of BRAF
mutation with negative prognostic indicators in papillary thyroid
carcinoma: the University of California, San Francisco, experience.
JAMA Otolaryngol Head Neck Surg. 139:1164–1170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witt RL, Ferris RL, Pribitkin EA, Sherman
SI, Steward DL and Nikiforov YE: Diagnosis and management of
differentiated thyroid cancer using molecular biology.
Laryngoscope. 123:1059–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu LY, Zhang C, Xu RJ, Zhou H and Ma LZ:
Diagnostic value of TPO, CK-19, Gal-3 and HBME-1 in fine needle
aspiration cytological specimens for thyroid carcinoma with
Hashimoto's disease. Zhejiang Medical. 32:1007–1010. 10132010.
|
|
14
|
Trovisco V, Soares P, Soares R, Magalhães
J, Sa-Couto P and Sobrinho-Simoes M: A new BRAF gene
mutation detected in a case of a solid variant of papillary thyroid
carcinoma. Hum Pathol. 36:694–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt J, Derr V, Heinrich MC, Crum CP,
Fletcher JA, Corless CL and Nose V: BRAF in papillary
thyroid carcinoma of ovary (struma ovarii). Am J Surg Pathol.
31:1337–1343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lupi C, Giannini R, Ugolini C, et al:
Association of BRAF V600E mutation with poor
clinicopathological outcomes in 500 consecutive cases of papillary
thyroid carcinoma. J Clin Endocrinol Metab. 92:4085–4090. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frau DV, Lai ML, Caria P, et al: Trisomy
17 as a marker for a subset of noninvasive thyroid nodules with
focal features of papillary carcinoma: cytogenetic and molecular
analysis of 62 cases and correlation with histological findings. J
Clin Endocrinol Metab. 93:177–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolff EF, Hughes M, Merino MJ, Reynolds
JC, Davis JL, Cochran CS and Celi FS: Expression of benign and
malignant thyroid tissue in ovarian teratomas and the importance of
multimodal management as illustrated by a BRAF-positive
follicular variant of papillary thyroid cancer. Thyroid.
20:981–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pennelli G, Vianello F, Barollo S, et al:
BRAF (K601E) mutation in a patient with a follicular thyroid
carcinoma. Thyroid. 21:1393–1396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohori NP, Singhal R, Nikiforova MN, et al:
BRAF mutation detection in indeterminate thyroid cytology
specimens: underlying cytologic, molecular and pathologic
characteristics of papillary thyroid carcinoma. Cancer Cytopathol.
121:197–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schulten HJ, Salama S, Al-Mansouri Z, et
al: BRAF mutations in thyroid tumors from an ethnically
diverse group. Hered Cancer Clin Pract. 10:10–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JY, Kim WY, Hwang TS, et al:
BRAF and RAS mutations in follicular variants of papillary
thyroid carcinoma. Endocr Pathol. 24:69–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim WY, Ko YS and Hwang TS: A case of
multifocal papillary thyroid carcinoma consisting of one
encapsulated follicular variant with BRAF K601E mutation and
three conventional types with BRAF V600E mutation. Korean J
Pathol. 47:293–298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barollo S, Pezzani R, Cristiani A, et al:
Prevalence, tumorigenic role and biochemical implications of rare
BRAF alterations. Thyroid. 24:809–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soares P, Celestino R, Melo M, Fonseca E
and Sobrinho-Simoes M: Prognostic biomarkers in thyroid cancer.
Virchows Archiv. 464:333–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roti E, Degli Uberti EC, Bondanelli M and
Braverman LE: Thyroid papillary microcarcinoma: a descriptive and
meta-analysis study. Eur J Endocrinol. 159:659–673. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee X, Gao M, Ji Y, et al: Analysis of
differential BRAF (V600E) mutational status in high
aggressive papillary thyroid microcarcinoma. Ann Surg Oncol.
16:240–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Virk RK, Van Dyke AL, Finkelstein A, et
al: BRAFV600E mutation in papillary thyroid microcarcinoma:
a genotype-phenotype correlation. Mod Pathol. 26:62–70. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim D and Park JW: Clinical implications
of preoperative thyrotropin serum concentrations in patients who
underwent thyroidectomy for nonfunctioning nodule(s). J Korean Surg
Soc. 85:15–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SK, Song KH, Lim SD, Lim YC, Yoo YB,
Kim JS and Hwang TS: Clinical and pathological features and the
BRAF (V600E) mutation in patients with papillary thyroid
carcinoma with and without concurrent Hashimoto thyroiditis.
Thyroid. 19:137–141. 2009. View Article : Google Scholar : PubMed/NCBI
|