Introduction
Bone is one of the most common sites of metastasis
and the spine is the most frequently affected painful skeletal site
(1). Spinal metastases are observed
in more than two-thirds of patients who succumb to cancer.
Pathological fractures therefore affect vertebrae in 10–20% of
cases that involve the posterior wall of the vertebral body,
causing them to protrude posteriorly and compromise the spinal
canal, resulting in neurological injury (2). The most common sites of disease are the
thoracic vertebrae (60–80%), followed by the lumbar (20%) and
cervical (10%) spine (3).
The invasiveness of metastatic lesions in the
vertebral body and its attachments may cause severe thoracic lumbar
and back pain, and even neurological dysfunction, reducing the
patient's quality of life and hastening mortality. Radiotherapy
weakens the bone remodeling ability and increases the risk of
vertebral collapse and nerve compression (4–6). By
contrast, surgery is more suitable for patients with an oppressed
spinal cord, but this causes more trauma and has a high incidence
of complications; in particular, corpectomy is not suitable for
patients with multiple vertebral metastases. Percutaneous
vertebroplasty (PVP) has been widely used in the treatment of
vertebral osteolytic tumors, and has become the main treatment of
these diseases (1,7); however, its application in the treatment
of multiple thoracic metastases is rarely reported.
In the present study, the clinical data of 28
patients with multiple thoracic metastases who received PVP at the
Department of Spinal Surgery at the Affiliated Hospital of Weifang
Medical College (Weifang, Shangdong, China), between March 2006 and
March 2008, were therefore retrospectively analyzed in order to
explore the treatment effectiveness of the injection of bone cement
on multiple thoracic metastases.
Materials and methods
General data
A total of 28 patients with multiple thoracic
metastases, consisting of 9 males and 19 females, aged 36–76 years
(mean, 60.5 years), were retrospectively analyzed. The study was
conducted in accordance with the Declaration of Helsinki and with
the approval of the Ethics Committee of Weifang Medical College.
Written informed consent was obtained from all participants. All
patients presented with thoracolumbar and back pain, percussion
pain and movement disorders of the thoracolumbar vertebrae, among
which, one patient presented with decreased superficial sensation
of the skin of one lower limb. X-ray revealed various degrees of
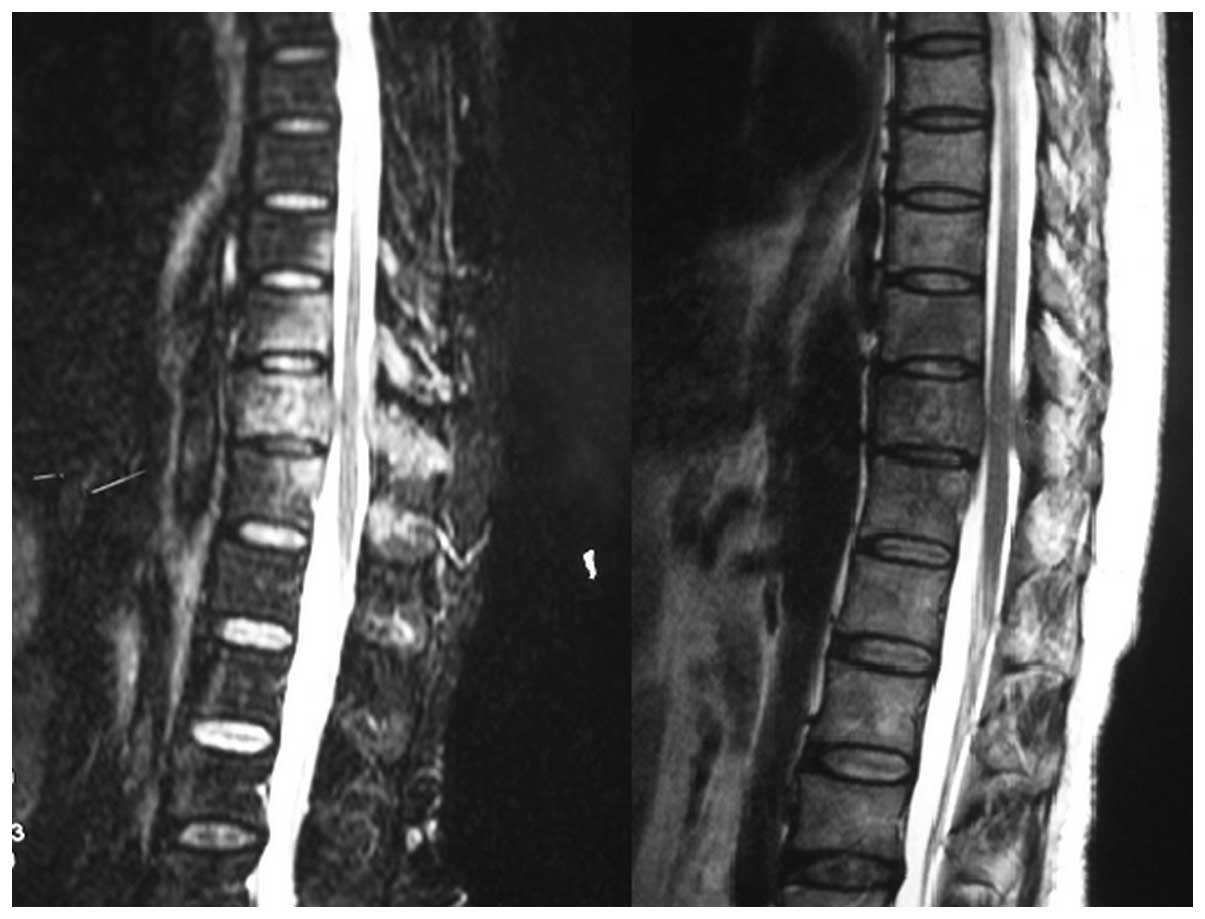
damage and/or collapse in the involved vertebral bodies. Computed
tomography (CT) and magnetic resonance imaging (MRI) scans revealed
osteolytic bone destruction, but the posterior wall of the involved
vertebral body was intact (Fig. 1).
Emission CT (ECT) revealed significant radioactivity accumulation
in the thoracic lesions. No cases were complicated with severe
bleeding, coagulation disorders or severe lung diseases. Patients
with advanced cachexia and extreme physical weakness, as well as
patients with spinal cord involvement and paraplegia, were
excluded. In total, the 28 patients exhibited 104 involved
vertebral bodies, spread amongst the T12 (n=16), T11 (n=21), T10
(n=23), T9 (n=19), T8 (n=11), T7 (n=6), T6 (n=3), T5 (n=1), T4
(n=3) and T2 (n=1) vertebrae. Among the 28 patients, there were 11
patients with continuous thoracic metastases and 17 patients with
interval metastases. The number of involved thoracic vertebrae in
each patient was between two and six. There were six thoracic
vertebrae involved in three cases, five thoracic vertebrae involved
in four cases, four thoracic vertebrae involved in 11 cases, three
thoracic vertebrae involved in six cases and two thoracic vertebrae
involved in four cases. The sources of metastases included breast
cancer (n=12), prostate cancer (n=3), gastric cancer (n=3), lung
cancer (n=3), kidney cancer (n=1), liver cancer (n=2) and thyroid
carcinoma (n=1). There were two cases with unknown origin. A total
of 16 cases of primary lesions had been surgically removed and
determined histologically, 10 cases were confirmed using needle
biopsy and endoscopy, and the other two had unknown origin.
Methods
Patients were asked to maintain a prone position.
The lower thoracic spine was punctured through the pedicle of the
vertebral arch, and the upper thoracic spine was also punctured
through the pedicle of the vertebral arch or through the gap
between the costal head and the vertebral body. Guided by the G-arm
X-ray machine, the needle (Guanlong Medical Supplies Co., Jinan,
China) was percutaneously inserted into the pedicle of the
vertebral arch at a 10–15° angle, and further passed into the
anterior third of the vertebral body. The point of the needle was
located in the upper or lower half of the vertebral body according
to the location of the tumor determined by MRI. For the two
patients with severe osteoporosis, in order to avoid the
difficulties of the G-arm X-ray machine in positioning the involved
vertebra and the needle insertion point, the puncture was performed
with the guidance of a large digital subtraction angiography
machine (Angiostar, Siemens, Munich, Germany). Bone cement (Heraeus
Medical GmbH, Wehrheim, Germany), which can be developed under
X-ray, was prepared with a ratio of powder (g) to liquid (m1) of
2:1. With the guidance of the X-ray, the bone cement in starch
paste state was fractionatedly and slowly injected into the
involved vertebral bodies at a volume of 0.5 to 1.0 ml per time
until the distribution of bone cement reached the edge of the
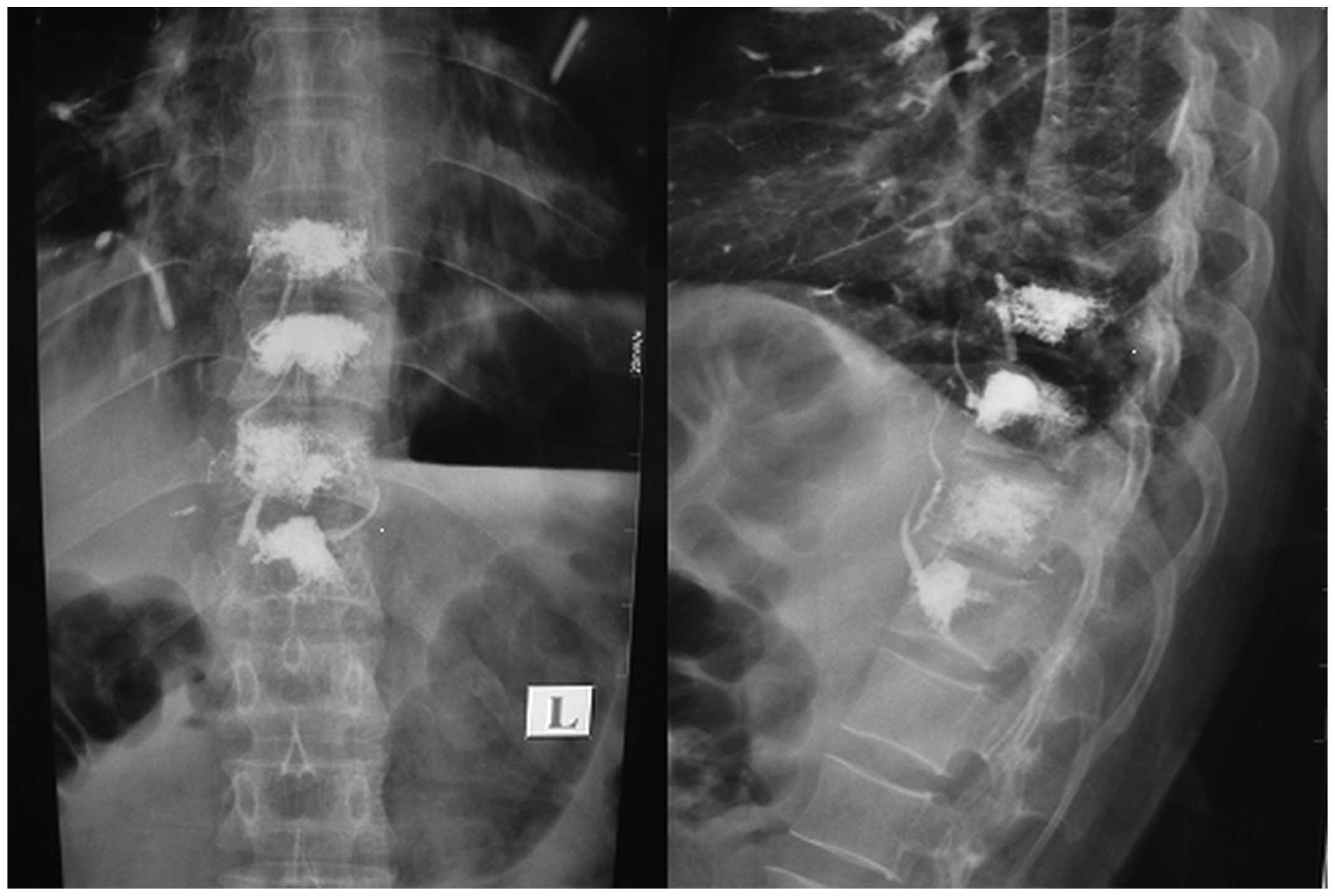
vertebral body. If any apparent bone cement leakage was observed at
the side or rear of the vertebral body, the injection was
immediately stopped (Fig. 2). The
total injection volume was 2.0 to 2.5 ml for the upper thoracic
spine and 2.5 to 3.5 ml for the lower thoracic spine. During the
injection of bone cement, patients were monitored by
electrocardiogram and the sensory and movement changes of their
lower extremity was observed at all time. Post-operative
application of antibiotics was administered for three days.
Patients were allowed to ambulate after 48 h of bed rest. Periodic
post-operative reviews of the distribution of bone cement in the
vertebral bodies were performed using X-ray or CT scans (Fig. 3). Following PVP, all patients received
appropriate chemotherapy and radiotherapy, with schemes specific
for each patient (Table I).
 | Table I.Radiotherapy and chemotherapy
treatments for patients with percutaneous vertebroplasty. |
Table I.
Radiotherapy and chemotherapy
treatments for patients with percutaneous vertebroplasty.
| Patient tumor
type | Chemotherapy | Radiotherapy | Other treatments |
|---|
| Breast cancer | TXT+EPI | Local
radiotherapy |
|
| Prostate cancer | DDP+VP-16 |
| Endocrine
therapy |
| Gastric cancer | L-OHP+5-FU+CF |
|
|
| Small cell lung
cancer | CBP+VP-16 |
|
|
| Non-small cell lung
cancer | GEMZ+DDP |
|
|
| Kidney cancer | GEMZ |
|
|
| Liver cancer | Regional 5-FU
perfusion |
|
|
| Thyroid
carcinoma |
| Local
radiotherapy |
|
| Unknown origin |
|
| Symptomatic
therapy |
Evaluation of therapeutic effect
To assess pain relief, the pain status of the
patients prior to and following treatment was measured according to
visual analog scales (8).
Post-operative pain relief was graded into six levels: Grade 0, no
relief; grade I, pain reduced by <25%; grade II, pain reduced by
25–50% (the dosage of analgesics should be reduced); grade III,
pain reduced by 5l-75% (the dosage of analgesics should be reduced
by one step); grade IV, pain reduced by 76–90% (the dosage of
analgesics should be reduced by two steps or the administration
should be stopped); and grade V, complete pain relief (the
analgesics should be stopped). Pain relief assessment was conducted
at 1 week, and 3, 6 and 12 months post-PVP. Grades IV and V were
considered as excellent, grade III as good, grade II as a response
and grades I and 0 as no response.
To assess quality of life, ratings of the activity
of daily life (ADL) (9) were
used.
To assess the morphological changes of the involved
vertebrae and the invasiveness of the intraspinal tumors, the
heights of the anterior border, center and posterior border of the
involved vertebrae were measured using X-ray examination one year
after PVP surgery to determine whether vertebral body collapse had
occurred. The attachments of the involved vertebrae, the tumor
invasion in the vertebral canal and the oppression of the spinal
cord were also observed using a CT or MRI scan.
Statistical analysis
Statistical analyses were performed independently by
a non-clinical research assistant and an outside party to ensure
objectivity using SPSS version 16.0 software (SPSS, Inc., Chicago,
IL, USA). Student's t-test was used and P≤0.05 was considered to
indicate a statistically significant result for continuous
variables.
Results
The 28 patients all received successful punctures,
including 4 bilateral thoracic pedicle punctures and 100 unilateral
thoracic vertebral punctures. Bone cement leakage occurred in one
thoracic vertebral body in the intervertebral space. The average
surgical time for each individual vertebra was 0.5 h (range,
0.3–0.9 h). The total injection volume of bone cement was 2.2 to
3.7 ml (mean, 3.1±0.4 ml). No spinal cord compression was observed,
nor were other complications, such as pulmonary embolism and
infection. Patients were followed up for 6 to 24 months (mean, 14
months). One patient succumbed at 6 months post-surgery and another
succumbed at 12 months post-surgery. All mortalities were due to
systemic metastases of malignant tumors.
Pain relief
Post-operative pain was graded according to the
degree of pain relief. The pain relief rates of the patients at 1
week, and 3, 6 and 12 months post-PVP were 82.1, 89.3, 81.5 and
88.5%, respectively (Table II).
 | Table II.Post-operative pain relief and its
response rate in patients treated for multiple thoracic
metastases. |
Table II.
Post-operative pain relief and its
response rate in patients treated for multiple thoracic
metastases.
| Pain relief
grades | 1 week | 3 months | 6 months | 12 months |
|---|
| Grade V, n | 8 | 11 | 9 | 7 |
| Grade IV, n | 8 | 9 | 8 | 6 |
| Grade III, n | 7 | 5 | 5 | 10 |
| Grade II, n | 3 | 2 | 2 | 1 |
| Grade I, n | 2 | 1 | 2 | 1 |
| Grade 0, n | 0 | 0 | 1 | 1 |
| Mortalities, n | 0 | 0 | 1 | 1 |
| Response rate, n
(%) | 82.1 (23/28) | 89.3 (25/28) | 81.5 (22/27) | 88.5 (23/26) |
ADL
At the end of follow-up, the ADL scores of the
remaining 26 patients were significantly different from those prior
to surgery (P<0.01). The ability to use the toilet, move, walk
horizontally for 50 m, and go up and down the stairs was
significantly improved compared with prior to surgery. In addition,
the ability to eat and dress were also statistically different
prior to and following surgery (Table
III).
 | Table III.Comparison of activity of daily life
scores of the 26 patients with multiple thoracic metastases prior
to and one year after surgery. |
Table III.
Comparison of activity of daily life
scores of the 26 patients with multiple thoracic metastases prior
to and one year after surgery.
| Time-points | Eating | Bathing | Personal care | Dressing | Bowel management | Bladder
management | Toileting | Moving bed or
chair | Walking horizontally
for 50 m | Moving up/down
stairs | Total scores |
|---|
| Prior to PVP
(n=26) | 7.6±1.3 | 3.0±1.4 | 3.6±0.7 | 6.3±1.1 | 8.9±1.7 | 8.9±2.1 | 4.3±0.7 | 5.9±1.2 | 4.5±0.6 | 2.3±0.5 | 54.3±12.7 |
| 1 year after PVP
(n=26) | 9.2±1.5 | 3.2±1.3 | 4.8±1.1 | 8.6±1.4 | 9.1±2.0 | 9.1±1.8 | 8.4±1.7 | 11.2±1.7 | 12.3±1.6 | 8.1±1.6 | 84.3±17.9 |
| t-value | 2.125 | 1.123 | 0.984 | 2.564 | 1.154 | 1.025 | 4.235 | 5.625 | 5.745 | 6.137 | 4.562 |
| P-value | 0.042 | 0.182 | 0.192 | 0.041 | 0.161 | 0.121 | 0.007 | 0.005 | 0.003 | 0.002 | 0.008 |
Vertebral height
One year after PVP, the heights of the anterior
border, center and posterior border of the 98 involved vertebrae of
the remaining 26 patients were not significantly different from
those prior to PVP (P>0.05; Table
IV). As demonstrated by the pre-operative CT and MRI scan one
year after PVP, there were five vertebrae in four patients that
presented with pedicle invasiveness and extending into the spinal
canal, but no obvious neurological symptoms were observed.
 | Table IV.Comparison of vertebral heights of the
98 involved vertebrae of the 26 patients prior to (n=26) and 1 year
after (n=26) surgery. |
Table IV.
Comparison of vertebral heights of the
98 involved vertebrae of the 26 patients prior to (n=26) and 1 year
after (n=26) surgery.
| Time-points | Anterior border | Center | Posterior border |
|---|
| Prior to PVP | 18.7±1.6 | 17.9±1.9 | 19.9±2.0 |
| 1 year after PVP | 19.1±1.7 | 17.8±1.5 | 19.8±1.8 |
| t-value | 1.452 | 1.637 | 1.836 |
| P-value | 0.095 | 0.121 | 0.134 |
Discussion
Spinal malignant tumors are mainly metastases. It
has been reported that 60 to 80% of malignant tumors metastasize to
the spine, mainly involving the thoracic vertebrae, followed by the
lumbar and then the cervical vertebrae (1–3). The
invasiveness of the metastasis to the vertebral body and its
accessories leads to severe thoracolumbar and back pain, and even
neurological dysfunction, seriously affecting a patient's daily
life. However, conventional radiotherapy and chemotherapy achieve a
less than ideal therapeutic effect. Although the effect of active
surgical treatment in recent years has been improved greatly and
the surgical indications have also been expanded along with the
improvement of spinal surgery techniques, treating multiple
thoracic metastases remains a challenge, particularly for interval
metastases (10,11). Since PVP was applied in the treatment
of spinal metastases by Kaemmerlen et al (12) in 1989, good results have been achieved
in China and abroad. However, its application in the treatment of
multiple thoracic metastases has never been reported.
Metastasis destroys the vertebral body by causing
micro-fractures and compression fractures, resulting in spinal
instability (13). The nerve endings
inside and outside the vertebral body are stimulated and damaged,
which is the most common reason for thoracolumbar and back pain
(14,15). The direct invasiveness of the tumor
tissues to the nerve endings also causes pain (16). The present study showed that PVP
quickly relieved thoracolumbar and back pain in patients with
multiple thoracic metastases. The mechanisms for this may be as
follows: i) The stable and supportive roles of
polymethylmethacrylate (PMMA) on the vertebral body. The PMMA
injected into the involved vertebrae solidifies into clumps in a
short time, inhibiting the reduction of the support force caused by
vertebral destruction and fixing the micro-fractures in the
involved vertebral bodies, thereby reducing the stimulation on the
nerve roots and sinus vertebral nerve due to the loss of stability
of the spine (17,18). ii) The thermogenic effect during the
polymerization of PMMA. The polymerization of PMMA increases the
local temperature of the vertebral body, reaching 52 to 93°C, which
leads to degeneration, necrosis and a loss of sensation in the pain
nerve endings within the vertebral body. Furthermore, the heat
effectively inactivates the tumor cells and reduces the production
of the mediators of inflammation and pain. The heat also prevents
the growth of cancer cells and reduces its compression on the nerve
endings (19). iii) The monomer
toxicity of PMMA. PMMA produces monomers with toxic side-effects in
the body, causing peripheral nerve endings and tumor cell necrosis
(20).
Simple chemotherapy or radiotherapy achieves only
limited results on spinal metastases. Moreover, it cannot relieve
the unstable spine and spinal cord compression caused by the
invasiveness of tumors (15,21,22). In
the present study, PVP significantly prevented the involved
vertebral bodies from further collapse or invasion into the spinal
canal, and then prevented it from nerve dysfunction induced by
spinal cord compression. Since the destruction of metastatic tumors
is mostly osteolytic, it often results in bone defects. The
vertebral compression fracture occurs when the spine bears the body
weight and consequently increases pain and even causes neurological
symptoms (5,6,19). The
injection of bone cement can strengthen the vertebral structure and
restore the height, proof pressure and intensity of vertebral
bodies. In addition, the heat and monomers produced locally have
antitumor effects, which reduce the local tumor burden and
consequently reduce the destruction of local tumors on bones
(18). All these effects
significantly prevent the vertebral bodies from further collapse
and from invasion of the spinal tumors.
Currently, the evaluation of the treatment efficacy
of PVP on vertebral metastases is mainly based on pain relief.
However, the simple application of pain relief assessment cannot
fully reflect the patient's quality of life. In the present study,
ADL scores were used to comprehensively assess quality of life
following PVP. The results indicated that the application of PVP in
the treatment of multiple thoracic metastases comprehensively
improves a patient's quality of life.
The major complication of PVP is the leakage of bone
cement to the surrounding area, causing mechanical compression on
nerve roots and the spinal cord or simultaneously causing thermal
damage. The leakage of bone cement into the peripheral veins,
particularly into the basivertebral venous plexus, can cause a
pulmonary embolism (17,20). The occurrence of complications is
associated with the injection rate of bone cement. Thus, the
injection of bone cement should be performed slowly with the
guidance of X-ray. Side monitoring can prevent bone cement
permeating into the spinal canal, and anteroposterior monitoring
can prevent bone cement leaking bilaterally into the intervertebral
foramen. In order to avoid leakage, bone cement should be injected
fractionatedly at a volume of 0.5 to 1.0 ml each time. If there are
any signs of leakage, the injection should be immediately stopped.
The puncture needle should be inserted into the anterior third of
the vertebral body, but not wear through the inner wall of the
pedicle. The consistency of bone cement for infusion is also a
significant factor. Its flow is difficult to control if it is too
thin, as it leaks easily and also refluxes with venous flow.
Moreover, a careful study of the preoperative imaging data is also
critical to prevent leakage.
Compared with surgical ablation of thoracic
metastases, PVP is less harmful to patients and is relatively easy
to administer; it effectively relieves the pain caused by
osteolytic vertebral metastases, increases vertebral strength and
improves spinal stability. Moreover, PVP can significantly prevent
the invasion of spinal tumors and improve the patient's quality of
life. This innovative technology for micro-spinal surgery is likely
to become one of the primary means of palliative treatment for
multiple thoracic metastases.
Acknowledgements
This study was supported by a grant from the Special
Training Fund for Domestic and Abroad High-level Health
Technological Talents in Shandong Province (grant no.
2013WS0277).
References
|
1
|
Barragán-Campos HM, Vallée JN, Lo D, et
al: Percutaneous vertebroplasty for spinal metastases:
complications. Radiology. 238:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masala S, Anselmetti GC, Muto M, Mammucari
M, Volpi T and Simonetti G: Percutaneous vertebroplasty relieves
pain in metastatic cervical fractures. Clin Orthop Relat Res.
469:715–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thanos L, Mylona S, Galani P, et al:
Radiofrequency ablation of osseous metastases for the palliation of
pain. Skeletal Radiol. 37:189–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarençon F, Jean B, Pham HP, et al: Value
of percutaneous radiofrequency ablation with or without
percutaneous vertebroplasty for pain relief and functional recovery
in painful bone metastases. Skeletal Radiol. 42:25–36. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anselmetti GC, Marcia S, Saba L, et al:
Percutaneous vertebroplasty: multi-centric results from everest
experience in large cohort of patients. Eur J Radiol. 81:4083–4086.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masala S, Massari F, Fiori R, Mammucari M,
Bartolucci DA and Simonetti G: Future directions in percutaneous
vertebroplasty. Radiol Med. 114:976–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anselmetti GC, Corgnier A, Debemardi F and
Regge D: Treatment of painful compression vertebral fractures with
vertebroplasty: results and complications. Radiol Med. 110:262–272.
2005.PubMed/NCBI
|
|
8
|
Burton AW, Reddy SK, Shah HN,
Tremont-Lukats I and Mendel E: Percutaneous vertebroplasty - a
technique to treat refractory spinal pain in the setting of
advanced metastatic cancer: a case series. J Pain Symptom Manage.
30:87–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lips P and van Schoor NM: Quality of life
in patients with osteoporosis. Osteoporos Int. 16:447–455. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simmons ED and Zheng Y: Vertebral tumors:
surgical versus nonsurgical treatment. Clin Orthop Relat Res.
443:233–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee B, Franklin I, Lewis JS, Coombes RC,
Leonard R, Gishen P and Stebbing J: The efficacy of percutaneous
vertebroplasty for vertebral metastases associated with solid
malignancies. Eur J Cancer. 45:1597–1602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaemmerlen P, Thiesse P, Bouvard H, Biron
P, Mornex F and Jonas P: Percutaneous vertebroplasty in the
treatment of metastases. Technic and results. J Radiol. 70:557–562.
1989.(In French). PubMed/NCBI
|
|
13
|
Oakland RJ, Furtado NR, Timothy J and Hall
RM: The biomechanics of vertebroplasty in multiple myeloma and
metastatic bladder cancer: a preliminary cadaveric investigation. J
Neurosurg Spine. 9:439–501. 2008. View Article : Google Scholar
|
|
14
|
Do HM, Kim BS, Marcellus ML, Curtis L and
Marks MP: Prospective analysis of clinical outcomes after
pereutaneous vertebroplasty for painful osteoporotic vertebral body
fractures. AJNR Am J Neuroradiol. 26:1623–1628. 2005.PubMed/NCBI
|
|
15
|
Chen LH, Hsieh MK, Niu CC, Fu TS, Lai PL
and Chen WJ: Percutaneous vertebroplasty for pathological vertebral
compression fractures secondary to multiple myeloma. Arch Orthop
Trauma Surg. 132:759–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao HQ, Yang HL, Geng DC, Bao ZH and Tang
TS: Spinal extradural arachnoid cyst following percutaneous
vertebroplasty. Eur Spine J. 20:(Suppl 2). S206–S210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trumm C, Jakobs T, Pahl A, et al: CT
fluoroscopy-guided percutaneous vertebroplasty in patients with
multiple myeloma: analysis of technical results from 44 sessions
with 67 vertebrae treated. Diagn Interv Radiol. 18:111–120.
2012.PubMed/NCBI
|
|
18
|
Kallmes DF, Comstock BA, Heagerty PJ, et
al: A randomized trial of vertebroplasty for osteoporotic spinal
fractures. New Engl J Med. 361:569–579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garland P, Gishen P and Rahemtulla A:
Percutaneous vertebroplasty to treat painful myelomatous vertebral
deposits-long-term efficacy outcomes. Ann Hematol. 90:95–100. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan D, Duan L, Li J, Soo C, Zhu H and
Zhang Z: Comparative study of percutaneous vertebroplasty and
kyphoplasty in the treatment of osteoporotic vertebral compression
fractures. Arch Orthop Trauma Surg. 131:645–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown DB, Gilula LA, Sehgal M and Shimony
JS: Treatment of chronic symptomatic vertebral compression fracture
with percutaneous vertebroplasty. AJR Am J Roentgenol. 182:319–322.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacofsky DJ, Papagelopoulos PJ and Sim FH:
Advances and challenges in the surgical treatment of metastatic
bone disease. Clin Orthop Relat Res. 415:(Suppl). S14–S18. 2003.
View Article : Google Scholar : PubMed/NCBI
|