Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of
the most common head and neck cancers in the world. Now, the main
treatment strategy for LSCC is still surgery or total laryngectomy
followed by radiotherapy. For the majority of patients with
advanced cases, existing scheme may seriously impair their
laryngeal function and further affect the quality of life (1). Although genetic and epigenetic
alterations were systematically analyzed to guide improvement in
survival rates and treatments, the molecular mechanisms leading to
LSCC development and progression are complex and not entirely
clear.
A new dimension of gene regulation mechanisms has
been identified since the discovery of microRNAs (miRNAs), which
are small non-coding single stranded RNAs of ~22 nucleotides in
length, which negatively regulate gene expression by repressing
translation or decreasing the stability of mRNAs depending on the
degree complementarity between the miRNA and its target (2,3).
Recent evidence indicated that miRNAs have important roles in many
biological processes, including cell differentiation, proliferation
and apoptosis (4). Altered miRNA
expression profiles are involved in various types of human cancers
and seem to function as oncogenes and tumor suppressors by
targeting mainly the 3'un-translation region (3'UTR) of associated
genes (5–7). Although miRNAs have been predicted to
regulate approximately 30% of all human genes, few miRNAs have been
assigned to their target mRNAs and specific functions.
Most evidence suggested that miR-24 is aberrantly
expressed in many kinds of cancers and the erythropoiesis process,
which lead to significantly malignancies and cell differentiation
alteration (8–11). Of clinical significance, miR-24 is
involved in the regulation of oral squamous cell carcinoma (OSCC)
growth and that the expression level of miR-24 in plasma might be
validated as a tumor marker for OSCC (12). Another study showed that miR-24
emerged as a biomarker specific for Kaposi sarcoma (13). However, the relationship of miR-24
to LSCC is not yet reported. In this study, we explored the role
and mechanism of miR-24 in the development and aggression of LSCC
by analyzing the biological characteristics and regulation manner
of miR-24 in LSCC.
Materials and methods
Materials, antibodies, cell lines and
patient tissues
Media/FBS were purchased from Invitrogen/Gibco
(Karlsruhe, Germany), pGL3-Promoter vector from Promega (Madison,
WI, USA), control miR and pre-miR-24 from Ambion (Austin, TX, USA),
Lipofectamine™ 2000 from Invitrogen (Carlsbad, CA, USA), and
Transwell chambers (1 cm2, 12 mm pores) from
Machery-Nagel (Düren, Germany). Power SYBR-Green PCR Master Mix was
obtained from Applied Biosystems (Foster City, CA, USA).
S100A8-antibody was purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA), and β-actin-antibody was from Sigma. Hep2 (human
laryngeal cancer) and HEK293 (human embryonic kidney) cell lines
were acquired from the Cell Biology Institute of Shanghai, Chinese
Academy of Science. Tissue specimens (tumor tissues, matched
non-tumor tissues) from 20 patients with LSCC were collected after
the patients gave informed consent. Verification of the specimens
was performed by a pathologist and the samples were immediately
frozen at −80˚C.
miRNA and gene expression analysis
microRNA and total RNA were isolated using the
mirVana™ miRNA isolation kit (Ambion, TX, USA) according to the
manufacturer's instructions. The concentrations of small and total
RNA were measured by reading the absorbance at OD260/280 nm.
microRNA cDNAs were synthesized with the QuantiMir RT Kit Small RNA
Quantitation System (System Biosciences, CA, USA). Briefly,
microRNA templates were polyadenylated followed by ligation to an
oligo-dT adaptor and reverse transcriptions were then performed to
generate small RNA cDNAs. Total RNA cDNAs were synthesized from 1
μg of total RNA in the presence of oligo-dT (12–18)
primer (Takara, Japan) and MMLV reverse transcriptase according to
the manufacturer's instructions (Promega).
Expression of miR-24 was determined by the
SYBR-Green-based real-time quantitative PCR (qPCR). U6-snRNA was
used as internal control. A miR-24 specific primer and a universal
reverse primer RTQ-UNIr were used for the amplification. The qPCR
was performed with Power SYBR-Green PCR Master Mix in a 30 μl
reaction volume on the Applied Biosystems 7500HT. All primer
sequences used for S100A8 and miR-24 detection are listed in
Table I. PCR reactions were
performed at 95˚C for 10 min, followed by 40 cycles of 95˚C for 15
sec and 60˚C for 1 min. ΔCt was calculated by subtracting the Ct of
U6 or β-actin mRNA from the Ct of the mRNA of interest. ΔΔCt was
then calculated by subtracting the ΔCt of the negative control from
the ΔCt of the samples. The fold change in mRNA or microRNA was
calculated according to the equation 2−ΔΔCt.
 | Table IPrimer sequences used in the present
study. |
Table I
Primer sequences used in the present
study.
| Gene | Primer sequence |
|---|
| miR-24 | Specific primer: F:
5′-TGGCTCAGTTCAGCAGGAACAG-3′
RTQ-UNr: CGAATTCTAGAGCTCGAGGCAGGCGACATGGCTGGCTAGTTAAGC
TTGGTACCGAGCTCGGATCCACTAGTCC (T) 25VN |
| S100A8 | F:
TTGCTAGAGACCGAGTGTCC
R: CTTTGTGGCTTTCTTCATGG |
| S100A8
3'UTR | F:
5′-GAATCTAGACTGAGTTACTGGGCCCAGAG-3′
R: 5′-CTTCTAGAGAGGTATTGATGACTTTATTAT-3′ |
| Mutagenic
S100A8 3'UTR | F:
5′-GAGTTACTGGGCCCAGAGGCCTTTTCCCTGGACATGTACCTGCAG-3′
R: 5′-CTGCAGGTACATGTCCAGGGAAAAGGCCTCTGGGCCCAGTAACTC-3′ |
Cell morphology, in vitro proliferation
and invasion assay
The Cell-based experiments were carried out by
transfection of 50 nM pre-miR-24 or control miR into Hep2 cells
using Lipofectamine™ 2000 in accordance with the manufacturer's
procedure. Cell morphology was evaluated at day 7 after
transfection by ×10 objective magnification using an Eclipse
TE2000-S microscope (Nikon).
For cell proliferation analysis, 2–3×103
Hep2 cells after transfection were plated into 96-well plates in
triplicate. Cells were then cultured for 1, 3, 5 and 7 days. The
absorbance at 570 nm was measured after incubation the cells with
100 μl sterile MTT dye (0.5 mg/ml, Sigma) for 4 h at 37˚C and 150
μl DMSO for 15 min. Then the cell growth curve was constructed by
using OD570 nm as ordinate axis.
In the invasion assay, cells transfected with
microRNA precursor were transferred to the upper compartment
(2×105 cells/well) of Transwell chamber in 25 μl
serum-free medium. The supernatant of mouse NIH3T3 (500 μl) was
added to the bottom compartment. Following incubation for 12 h at
37˚C, cells invaded into the lower surface of the membrane were
fixed and stained with haematoxylin and eosin. Then cell numbers in
5 randomly selected fields were counted under a light
microscope.
microRNA target prediction
The prediction of S100A8 mRNA as a target of
microRNAs was made with miRanda (http://cbio.mskcc.org/cgi-bin/mirnaviewer/mirnaviewer.pl?type=miRanda)
and RNA22 (http://cbcsrv.watson.ibm.com/rna22.html) software. The
default stringency settings included the maximum number of allowed
UN-base paired bases (equal to 0 in seed/nucleus of 7 nucleotides),
the minimum number of paired-up bases in heteroduplex (equal to 14)
and the maximum folding energy for hetero-duplex
(225/Kcal/mol).
3'UTR-luciferase plasmid construction and
reporter assays
The full-length 3'UTR of S100A8 gene was amplified
using cDNA from Hep2 cells (primer sequences are listed in Table I). Then, the PCR product was cloned
into the XbaI-site of pGL3-Promoter vector, checked for
orientation, sequenced and named Lut-S100A8-Wt. Site-directed
mutagenesis of the miR-24 target site in the S100A8 3'UTR
(Lut-S100A8-Mut) was carried out using the Quikchange Mutagenesis
Kit (Stratagene, Heidelberg, Germany), with Lut-S100A8-Wt serving
as a template (mutagenic oligonucleotide primer sequences are
listed in Table I.
For reporter assays, Hep2 cell were transiently
co-transfected with luciferase plasmid and microRNA precursor
(control miR: 5′-UGGAAUGUAAAGAAGUAUGUA-3′, pre-miR-24:
5′-UGGCUCAGUUCAGCAGGAACAG-3′) using Lipofectamine™ 2000. Reporter
assays were performed after 36 h post-transfection using the Dual
Luciferase assay system (Promega), normalized for transfection
efficiency by co-transfecting renilla luciferase. The group of
cells transfected with control miR and mutant reporter plasmid
served as negative control for specificity. Each experiment was
conducted in triplicate.
Cell lysate preparation and Western blot
analysis
After transfection of the luciferase plasmid and
miRNA precursor, Western blot analysis was performed as previously
described (14). Briefly, cells
were washed with PBS and lysed in RIPA buffer. Protein
concentration was determined by BCA (Pierce, IL, USA). Aliquots (25
mg) were separated on 12% SDS-PAGE and transferred to PVDF
membrane. The membrane was then blocked and incubated with S100A8
antibody (1:1000) followed by horseradish peroxidase-conjugated
antibody (1:5000). Detection was performed by enhanced
chemi-luminescence (ECL) using a Western blotting luminological
reagent (Santa Cruz Biotechnology) according to the manufacturer's
instructions. β-actin was used as a reference protein, and was
determined following the same procedure as above.
Statistical analysis
All values were reported as the means ± standard
deviation. Differences were assessed by one-way analysis of
variance (ANOVA) and Student's unpaired t-test using software SPSS
13.0. P<0.05 was considered to be statistically significant.
Results
miR-24 is down-regulated in human LSCC
specimens
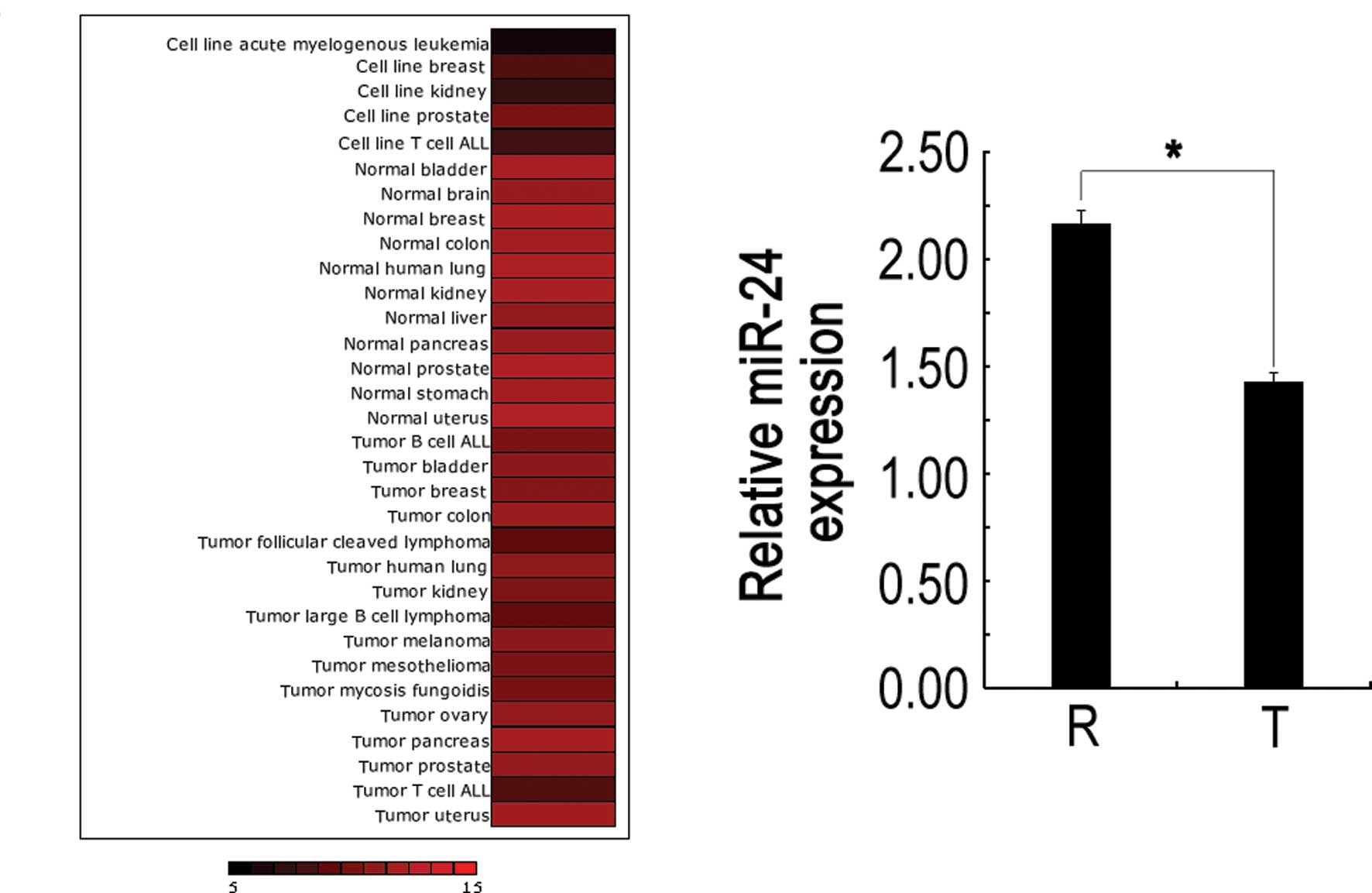
It has been observed that miR-24 was aberrantly
expressed in human malignancies, including tongue squamous cell
carcinoma. In addition, we investigated miR-24 expression in normal
vs. cancer tissue using miRNAMap-2.0 (15), and found that miR-24 levels were
frequently up-regulated in normal but down-regulated in tumor
samples (Fig. 1A). To explore the
possible role of miR-24 in LSCC development, we tested miR-24
expression in LSCC obtained from 20 patients by SYBR qRT-PCR. As
shown in Fig. 1B, 75% (15 of 20) of
carcinoma tissues showed reduced miR-24 expression with respect to
normal counterparts, and the average expression level in carcinoma
tissues was significantly lower than that in normal larynx tissues,
which is consistent with the miRNAMap-2.0 gene chip results.
Together, these results suggest that miR-24 plays an important role
in LSCC development.
miR-24 induces morphological change and
impairs proliferation and invasion properties in Hep2 cells
Given that miR-24 is markedly down-regulated in
laryngeal carcinoma, it may thus function as a potential tumor
suppressor. To investigate whether miR-24 down-regulation played a
causative role in Hep2 cells, we assessed the biological effect of
miR-24 on the development and/or progression of LSCC using a gain
of function approach. As shown in Fig.
2A and B, qRT-PCR revealed that miR-24 precursor (pre-miR-24)
enhanced miR-24 level, suggesting that pre-miR-24 is efficiently
introduced into the cells and the following detection is
invincible. Microscope observed result showed that transfection of
Hep2 cells with pre-miR-24 resulted in morphological changes,
including the increase of round-shaped cells and the reduction of
cell number compared with control groups (Fig. 2C–E). MTT assay showed that the cell
proliferation ability displayed a time-dependent tendency among the
three groups. Especially on day 7, the cells transfected with
pre-miR-24 showed significantly lower proliferation ability than
those in control groups (Fig. 2F).
The above results indicate that the expression level of miR-24 has
an influence on cell growth in vitro.
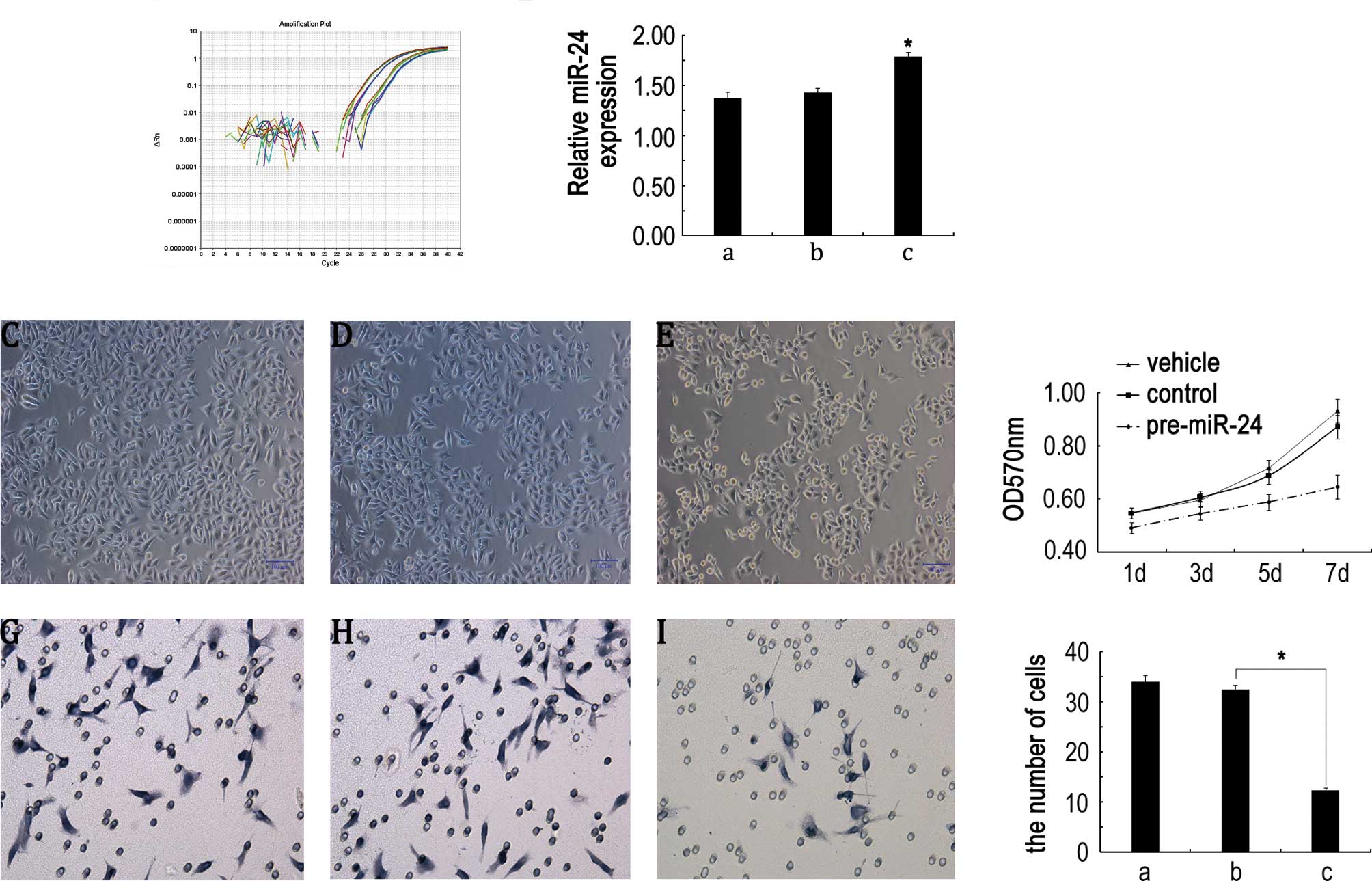 | Figure 2miR-24 induces morphological changes
and impairs proliferation and invasion properties in Hep2 cells.
(A) Amplification plot of miR-24. (B) Statistical analysis of
miR-24 expression. a, vehicle group; b, control miR transfection
group; c, pre-miR-24 transfection group. (C–E) Representative
images showing the morphology of Hep2 cells transfected with
vehicle, control miR and pre-miR-24, respectively. Scale bar, 100
μm. (F) Cell proliferation was measured by the MTT assay. Results
are means of three independent experiments ± SD. (G–I)
Representative images of invasive cells on the membrane by
transfection vehicle, control miR and pre-miR-24 for 7 days,
respectively (magnification, ×400). (J) Statistical analysis of
average invasive cell number of three independent experiments ± SD
(*P<0.05). a, vehicle group; b, control miR group; c,
pre-miR-24 group. |
Meanwhile, we assessed the effects of miR-24 on cell
invasion, a key determinant of malignant progression and
metastasis. As shown in Fig. 2G–I,
the invasion effect of pre-miR-24 on Hep2 cells by Transwell showed
the number of trans-membrane Hep2 cells undergoing pre-miR-24
transfection was much lower than those in control groups on day 7.
The transmembrane Hep2 cell number ranged from 34±1.25 and
32.48±0.95 to 12.37±0.52 in the vehicle, control miR and pre-miR-24
transfection groups, respectively. ANOVA analysis showed a
significant statistical difference between the pre-miR-24
transfection and control groups (P<0.05, Fig. 2J), which implies that
down-regulation of miR-24 may contribute to tumor metastasis in
LSCC.
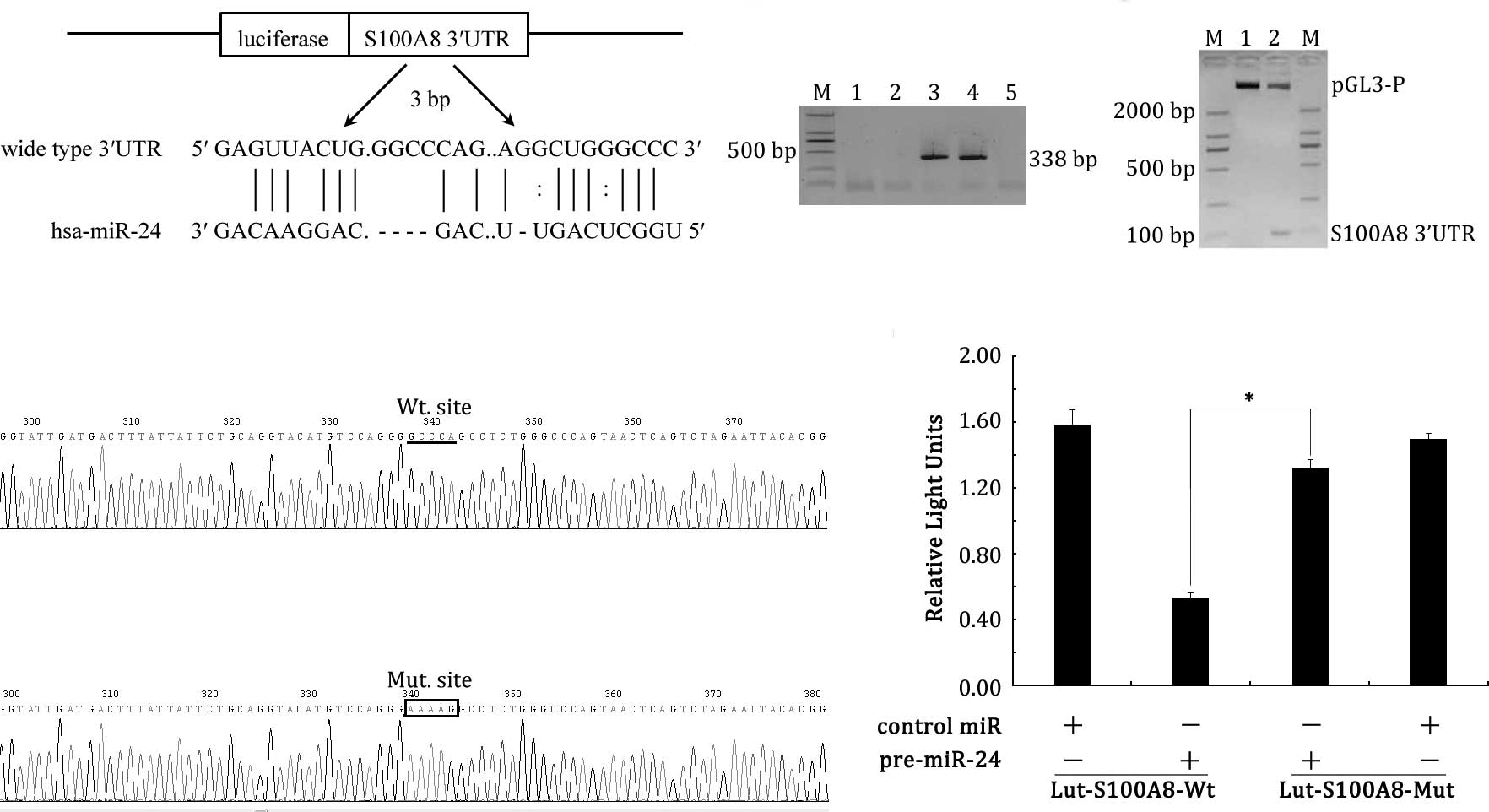
S100A8 mRNA is a target of miR-24
As miR-24 has a pivotal function in LSCC, the
question how the miRNA exerts its role in LSCC needs to be
investigated. In this study, S100A8 mRNA was predicted to be
a potential target of miR-24 after computational analysis using two
different programs (miRanda and RNA22). The programs returned a hit
between the 455 bp sequences of S100A8 and miR-24 (Fig. 3A).
To confirm whether the 3'UTR of S100A8 was a
functional target of miR-24 in LSCC, we set up a luciferase
reporter assay. The wild-type of S100A8 gene 3'UTR,
harboring miR-24 target sites, was cloned into the downstream of
pGL3 plasmid, which was driven by the SV40 basal promoter,
Lut-S100A8-Wt. PCR screening, enzyme digestion and sequencing
identified that Lut-S100A8-Wt had the correct origin, right
sequence compared with GeneBank database, with no frame shift
(Fig. 3B–D). In parallel, we cloned
a second reporter construct, named Lut-S100A8-Mut, in which the
conserved targeting site of miR-24 was specifically mutated,
putatively to abolish the miRNA binding ability. Fig. 3E showed that the Lut-S100A8-Mut
plasmid was constructed successfully. Then the Hep2 cells (which
have low endogenous miR-24 expression) were co-transfected with the
reporter vector and miRNA mimics. As shown in Fig. 3F, Hep2 cells with Lut-S100A8-Wt and
pre-miR-24 led to a significant decrease in reporter activity
compared to the controls (P<0.05). However, the activity of the
reporter construct with a mutation at the miR-24 target site was
unaffected when Hep2 cells were co-transfected with pre-miR-24
(P>0.05). Together, these results indicate that 3'UTR of
S100A8 carries a direct and specific binding site of miR-24
in vitro.
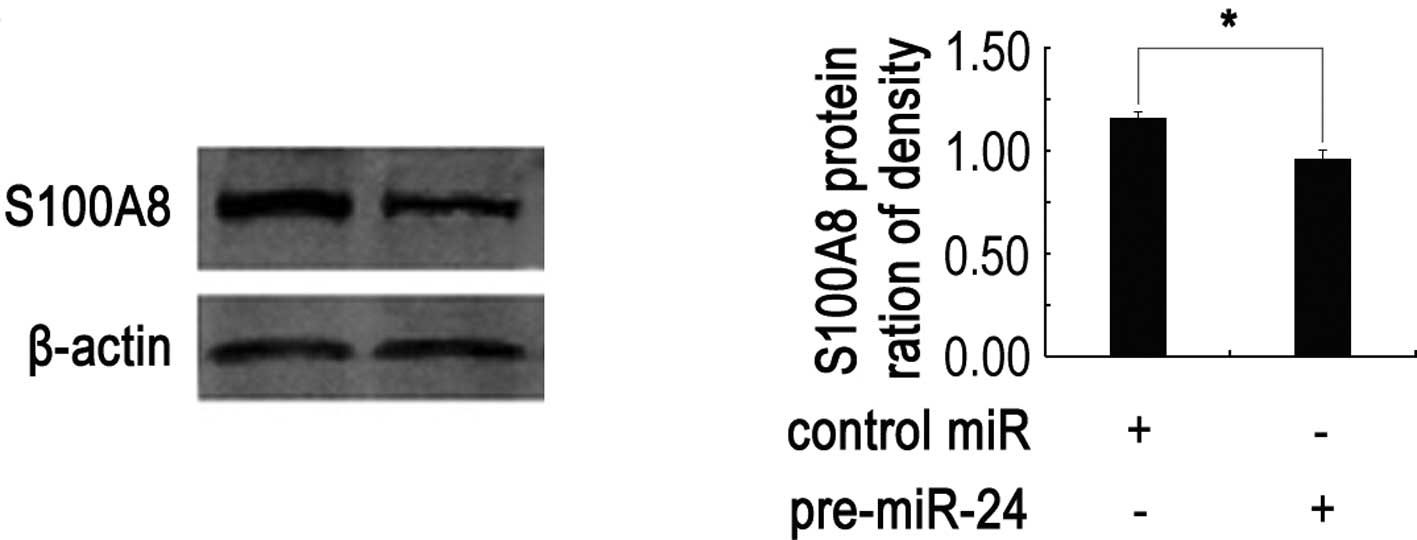
miR-24 down-regulates S100A8 expression
at translational level
miRNAs can regulate gene expression through
decreased translation of target mRNA, increased degradation of
target mRNA, or both. To test at which level S100A8 was
down-regulated by miR-24, qRT-PCR and Western blotting assays were
performed. Compared with controls, miR-24 had no significant effect
on the S100A8 mRNA expression (data not shown), while the
S100A8 protein level was significantly down-regulated when Hep2
cells were cotransfected with miR-24 and Lut-S100A8-Wt (P<0.05)
(Fig. 4). These results strongly
suggest that miR-24 negatively regulates S100A8 expression
through translational repression pathways.
miR-24 inhibits Hep2 cell invasion partly
through S100A8 blockage
S100A8 gene is one of the important
inflammation factors, which has the ability to promote tumor cell
invasion. Therefore, we further down-regulated endogenous S100A8
protein level using S100A8 antibody blocking, and then detected the
role of S100A8 in miR-24 mediated Hep2 cell invasion after
cells were transfected with pre-miR-24 36 h. Transwell results
showed that the transmembrane cells of pre-miR-24 and control miR
transfection groups were 12.37±0.52 and 32.48±0.95, respectively.
The transmembrane cells of S100A8 protein that blocked following
pre-miR-24 transfection group was much less, with only some cells
observed, and no more than 15 cells in the whole membrane. ANOVA
analysis showed significant statistic difference among the groups,
P<0.05. This result indicates that S100A8 protein plays a
critical role in miR-24 mediated Hep2 cell invasion.
Discussion
miRNAs are essential regulators of many cellular
processes including proliferation, differentiation, metastasis, and
morphogenesis. They are usually overexpressed or show loss of
expressed in almost all carcinogenesis and tumor development.
Moreover, half of these aberrant miRNAs located in the genetic
region of oncogenes or tumor suppressor genes, functions as
proto-oncogenes or tumor suppressors. Substantial evidence shows
that miR-24 encoding gene maps to human chromosome 9q22 and 19p13,
regions that are unstable and frequently altered in head and neck
squamous cell carcinoma (16,17).
Several studies have shown that miR-24, functioning
as a tumor suppressive gene, plays an important role in human
cancer and other diseases. For example, Mishra et al found
that miR-24 was abnormally down-regulated in human colorectal
cancer tumors and showed a p53-independent cellular proliferation
(8). Rogler et al showed
that miR-24 was up-regulated in hematopoietic carcinoma and induced
tumor suppressive activities (7).
However, Liu et al reported the up-regulation of miR-24 in
tongue squamous cell carcinoma (TSCC) and the miR-24 mediated
changes led to enhanced proliferation and reduced apoptosis in TSCC
cells (19). Although the
expression of miR-24 in cancers is controversial, the functional
evidence for a role of miR-24 has been documented consistently. Our
results show that the up-regulation of miR-24 leads to significant
cell morphological changes, reduced cell number, low proliferation
and enhanced cell invasion potential of LSCC, which implies that
miR-24 is associated with the biological effect of LSCC and retards
the development of LSCC. Importantly, studies have clarified the
high stability of miRNAs in blood and the increase in plasma miR-24
is detectable in patients with a low level of miR-24 up-regulation
in tumors (12,20). Since the sampling of blood is
relative non-invasive and the examination of blood can facilitate
early diagnosis, our findings further reveal that plasma miR-24
might be a potential useful LSCC biomarker.
The discrepancy of miR-24 expression and function
might be mainly due to its effect on the multiple target genes
(2,21). Recent studies found that FAF1,
DHFR, E2F2, MYC and other cell cycle regulatory genes are
target genes of miR-24 (8,9,21,22).
However, given that a single miRNA has multiple targets, we believe
that miR-24 also has other targets. Computational algorithms have
been the major driving force in predicting miRNA targets (23). Through analysis using miRanda and
RNA22, we identified S100A8 as a possible target of miR-24
among the regulated genes. Further, we used series of experiments
to confirm that S100A8 is a direct negative target gene of
miR-24 in LSCC. First, overexpression of miR-24 significantly
reduces the luciferase activity of 3'UTR sequence of S100A8,
while mutation at the miR-24 target site in the 3'UTR of
S100A8 could significant decrease the miR-24 regulation
effect. These results indicate that S100A8 3'UTR carries a
direct and specific miR-24 binding site. Second, over-expression of
miR-24 significantly down-regulates S100A8 protein expression,
while has no effect on S100A8 mRNA level, which further
suggests that miR-24 directly regulate S100A8 gene through
translational level.
As a new target gene of miR-24, S100A8 is an
important inflammation factor, localized in the cytoplasm and/or
nucleus of a wide range of cells. S100A8 and S100A9 are often
co-expressed as a complex, suggesting a common mechanism of
transcriptional regulation in inflammatory diseases and cancers
(24). Accumulating evidence
suggests that infection and inflammation contribute to 15–20% of
all malignancies. Recent clinical and experimental data have
suggested that changes in the expression and/or function of the
S100A8 protein may represent a key step during cancer development
(25,26). Yao et al concluded that
overexpression of S100A8 is associated with stage,
progression, invasion, metastasis and poor survival in human
bladder cancer (27). Additionally,
obvious up-regulation of S100A8 has been found in breast,
lung, gastric, colorectal, pancreatic and prostate, while
down-regulation of S100A8 has been detected in squamous
esophageal carcinomas (24). Our
previous research also validated the up-regulation of S100A8
in LSCC, leading to Hep2 cells invasion and enhanced metastasis
ability (28). Collectively our
present results indicate that miR-24 down-regulated in LSCC leads
to loss of tumor suppressor function, which enhanced Hep2 cell
invasion. As a direct target of S100A8, miR-24 negatively
regulates S100A8 expression at translational level. We
speculate that miRNA-24 inhibits LSCC Hep2 cell invasion through
regulating S100A8 protein expression.
Additionally, previous studies have found that
down-regulation of S100A8 via RNA interference could promote
apoptosis and inhibit metastasis in LSCC (28). In the present study, we found that
miR-24 acts as an endogenous siRNA for S100A8, and cell
invasion ability induced by S100A8 can be subtracted by the
overexpression of miR-24. Thus, the identification of S100A8
as a miR-24 target gene provides a possible explanation as to why
the overexpression of miR-24 can inhibit LSCC Hep2 cell
invasion.
In conclusion, miR-24 was down-regulated in LSCC,
and functions as a tumor suppressor in Hep2 cells partly through
targeting the S100A8 gene. Our findings revealed that the
identification of miR-24 and its target gene S100A8 in LSCC
may help to understand the molecular mechanism of carcinogenesis,
and also give us strong rationale to further investigate miR-24 as
a potential treatment target for LSCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation (30700980) and Research Starting Foundation of
China Medical University Stomatology College (K101593-11-28), P.R.
China.
References
|
1
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchesex F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim J, Kim H, Lee Y, Yang K, Byun S and
Han K: A simple and economical short-oligonucleotide-based approach
to shRNA generation. J Biochem Mol Biol. 39:329–334. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendell JT: MicroRNAs: critical regulators
of development, cellular physiology and malignancy. Cell Cycle.
4:1179–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT,
Xia YJ, Ye ZY and Tao HQ: MicroRNA-101 is down-regulated in gastric
cancer and involved in cell migration and invasion. Eur J Cancer.
46:2295–2303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Majid S, Dar AA, Saini S, Yamamura S,
Hirata H, Tanaka Y, Deng G and Dahiya R: MicroRNA-205-directed
transcriptional activation of tumor suppressor genes in prostate
cancer. Cancer. 116:5637–5649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rogler CE, Levoci L, Ader T, Massimi A,
Tchaikovskaya T, Norel R and Rogler LE: MicroRNA-23b cluster
microRNAs regulate transforming growth factor-beta/bone
morphogenetic protein signaling and liver stem cell differentiation
by targeting Smads. Hepatology. 50:575–584. 2009. View Article : Google Scholar
|
|
8
|
Mishra PJ, Humeniuk R, Mishra PJ,
Longo-Sorbello GS, Banerjee D and Bertino JR: A miR-24 microRNA
binding-site polymorphism in dihydrofolate reductase gene leads to
methotrexate resistance. Proc Natl Acad Sci USA. 104:13513–13518.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lal A, Navarro F, Maher CA, Maliszewski
LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O,
Becker KG, Gorospe M, Hide W and Lieberman J: miR-24 inhibits cell
proliferation by targeting E2F2, MYC, and other cell-cycle genes
via binding to ‘seedless’ 3'UTR microRNA recognition elements. Mol
Cell. 35:610–625. 2009.PubMed/NCBI
|
|
10
|
Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han
JD and Chen YG: MicroRNA miR-24 inhibits erythropoiesis by
targeting activin type I receptor ALK4. Blood. 111:588–595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lal A, Kim HH, Abdelmohsen K, Kuwano Y,
Pullmann R Jr, Srikantan S, Subrahmanyam R, Martindale JL, Yang X,
Ahmed F, Navarro F, Dykxhoorn D, Lieberman J and Gorospe M:
p16(INK4a) translation suppressed by miR-24. PLoS One. 3:e18642008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin SC, Liu CJ, Lin JA, Chiang WF, Hung PS
and Chang KW: miR-24 up-regulation in oral carcinoma: positive
association from clinical and in vitro analysis. Oral Oncol.
46:204–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Hara AJ, Chugh P, Wang L, Netto EM, Luz
E, Harrington WJ, Dezube BJ, Damania B and Dittmer DP: Pre-micro
RNA signatures delineate stages of endothelial cell transformation
in Kaposi sarcoma. PLoS Pathog. 5:e10003892009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Y, Liu J, Xu Z, Sun K and Fu W: HLA-B
gene participates in the NF-kappaB signal pathway partly by
regulating S100A8 in the laryngeal carcinoma cell line Hep2. Oncol
Rep. 19:1453–1459. 2008.PubMed/NCBI
|
|
15
|
Hsu SD, Chu CH, Tsou AP, Chen SJ, Chen HC,
Hsu PW, Wong YH, Chen YH, Chen GH and Huang HD: miRNAMap 2.0:
genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res.
36:D165–D169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smeets SJ, Braakhuis BJ, Abbas S, Snijders
PJ, Ylstra B, van de Wiel MA, Meijer GA, Leemans CR and Brakenhoff
RH: Genome-wide DNA copy number alterations in head and neck
squamous cell carcinomas with or without oncogene-expressing human
papillomavirus. Oncogene. 25:2558–2564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu YH, Kuo HK and Chang KW: The evolving
transcriptome of head and neck squamous cell carcinoma: a
systematic review. PLoS One. 3:e32152008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang S, He X, Ding J, Liang L, Zhao Y,
Zhang Z, Yao X, Pan Z, Zhang P, Li J, Wan D and Gu J: Upregulation
of miR-23a approximately 27a approximately 24 decreases
transforming growth factor-beta-induced tumor-suppressive
activities in human hepatocellular carcinoma cells. Int J Cancer.
123:972–978. 2008. View Article : Google Scholar
|
|
19
|
Liu X, Wang A, Heidbreder CE, Jiang L, Yu
J, Kolokythas A, Huang L, Dai Y and Zhou X: MicroRNA-24 targeting
RNA-binding protein DND1 in tongue squamous cell carcinoma. FEBS
Lett. 584:4115–4120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt
DL, Gentleman R, Vessella RL, Nelson PS, Martin DB and Tewari M:
Circulating microRNAs as stable blood-based markers for cancer
detection. Proc Natl Acad Sci USA. 105:10513–10518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guan Y, Yao H, Zheng Z, Qiu G and Sun K:
miR-125b targets BCL3 and suppresses ovarian cancer proliferation.
Int J Cancer. 128:2274–2283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin W, Shi Y, Zhao B, Yao C, Jin L, Ma J
and Jin Y: miR-24 regulates apoptosis by targeting the open reading
frame (ORF) region of FAF1 in cancer cells. PLoS One. 5:e94292010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sethupathy P, Megraw M and Hatzigeorgiou
AG: A guide through present computational approaches for the
identification of mammalian microRNA targets. Nat Methods.
3:881–886. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gebhardt C, Breitenbach U, Tuckermann JP,
Dittrich BT, Richter KH and Angel P: Calgranulins S100A8 and S100A9
are negatively regulated by glucocorticoids in a c-Fos-dependent
manner and overexpressed throughout skin carcinogenesis. Oncogene.
21:4266–4276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gebhardt C, Németh J, Angel P and Hess J:
S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol.
72:1622–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahman I: Oxidative stress, chromatin
remodeling and gene transcription in inflammation and chronic lung
diseases. J Biochem Mol Biol. 36:95–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao R, Davidson DD, Lopez-Beltran A,
MacLennan GT, Montironi R and Cheng L: The S100 proteins for
screening and prognostic grading of bladder cancer. Histol
Histopathol. 22:1025–1032. 2007.PubMed/NCBI
|
|
28
|
Huang DF, Fu WN, Guo Y, Xu ZM, Sun XH and
Sun KL: S100A8 mediates the activation of
P65/HLA-B/S100A8/BCL-2/Caspase-9(-3) pathway in laryngeal
carcinogenesis. Chinese Sci Bull. 53:2017–2024. 2008. View Article : Google Scholar
|