Introduction
Many epidemiological studies show a relationship
between diet and the incidence of colorectal cancer (CRC). In
particular, it is known that approximately 60–75% of all sporadic
CRC cases are directly influenced by diet (1). For this reason, chemoprevention
presents a major strategy for the medical management of CRC and
several natural compounds are being investigated by many
researchers as possible inhibitory agents for CRC initiation and
progression (2–4).
It is widely known that tumor formation is a
complex, multistep process involving the accumulation of genetic
lesions in genes that regulate the pathways of cell proliferation,
adhesion, differentiation and death required for normal
development. In order to invade, epithelial cancer cells need to
penetrate through the basement membrane and to disorganize the
extracellular matrix (ECM). In this context, proteases play a key
role since they can either degrade or process the ECM components
and thereby support cancer cell invasion (5). It is well known that tumor cells
produce higher amounts of proteolytic enzymes than their normal
counterparts. In particular, the matrix metalloproteinase (MMP)
MMP-2 and MMP-9 and the urokinase plasminogen activator (uPA) are
responsible for the degradation of several ECM components and play
important roles in the process of human colon cancer invasion and
metastasis (6).
Cyclooxygenase (COX) enzymes catalyze the enzymatic
conversion of arachidonic acid to prostaglandins (PG). Constitutive
COX-1 is responsible of physiological PG levels, whereas inducible
COX-2 is expressed upon stimulation and accounts for high PG
levels. COX-2 is overexpressed in a number of human and murine cell
lines and tumors (7). In relation
to cancer, PG are able to promote tumor growth by inducing cell
proliferation and/or inhibiting the apoptosis of tumor cells,
stimulating the release of MMPs and/or tumor cell migration,
finally favoring metastatic dissemination (8). For instance, it was observed that
COX-2 overexpression in Caco-2 human colon cancer cells, stimulates
cell migration and invasion, associated with higher expression of
several proteases of the MMP family (9).
Plant-derived polyphenols are a large group of
naturally occurring antioxidants. Epidemiological studies have
suggested that a polyphenol-rich intake from fruits and vegetables
is associated with decreased risk of different diseases, including
cancer (10). This may be due to
the fact that polyphenols are able to act as negative regulators of
inflammation or because they may serve as signaling agents
themselves (11). In particular,
Rosmarinus officinalis L. (rosemary), the most popular spice
of the Lamiaceae family, is a rich source of polyphenols as
carnosic acid (CA), carnosol (COH) and rosmarinic acid (RA). It was
reported that CA has many pharmacological activities (12,13),
as inhibiting the proliferation of the human promyelocytic leukemia
cells HL-60 and U937 (14–16). Furthermore, CA has been shown to
have anti-inflammatory properties, to reduce the expression of
cytokine-induced adhesion molecules, to block the adhesion of
monocytes to endothelial cells (17), and to prevent the migration of human
aortic smooth muscle cells by suppressing the expression of MMPs
(18).
Previously, we have studied the antioxidant and
antibacterial activities of the more conspicuous non-volatile
polyphenols isolated from Rosmarinus officinalis L., as CA
and RA, employing different in vitro and in vivo
approaches (19–21).
In the present study, we demonstrated the
antitumoral action of CA on three human colon cancer lines with
different genetic background: Caco-2 (p53m), LoVo
(p53wt) y HT29 (p53wt). We found that CA
reduces cell viability by inducing apoptosis in Caco-2 cell line,
and inhibits cell migration ability, probably due to the inhibition
of uPA and MMP-9 protease activities. In addition, CA inhibited
COX-2, at mRNA and protein levels. These findings suggest that CA
may provide a new therapeutic strategy useful for the treatment of
CRC disease.
Materials and methods
Reagents and rosemary plant
compounds
Carnosic acid (CA) and rosmarinic acid (RA) were
purchased from Alexis Biochemicals (USA). R. officinalis L.
extract (RE) was obtained from dried leaves by ethanol extraction
and the identification of RE compounds was performed by HPLC as
previously described (19). Stock
solutions were prepared in ethanol 100% and stored at −20°C.
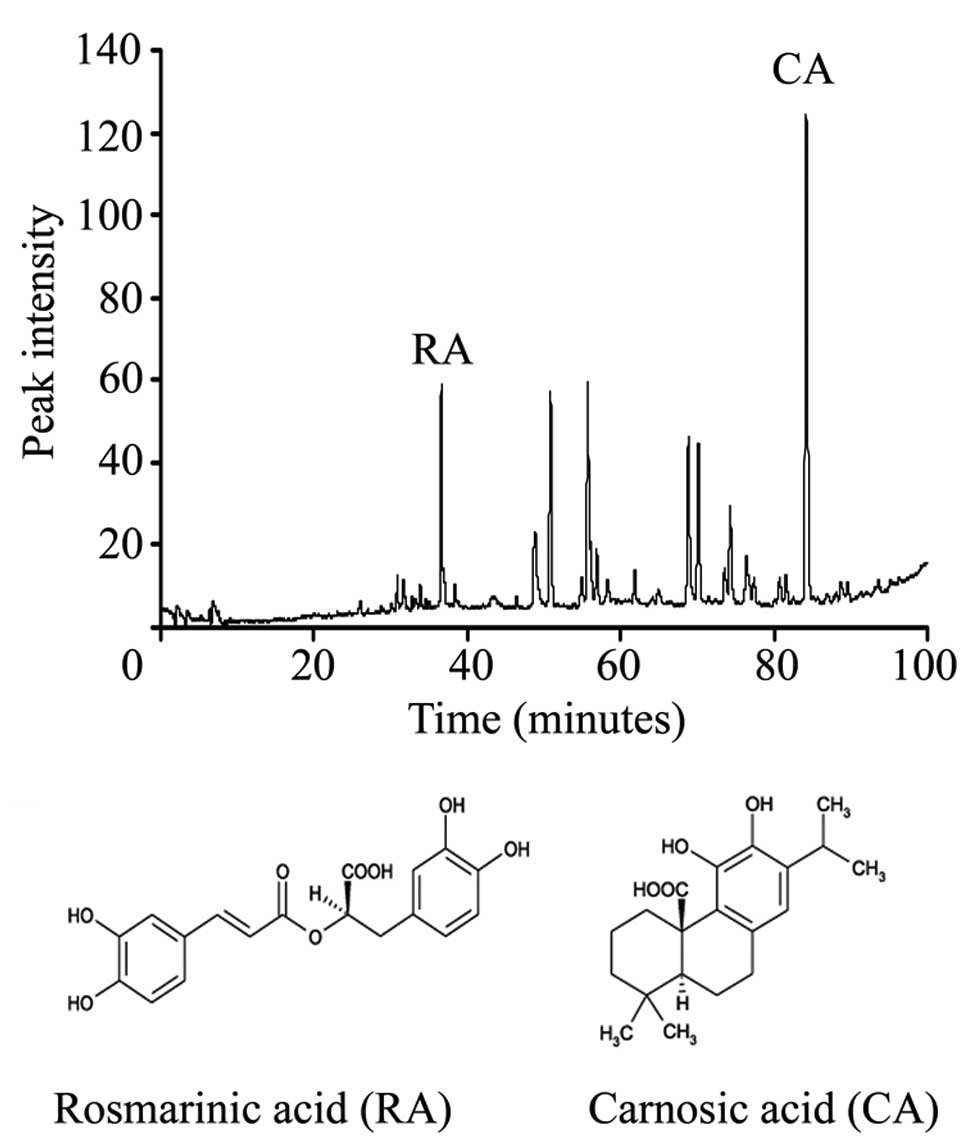
Fig. 1A shows that the RE contained
two main peaks corresponding to 10% CA and 3% RA, and Fig. 1B shows the structures of RA and
CA.
Cell culture
Human colon carcinoma cell lines, Caco-2, HT29 and
LoVo were grown in DMEM (Gibco/Invitrogen, USA) with HyQ Ham’s/F-12
(HyClone, Thermo Scientific, USA) and supplemented with 10% fetal
bovine serum (Internegocios, Argentina), 100 μg/ml streptomycin and
100 U/ml penicillin-G at 37°C in a humidified 5% CO2-air
atmosphere. Cells were grown to 70% confluence and subcultured 2–3
times a week using 0.25% trypsin-EDTA (Gibco/Invitrogen).
Cell viability assay
Cells (1×104) were seeded in 96-well
microplates in complete medium. After 48 h, cells were washed twice
with PBS and treated with RE, RA and CA (concentration range from 0
to 388 μM) in complete medium for 24 h. Cell viability was assessed
by the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation
Assay (Promega, Madison, WI) following the manufacturer’s
recommendations and monitored by absorbance at 595 nm in a
microtiter plate reader (Beckman-Coulter DTX880 Multimode
Detector). IC50 was produced using Microcal Origin 6.0
Professional analysis software.
Annexin-V-Cy3/6-carboxyfluorescein
diacetate staining
Phosphatidylserine translocation from the inner to
the outer leaflet of the plasma membrane is one of the early
apoptotic features. Cell surface phosphatidylserine was detected by
phosphatidylserine-binding protein Annexin-V conjugated with Cy3.18
using the Annexin-V-Cy3 apoptosis detection kit (Sigma-Aldrich,
USA) (22). Briefly, Caco-2 cells
(3×104) were cultured in glass coverslips on 24-well
microplates. After 24 h cells were washed with PBS and treated or
not with CA (IC50 dose) for additional 24 h. Then, cells
were washed with PBS and incubated with 50 μl of double label
staining solution (containing 1 mg/ml AnnCy3 and 100 mM
6-carboxyfluorescein diacetate) for 10 min at room temperature in
the dark. Cells were then washed three times with 50-μl binding
buffer followed by immediate observation using a confocal and
fluorescence microscope (LSM 5 Pascal, Axioplan 2 Imaging). The
combination of 6-carboxyfluorescein diacetate (6-CFDA) with
Cy3-conjugated Annexin-V allowed the differentiation between live
(green), necrotic (red), and apoptotic cells (red and green).
DAPI nuclear staining
Caco-2 cells were cultured on 24-well microplates
(3×104/well) for 24 h. Cells were then washed with PBS
and treated or not with CA (concentration range from 0 to 388 μM).
Cells were washed with PBS twice and fixed with 4% formaldehyde in
PBS for 1 h at room temperature, washed twice with H2O
and then kept in PBS for 30 min at room temperature. Finally, cells
were stained with 300 μl of 30 nM DAPI (Molecular Probes, USA) in
PBS for 5 min in the dark. Cells were observed for nuclear
condensation/fragmentation indicative of apoptosis with an inverted
and fluorescence microscope (Axiovert 135M, Carl Zeiss) and
photographed using a high resolution camera. Three fields were
photographed and the percentage of total apoptotic cells compared
to the total number of cells (100–300 cells) was determined. Each
condition was assayed in triplicate.
Adhesion assay
Cell adhesion assay was performed with modifications
of previously described methods (5,23).
Ninety-six-well microplates were coated with matrix proteins (40
μg/ml type I collagen or 2 μg/cm2 fibronectin in PBS) at
room temperature for 1 h, washed twice with PBS and blocked with
100 μl of 1% BSA in PBS for 2 h at 37°C. BSA-coated wells were used
as negative controls. Wells were washed twice with 100 μl PBS.
Cells (2.5×104 cells/100 μl) were suspended in culture
medium with CA (concentration range from 0 to 388 μM) and added to
each well. After 1-h incubation at 37°C, cells were inspectioned
using a microscope, washed gently with PBS, fixed with 50 μl of
methanol, washed twice with H2O, stained with 2% crystal
violet for 10 min and washed twice with water. Finally, 50 μl of
10% methanol and 5% glacial acetic acid solution was added to each
well and the optical density at 595 nm was measured in a microplate
reader (Beckman Coulter DTX880 Multimode Detector). Adhesion of
CA-treated cells was related to the control set at 100%.
In another set of experiments, 24-h-CA pre-treated
cells (same concentration range) were washed, suspended in culture
medium and then seeded onto the coated wells. Then, the adhesion
assay was performed as described above.
The morphology of control and pre-treated cells with
CA at IC50 were photographed using an inverted
microscope (Axiovert 135M, Carl Zeiss).
Migration assay
Caco-2 confluent monolayers were manually scratched
with a pipette tip to create scratches in the center of the dishes.
Detached cells were removed by washing the cells twice with PBS and
serum-free medium, with or without CA (concentration range from 0
to 388 μM), was added to each dish. Each treatment was performed in
triplicate. Four images per well were taken immediately after
adding treatments and 24 h later using an inverted microscope
(Axiovert 135M, Carl Zeiss). Unpopulated areas were analyzed using
Image-Pro Plus analysis software by measuring unpopulated area at 0
and 24 h and cell advancement area was derived for each treatment.
Data were expressed as a percentage from untreated control cells
(set as 100%).
Urokinase plasminogen activator
activity
A radial caseinolysis assay (24), using plasminogen-rich (2 μg/ml)
casein-agarose plates, was employed to quantitate uPA activity in
the conditioned media of control or CA-treated cells obtained as
described above. Radial caseinolysis of conditioned media were
photographed with a digital densitometer and quantified using
Image-Pro Plus 5.1 analysis software. uPA activities were
referenced to a standard urokinase curve (0.1–50 UI/ml), normalized
to the original protein concentration content by Bradford assay and
uPA activities were expressed as a percentage from untreated
control cells (set as 100%).
Gelatin zymography
Caco-2 subconfluent (70%) 35-mm plates were washed
with PBS to remove growth factors, and the cells were fed with 1-ml
serum-free medium with or without CA (concentration range from 0 to
388 μM). After 24 h, the conditioned medium was collected,
centrifuged to remove cellular debris, and stored at −20°C until
use. MMP enzymatic activity was determined on substrate-impregnated
gels. Briefly, 10 μl of conditioned mediums were separated on 9%
SDS polyacrylamide gels containing 1 mg/ml of gelatin
(Sigma-Aldrich), under non-reducing conditions. After
electrophoresis, gels were washed for 20 min in 2.5% Triton X-100
and incubated for 24 h at 37°C in 50 mM Tris-HCl (pH 7.4), 200 mM
NaCl, 5 mM CaCl2 and 0.02% Triton. Gels were fixed and
stained with 0.5% Coomassie brilliant blue G-250 (Bio-Rad
Laboratories, Richmond, CA) in methanol/acetic acid/H2O.
Activity bands were visualized by negative staining. Gelatinolytic
bands were measured with a digital densitometer and quantified
using Image-Pro Plus 5.1 analysis software. Data were expressed as
arbitrary units and normalized regarding control cell values (set
as 100%) and the original cell lysate protein content, determined
by Bradford assay.
RNA extraction and reverse
transcriptase-polymerase chain reaction (RT-PCR)
Caco-2 cells were cultured on 6-well microplates
(5×104/well) for 24 h. Cells were then washed with PBS
and treated or not with CA (concentration range from 0 to 388 μM)
for 24 h. Total RNA was extracted using TRIzol reagent (Invitrogen)
according to the manufacturer’s instructions. For RT-PCR, cDNA was
made from total RNA using random primers and the M-MLV Reverse
Transcriptase kit (Promega) according to the manufacturer’s
protocol. PCR amplification was performed with the following
primers (forward and reverse): 5′-GAGCGTCAGTATCAACTGCG-3′ and
5′-ATTGGAACTGGACACCGAAC-3 for COX-1, 5′-TTC
AAATGAGATTGTGGGAAAATTGCT-3′ and 5′-AGATCA TCTCTGCCTGAGTATCTT-3′ for
COX-2; 5′-CCACCCAT GGCAAATTCCATGGCA-3′ and 5′-TCTAGACGGCAGGT
CAGGTCCAC-3′ for GAPDH. PCR amplification was carried out with 30
ng of cDNA template in a volume of 50 μl reaction mixture
containing 5 μl reaction buffer (10x), 0.25 mM dNTPs (Promega),
0.25 μM of each primer, and 1 U of Pfu DNA polymerase (Institute
Leloir, Argentina). PCR was performed with a thermal cycler
(GeneAmp PCR System 9600, Perkin-Elmer) under the following
conditions: 94°C/1 min; 60°C/1 min, 72°C/1 min for 35 cycles.
Amplified cDNAs were electrophoresed on 1% agarose gel stained with
ethidium bromide. Results were quantified with Scion Image analysis
software.
Western blot analysis
Caco-2 cells were cultured on 6-well microplates
(5×104/well) for 24 h. Cells were then washed with PBS
and treated or not with CA (concentration range from 0 to 388 μM)
for 24 h. Then, the cells were washed with PBS and lysed with RIPA
buffer containing 150 mM NaCl, 1% NP-40, 50 mM Tris-HCl (pH 8.0), 1
mM EDTA, 0.5% deoxycholate, 100 μg of phenylmethylsulfonyl fluoride
for 30 min on ice, centrifuged at 13000 rpm at 4ºC for 15 min, and
equal amounts (50 μg) of the supernatant proteins were used in
western blots with rabbit polyclonal COX-2 (1:1000) and rabbit
polyclonal β-actin (1:1000, Santa Cruz Biotechology) as loading
control. Proteins were separated by 10% SDS-PAGE and transferred
onto nitrocellulose (Amersham Hybond-P, GE Healthcare). The
membranes were blocked for non-specific binding for 1 h in 5% milk
(w/v) diluted in PBS Tween-20. The blots were then incubated
overnight with primary antibodies. Subsequently, the blots were
washed and ECL anti-rabbit IgG, horseradish peroxidase-linked whole
antibody from donkey (1:5000) (GE Healthcare UK). After further
washing, the blots were subjected to enhanced chemiluminescence
detection system (ECL Plus, GE Healthcare) reagent and monitored
with Molecular Dynamics Storm B40 scanner. Results were quantified
with Scion Image analysis software.
Statistical analysis
Results are expressed as means ± SD. Differences
among groups were analyzed by Student’s t-test and ANOVA test.
Values of p≤0.05 were considered statistically significant.
Results
Effect of CA the main bioactive of R.
officinalis on the viability of three CRC cell lines
Previously we studied the antiproliferative activity
of several rosemary extracts (RE) using the microplate colorimetric
MTS assay employing human colon cancer cells (Biocell).
We evaluated whether CA was able to modulate the
in vitro growth of three human CRC cell lines with similar
population doubling time: Caco-2, HT29 and LoVo cells. Later, we
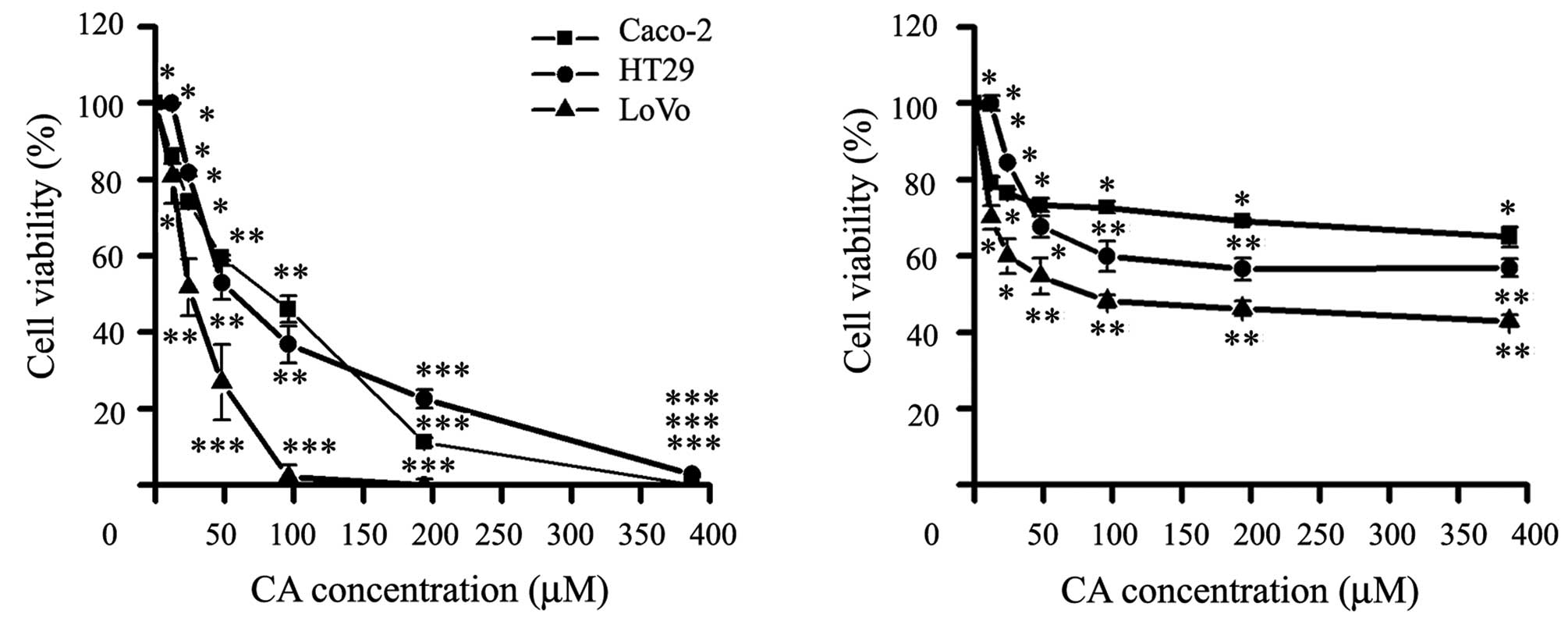
tested the two main constituents of the RE, the CA and RA (Fig. 1), CA was the main bioactive compound
inhibiting the viability of the three CCR cell lines, while RA
showed a significant activity at high concentrations (Fig. 2).
As shown in Table I,
CA was able to inhibit cell viability at different degrees after 24
h. CA significantly inhibited growth in a dose-dependent manner
IC50 (μM): Caco-2, 92.1±6.4; HT29, 48.5±8.2 and LoVo,
26.4±2.7. Therefore, CA compound was selected for the subsequent
studies.
 | Table IIC50 of CA on CRC cell
lines. |
Table I
IC50 of CA on CRC cell
lines.
| Cell lines |
|---|
|
|
|---|
| Carnosic acid | Caco-2 | HT29 | LoVo |
|---|
| IC50
(μM) | 92.1+6.4 | 48.5+8.2 | 26.4+2.7 |
Induction of apoptosis by CA
It is well known that cell viability of tumor cell
populations is determined by the balance between proliferation and
death; here, we studied the effect of CA on survival of Caco-2
cells using two different approaches: Annexin-V and DAPI
staining.
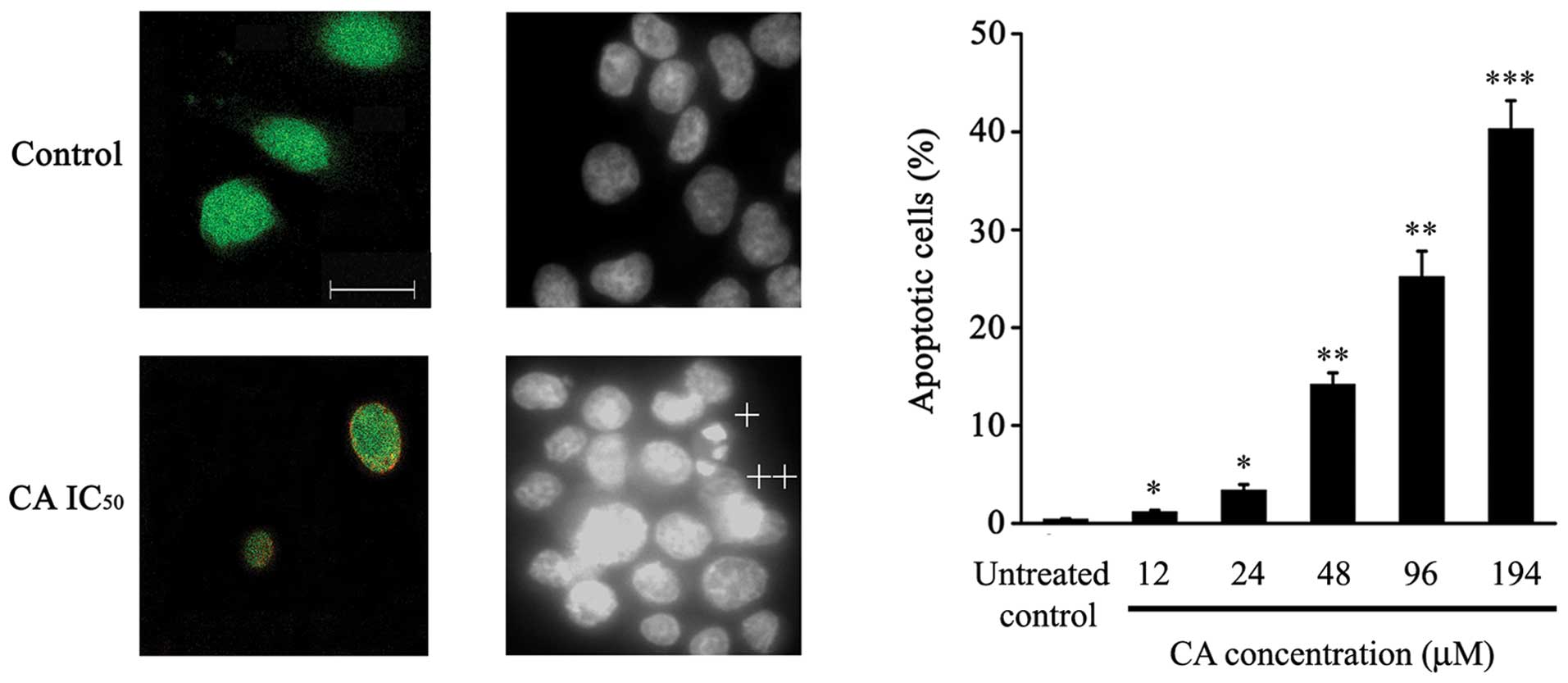
As depicted in Fig.
3A, intact control cells only showed 6-CFDA staining, while
24-h-CA-treated cells (IC50) showed increased numbers of
cells stained with both Annexin-V-Cy3 and 6-CFDA (Fig. 3A), suggesting that these cells were
undergoing apoptotic cell death.
DAPI staining assessment showed that CA treatment
induced typical apoptotic features in Caco-2 cells, such as
chromatin condensation, loss of normal nuclear architecture and
apoptotic bodies (Fig. 3B).
Furthermore, a significant increase in the percentage of apoptotic
cells dependent on CA dose was observed (Fig. 3C).
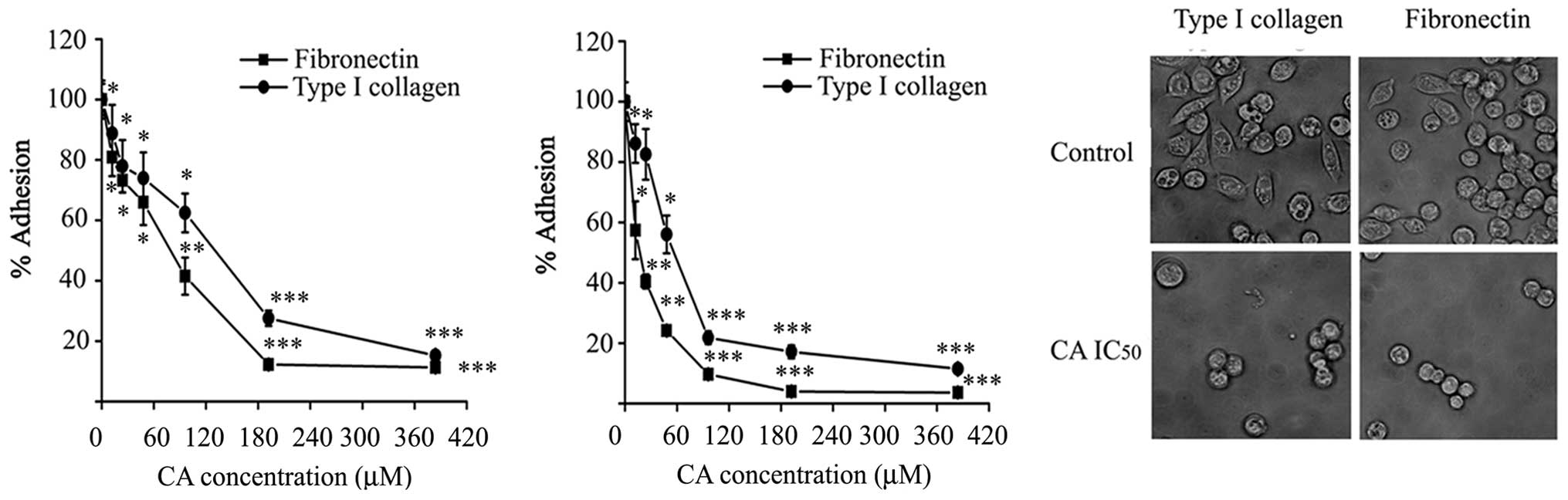
Inhibition of cell adhesion by CA
Adhesion of Caco-2 cells was measured on immobilized
ECM proteins (type I collagen and fibronectin) in the presence of
CA. As shown in Fig. 4A, adhesion
of Caco-2 cells to type I collagen and fibronectin was
significantly impaired in the presence of CA, compared with
adhesion to BSA used as control, when the assay was performed for 1
h. The effect of CA on cell adhesion was dose-dependent, the doses
were able to inhibit adhesion by half of control values (124±6.54
μM) on type I collagen and 77.57±5.22 μM) on fibronectin.
In a different approach, cells were pre-treated with
CA for 24 h prior to the adhesion assay. As expected, CA-pretreated
cells showed 2- to 4-fold lower adhesion to the substrates in this
condition. In addition, CA pre-treatment completely and
significantly inhibited the spreading of Caco-2 cells within 1 h of
incubation on type I collagen and fibronectin surfaces compared to
untreated control cells (Fig. 4C).
These results indicate that CA is able to impair the adhesion of
Caco-2 cells to ECM proteins.
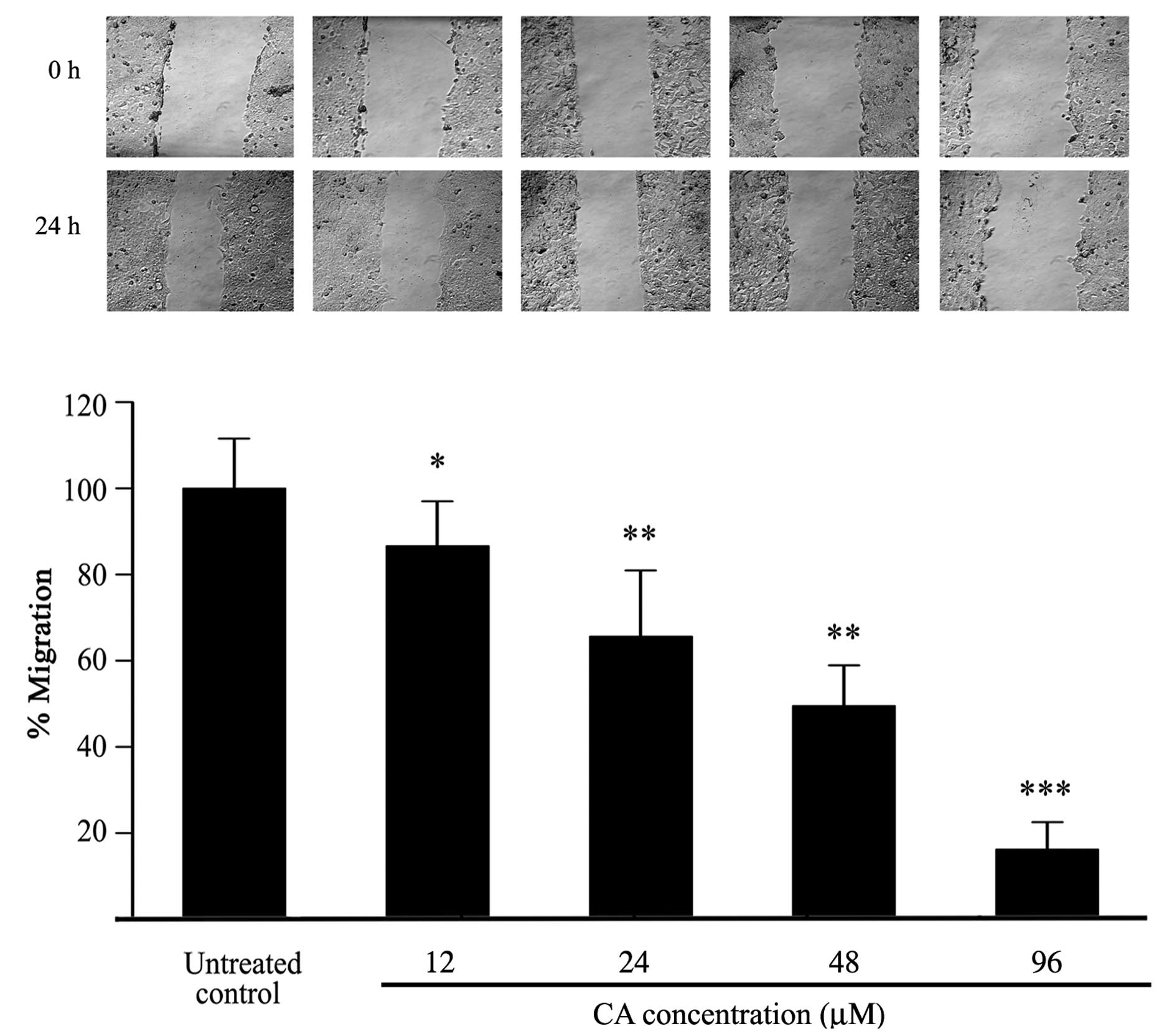
Inhibition of cell migration by CA
To evaluate the effects of CA on the migration
ability of Caco-2 cells, a wound healing assay was performed on
confluent cell monolayers. Cells were treated with increasing
concentrations of CA for 24 h. Cell migration was clearly inhibited
by CA after of treatment in comparison with untreated controls as
observed by optical microscopy (Fig.
5A). As shown in Fig. 5B, CA
significantly inhibited migration of Caco-2 cells dose-dependently,
with 48 μM CA inhibiting migration by 50%, although significant
inhibition of migration was already evident at 24 μM which had
little effect on cell viability.
Inhibition of secreted protease activity
by CA
The effect of CA on secreted uPA activity was
analysed by radial caseinolysis assay. CA treatment significantly
inhibited this activity in the conditioned media, since 8 μg/ml (24
μM) of CA was able to reduce the uPA activity approximately by a
half (Fig. 6). Preliminary results
using gelatin zymography showed that 48 μM of CA inhibited
approximately 50 and 80% the MMP-9 and MMP-2 activities,
respectively.
Inhibition of COX-2 expression by CA
COX-2 has been strongly implicated in intestinal and
colon tumor growth (9,31).The effect of CA on the levels of
COX-1 and COX-2 expression in Caco-2 cells was studied by RT-PCR
and western blot analysis. Amplification by PCR of Caco-2 cDNA with
COX-1, COX-2 and GAPDH primers produced bands of 0.3, 0.4 and 0.6
kb, respectively, as expected for the respective mRNAs. As shown in
Fig. 7A, CA treatment had no effect
on the expression of COX-1 mRNA (a constitutively expressed gene
responsible for housekeeping PG biosynthesis), whereas it
significantly downregulated COX-2 mRNA expression.
Fig. 7B shows that
Caco-2 cells express detectable levels of COX-2 protein. However,
the expression of COX-2 was reduced after 24-h CA treatment,
inhibiting approximately by 50% its expression with 24–48 μM of CA.
A strong inhibition effect on COX-2 expression at both protein and
mRNA levels were observed assaying 96 μM of CA. These findings
suggest that CA could act as a COX-2 inhibitor.
Discussion
Rosmarinus officinalis
L. is a medicinal plant with an elevated content of
anti-oxidant polyphenols as CA and RA. In the present study, we
demonstrated in human CRC cell lines the antiproliferative and
apoptotic effects of CA as well as its inhibitory effect on other
hallmarks of tumor progression such as migration and adhesion.
Although RA and CA were capable of suppressing cell
growth to different degree; CA was the most active polyphenol since
its antiproliferative effect was accompanied by substantial
dose-dependent cytotoxicity in the three CRC lines examined: LoVo,
HT29 and Caco-2 with different genetic backgrounds. In Caco-2 cell
line, we showed that CA at IC50 (92.1 μM) was associated
with induction of apoptosis, as evidenced by the translocation of
phosphatidylserine in the plasma membrane (Fig. 3A), chromatin condensation, and loss
of normal nuclear architecture (Fig.
3B). Other authors have described that CA inhibits DNA
synthesis on Caco-2 cells at 23 μM using [3H]thymidine
incorporation assay, and transient cell cycle arrest in G2/M phase
with 50 μM CA (25). The
antiproliferative effect of CA (2.5–10 μM) was also reported on
HL-60 and U937 human myeloid leukemia cells attributed to
inhibition of cell cycle progression with a transient blockage in
the G1 phase (14), while another
study with HL-60 cell reported that a high dose of CA (100 μM)
induces apoptosis associated with activation of caspase-9 and -3
(26). We found that Caco-2 cells
are arrested in G2/M after incubation with a RE containing
approximately 30 μM of CA (data not shown).
Tumor invasion requires degradation of basement
membranes (BM), which separates the epithelial and mesenchymal cell
compartments, and is composed of macromolecules such as collagen,
laminin, and heparan sulfate. A number of proteolytic enzymes,
including MMPs and serine proteases, are involved in the
degradation of the BM. In particular, activated MMP-2 and MMP-9
play an important role in BM degradation because of their ability
to cleave collagen. Among serine proteases, the urokinase type
plasminogen activator (uPA), which triggers a proteolysis cascade
by accelerating the conversion of plasminogen into plasmin, is
important for tumor invasiveness and metastasis and its expression
is increased in solid tumors. Plasmin can degrade fibrin,
fibronectin, proteoglycans, and laminin found in the
tumor-surrounding matrix, activates collagenases and indirectly
degrades collagens (27). We determined that CA has an inhibitory
effect on Caco-2 cell adhesion to type I collagen and fibronectin
surfaces (Fig. 4A and B). In
addition, we documented the inhibition of spreading and
pseudopodial extension of cells pre-treated with CA IC50
(Fig. 4C). Nevertheless, additional
experiments will be required to identify the precise underlying
mechanism.
Tumor cell migration is necessary at the first steps
of the metastatic cascade, when cancer cells leave the primary
tumor and gain access to the circulation, and also when malignant
cells extravasate into the parenchyma of the secondary site. Tumor
cells have a motile response to many agents, including host-derived
motility and growth factors, ECM components, and tumor-secreted
factors. In this study, we demonstrated by the wound healing assay
that CA can inhibit Caco-2 cell migration in a dose-dependent
manner (Fig. 5). Migration
inhibition (50%) was reached at 48 μM of CA, approximately half the
CA IC50 dose. Moreover, we found that at identical
concentrations CA decreases the activity of important ECM-degrading
proteases, as uPA, MMP-9 and MMP-2 which are closely associated
with tumor progression. Our results show that CA treatment after 24
h decreased Caco-2 conditioned media uPA activity and MMP-9 and
MMP-2.
Natural compounds as potential inhibitors of key
cell signaling pathways such as COX-2 have gained much attention
and therapeutic regimens with either the compounds alone or in
combination with existing chemotherapeutic agents have been
investigated (28). The expression
of COX-2 is involved in tumor promotion during CRC progression
(23,29-31).
We have determined that CA downregulates the expression of COX-2 in
Caco-2 cells at both mRNA and protein levels (Fig. 7A and B). Interestingly, COX-1 mRNA
level was not affected by CA. The expression of COX-2 protein was
reduced about 2-3-fold after the treatment with the IC50
dose of CA. Therefore, the growth inhibitory effect of CA may be
mediated through a mechanism that probably involves inhibition of
the COX-2 pathway.
The clinical importance of this effect lies in the
fact that CA could offer therapeutic benefits of non-steroidal
anti-inflammatory drugs (NSAIDs) (30) with reduced toxicity to the
gastrointestinal mucosa.
In conclusion, we have demonstrated that CA
inhibited Caco-2 cell growth by inducing apoptosis and reducing
adhesion, migration and proteolytic enzyme activities, most
probably by downregulation of COX-2 mRNA expression. Collectively,
our results suggest that CA might modulate different targets
involved in proliferation and apoptotic pathways. These findings
indicate that CA may serve as chemopreventive and/or
chemotherapeutic agent against colorectal cancer progress.
Acknowledgements
We would like to thank the ANCyT, Argentina for the
financial support through Grants PICT 2005-35401 and CONICET.
References
|
1
|
Bruce WR, Giacca A and Medline A: Possible
mechanisms relating diet and risk of colon cancer. Cancer Epidemiol
Biomarkers Prev. 9:1271–1279. 2000.PubMed/NCBI
|
|
2
|
Storz P: Reactive oxygen species in tumor
progression. Front Biosci. 10:1881–1896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kundu JK and Surh YJ: Epigallocatechin
gallate inhibits phorbol ester-induced activation of NF-κB and CREB
in mouse skin. Ann NY Acad Sci. 1095:504–512. 2007.PubMed/NCBI
|
|
4
|
Kellof GJ, Crowell JA, Steele VE, Lubert
RA, Malone WA, Boone CW, Koplovich L, Hawk ET, Liberman R, Lawrence
JA, Ali I, Viner JL and Sigman CC: Progress in cancer
chemoprevention: development of diet-derived chemopreventive
agents. J Nutr. 130:S467–S471. 2000.PubMed/NCBI
|
|
5
|
Friedl P and Wolf K: Tumor-cell invasion
and migration: diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS,
Wu SH and Chung JG: Benzyl Isothiocyanate (BITC) Inhibits migration
and invasion of human colon cancer HT29 cells by inhibiting matrix
metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC
and MAPK signaling pathway. J Agric Food Chem. 58:2935–2942.
2010.
|
|
7
|
Cao Y and Prescott SM: Many actions of
cyclooxygenase-2 in cellular dynamics and in cancer. J Cell
Physiol. 190:279–286. 2002.PubMed/NCBI
|
|
8
|
Philip M, Rowley DA and Schreiber H:
Inflammation as a tumor promoter in cancer induction. Semin Cancer
Biol. 14:433–439. 2004.PubMed/NCBI
|
|
9
|
Tsujii M, Kawano S and Dubois RN:
Cyclooxygenase-2 expression in human colon cancer cells increases
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340.
1997.PubMed/NCBI
|
|
10
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phytochemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aggarwal BB and Shisodia S: Suppression of
the nuclear factor-kappaB activation pathway by spice-derived
phytochemicals: reasoning for seasoning. Ann N Y Acad Sci.
1030:434–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung S and Tai J: Anti-proliferative and
antioxidant properties of rosemary Rosmarinus officinalis.
Oncol Rep. 17:1525–1531. 2007.PubMed/NCBI
|
|
13
|
Mengoni ES, Vichera G, Rigano LA,
Rodriguez Puebla ML, Galliano SR, Cafferata EE, Pivetta OH, Moreno
S and Vojnov AA: Suppression of COX-2, IL-1β and TNF-α expression
and leukocyte infiltration in inflamed skin by bioactive compounds
from Rosmarinus officinalis L. Fitoterapia. 8:414–421.
2011.
|
|
14
|
Steiner M, Priel I, Giat J, Levy J,
Sharoni Y and Danilenko M: Carnosic acid inhibits proliferation and
augments differentiation of human leukemic cells induced by
1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer.
41:135–144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danilenko M, Wang X and Studzinski GP:
Carnosic acid and promotion of monocytic differentiation of HL60-G
cell initiated by other agents. J Natl Cancer Inst. 93:1224–1233.
2001.PubMed/NCBI
|
|
16
|
Danilenko M, Wang Q, Wang X, Levy J,
Sharoni Y and Studzinski GP: Carnosic acid potentiates the
antioxidant and prodifferentiation effects of
1α,25-dihydroxyvitamin D3 in leukemia cells but does not
promote elevation of basal levels of intracellular calcium. Cancer
Res. 63:1325–1332. 2003.
|
|
17
|
Yu YM, Lin CH, Chan HC and Tsai HD:
Carnosic acid reduces cytokine-induced adhesion molecules
expression and monocyte adhesion to endothelial cells. Eur J Nutr.
48:101–106. 2009.PubMed/NCBI
|
|
18
|
Yu YM, Lin HC and Chang WC: Carnosic acid
prevents the migration of human aortic smooth muscle cells by
inhibiting the activation and expression of matrix
metalloproteinase-9. Br J Nutr. 100:731–738. 2008.PubMed/NCBI
|
|
19
|
Moreno S, Scheyer T, Romano CS and Vojnov
A: Antimicrobial and antioxidant activities of Argentinean
Rosmarinus officinalis L. extracts. Free Radic Res.
40:223–231. 2006.
|
|
20
|
Romano CS, Abadi K, Repetto V, Vojnov A
and Moreno S: Synergistic antioxidant and antibacterial activity of
rosemary plus butylated derivatives. Food Chem. 115:456–461. 2008.
View Article : Google Scholar
|
|
21
|
Barni MV, Fontanals A and Moreno S: Study
of the antibiotic Efficacy of an ethanolic extract from
Rosmarinus officinalis against Staphylococcus aureus
in two skin infection models in mice. BLACPMA. 8:219–223. 2009.
|
|
22
|
He J, Xiao Y, Casiano CA and Zhang L: Role
of mitochondrial cytochrome c in cocaine-induced apoptosis in
coronary artery endothelial cells. J Pharmacol Exp Ther.
295:896–903. 2000.PubMed/NCBI
|
|
23
|
Huang SS and Zheng RL: Rosmarinic acid
inhibits angiogenesis and its mechanism of action in vitro. Cancer
Lett. 239:271–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aguirre Ghiso JA, Farías EF, Alonso DF and
Bal de Kier Joffé E: Secretion of urokinase and metalloproteinase-9
induced by staurosporine is dependent on tyrosine kinase pathway in
mammary tumor cells. Int J Cancer. 76:362–367. 1998.PubMed/NCBI
|
|
25
|
Visanji JM, Thompson DG and Padfield PJ:
Induction of G2/M phase cell cycle arrest by carnosol and carnosic
acid is associated with alteration of cyclin A and cyclin B1
levels. Cancer Lett. 237:130–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pesakhov S, Khanin M, Studzinski GP and
Danilenko M: Distinct combinatorial effects of the plant
polyphenols curcumin, carnosic acid and silibinin on proliferation
and apoptosis in acute myeloid leukemia cells. Nutr Cancer.
62:811–824. 2010. View Article : Google Scholar
|
|
28
|
Limab YC, Park HY, Hwang HS, Kang SU,
Pyunc JH, Lee MH, Choi EC and Kimc C:
(-)-Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion
and metastasis in hypopharyngeal carcinoma cells. Cancer Lett.
271:140–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park C, Moon DO and Choi IW: Curcumin
induces apoptosis and inhibits prostaglandin E2 productionin
synovial fibroblasts of patients with rheumatoid arthritis. Int J
Mol Med. 20:365–372. 2007.PubMed/NCBI
|
|
29
|
Gupta RA and DuBois RN: Colorectal cancer
prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev
Cancer. 1:11–21. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sobolewski C, Cerella C, Dicato M,
Ghibelli M and Miederich M: The role of cyclooxygenase-2 in cell
proliferation and cell death in human malignancies. Int J Cell
Biol. 2010:1–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leung E, McArthur D, Morris A and Williams
N: Cyclooxygenase-2 inhibition prevents migration of colorectal
cancer cells to extracellular matrix by down-regulation of matrix
metalloproteinase-2 expression. Dis Colon Rectum. 51:342–347. 2007.
View Article : Google Scholar : PubMed/NCBI
|