Introduction
Histone deacetylase (HDAC) inhibitors represent a
structurally diverse group of compounds that inhibit the
deacetylation of histones, permitting the chromatin scaffolding to
assume a more relaxed, open conformation, which generally promotes
gene transcription. HDAC inhibitors induce apoptosis in cancer
cells through multiple mechanisms, and thus are emerging as a
promising new therapeutic tool for the treatment of a variety of
human cancers (7). Sodium butyrate
(NaB), a short chain fatty acid, occurs naturally in the body and
is synthesized through the acetyl-CoA-dependent catabolic oxidation
of long chain saturated fatty acids (1,2).
Sodium butyrate, acting as an HDAC inhibitor, is known to exhibit
anticancer effects via differentiation of carcinoma cells (3–6). NaB
is a novel chemotherapeutic agent as it is able to activate a
variety of anticancer mechanisms, including cell cycle arrest and
cellular differentiation (8–10).
Sodium butyrate induces apoptosis, decreases Bcl-2 transcription
(11), increases TNF-related
apoptosis-inducing ligand receptor 2 gene transcription to
accelerate the death-inducing signaling complex formation,
activates caspase, and inhibits the mitochondrial membrane
potential of cancer cells (12).
These results suggest that NaB may represent a potential new class
of anticancer agents with low toxicity; however, the mechanisms of
action are not fully understood.
DAPK induces programmed cell death through various
signaling pathways. FAK participates in signaling pathways involved
in adhesion between cells and the extracellular matrix, including
proteins such as fibronectin, laminin, actin, and fodrin. FAK is a
survival protein that suppresses apoptosis and maintains cell
suspended growth (26). In this
study, we attempted to elucidate NaB-induced death-associated
protein kinase expression and its association with human gastric
cancer cell apoptosis.
Materials and methods
Cell culture and treatments
A total of nine human gastric cancer cell lines
(AGS, Kato III, MKN28, MKN45, MKN74, NCI-N87, SNU1, SNU16 and
SNU638) were obtained from the Korean Cell Line Bank (Korea) and
the American Type Culture Collection (Manassas, VA, USA). Cells
were cultured in RPMI-1640 medium (Hyclone Laboratories, Inc., USA)
containing 10% fetal bovine serum (Hyclone) and 1% penicillin
streptomycin sulfate (Hyclone) at 5% CO2, 37°C, and 95%
humidity. Sodium butyrate, 5′-Aza-2-deoxycytidine (5′-Aza-dC), and
trichostatin A were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Working concentrations were as follows: sodium butyrate
(NaB), 2 μM; 5-Aza-dC, 2 μM; and trichostatin A, 200 nM.
RNA isolation and reverse transcription
polymerase chain reaction
Total RNA was prepared using TRIzol reagent
(Invitrogen). The RNA was reverse transcribed using oligo (dT)
primers and SuperscriptT™ II reverse transcriptase (Invitrogen,
Carlsbad, CA, USA). Primers used for PCR amplification of this cDNA
were caspase-3: forward, 5′-GGC ATT GAG ACA GAC AGT GGT G-3′ and
reverse, 5′-GCA CAA AGC GAC TGG ATG AAC C-3′; Bcl-2: forward,
5′-GAG TAC CTG AAC CGG CAC CT-3′ and reverse, 5′-CAG GGT GAT GCA
AGC TCC CA-3′; DAPK1: forward, 5′-TCT ACC AGC CAC GGG ACT TC-3′ and
reverse, 5′-GCT GGC CTG TGA GTA GAC GT-3′; DAPK2: forward, 5′-GCA
TCG TGT CCC TGT GCA AC-3′ and reverse, 5′-GCT TTC CTC CTG GCG ATG
TC-3′; DAPK3: forward, 5′-CCC AAC CCA CGA ATC AAG CTC-3′ and
reverse, 5′-GCT GAG ATG TTG GTG AGC GTC-3′; p21: forward, 5′-GTA
CCC TTG TGC CTC GCT CA-3′ and reverse, 5′-CCG GCG TTT GGA GTG GTA
GA-3′; p53: forward, 5′-AGC GAT GGT CTG GCC CCT CCT-3′ and reverse,
5′-CTC AGG CGG CTC ATA GGG CAC-3′; and β-actin: forward, 5′-TTG CCG
ACA GGA TGC AGA AG-3′ and reverse, 5′-AGG TGG ACA GCG AGG CCA
GG-3′. PCR reactions were performed with the PCR Maxi kit (Intron,
Sungnam, Korea). PCR reactions in the linear range of amplification
were analyzed by agarose gel electrophoresis and quantified by
densitometry, if needed.
Western blot analysis
Prepared cells were harvested after washing with
PBS. Collected cells were lysed with buffer [50 mM Tris-Cl (pH
7.5), 150 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton X-100, 1 mM PMSF,
1 mM Na3VO4, and protease inhibitor cocktail
(Roche Molecular Biochemicals, Indianapolis, IN, USA)].
Fractionation was performed by sequential extraction of cytosolic
and nuclear proteins in non-ionic detergent for analysis of
β-catenin. The same amount of protein was boiled at 95°C after
adding SDS sample buffer [62.5 mM Tris-Cl (pH 6.8), 2% sodium
dodecyl sulfate, 10% glycerol, β-mercaptoethanol, and 0.002%
bromophenol blue]. Samples were loaded on 8% SDS-PAGE gels for
DAPK1 and FAK and on 10% SDS-PAGE gels for DAPK2 analyses and
transferred to PVDF membranes (Amersham Biosciences, Pisctaway, NJ,
USA). Anti-DAPK1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA),
Anti-DAPK2 (Santa Cruz Biotechnology), and anti-FAK (Santa Cruz
Biotechnology) were used as the primary labeling antibodies, and
the appropriate horseradish peroxidase-conjugated antibodies (Santa
Cruz Biotechnology) were used as secondary antibodies. An enhanced
chemiluminescence detection system (ECL-Plus, Intron, Seoul, Korea)
was used for detection according to the manufacturer’s
protocol.
Immunofluorescence microscopy
Human gastric cancer cells were cultured on chamber
slides and then washed with phosphate-buffered saline (PBS) and
fixed with 10% formaldehyde. They were then incubated with
anti-DAPK1, anti-DAPK2, and anti-FAK antibodies and stained with
anti-goat IgG-FITC and anti-rabbit IgG-FITC antibodies (all from
Santa Cruz Biotechnology). Cells were visualized using a Zeiss LSM
510 confocal laser-scanning microscope (Carl Zeiss, USA).
Flow cytometry
Human gastric cancer cells were treated with 2 μM
NaB, 2 μM 5′-Aza-dC for 48 h, replacing the drug and medium every
24 h. Prepared cells were harvested after washing with PBS.
Collected cells were lysed with 1X binding buffer (BD Biosciences,
USA). One hundred microliters of the lysate was transferred to a 5
ml culture tube and treated with FITC Annexin-V and PI (BD
Biosciences) for 15 min at 25°C in the dark. The cell cycle
distribution was determined using a FACScan flow cytometer
(Becton-Dickinson, Mountain View, CA, USA) and 10,000 cells were
analyzed with the MultiCycle software package (Phoenix Flow
Systems, San Diego, CA, USA).
Cell proliferation assay
Human gastric cancer cells were seeded in 96-well
plates. Cells were treated with 2 μM NaB, 2 μM 5′-Aza-dC and 200 nM
trichostatin A for 48 h, replacing the drug and medium every 24 h.
The colorimetric MTS (Promega) assay was used to measure cell
numbers at 48 h, according to the manufacturer’s manual.
Experiments were performed in triplicate.
Results
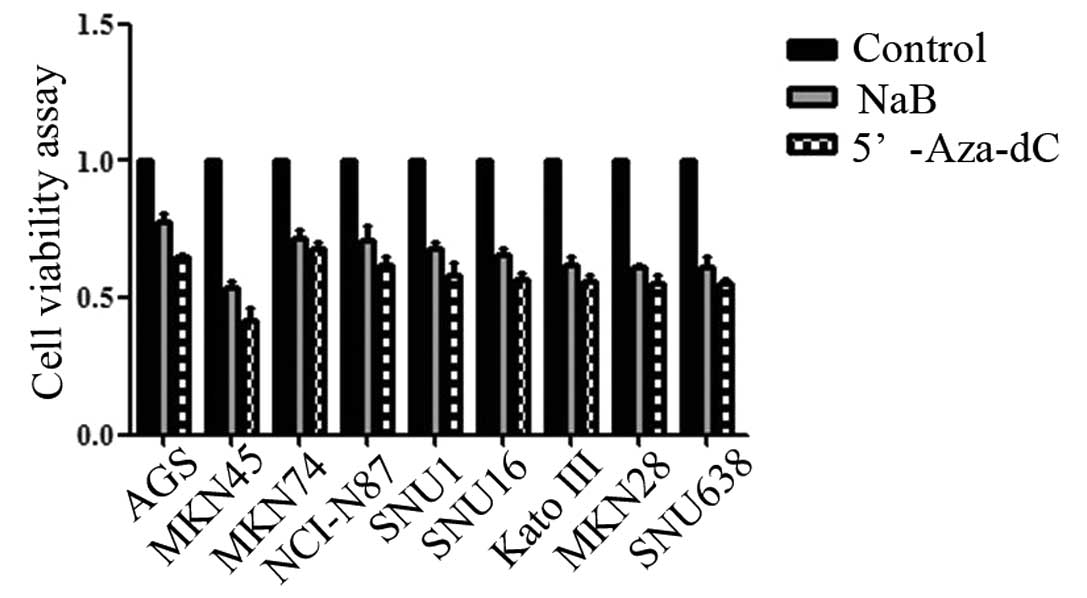
Sodium butyrate inhibited cell
proliferation
We examined the effects of NaB on human gastric
cancer cell proliferation. After 48 h of NaB treatment, cell
viability was reduced from 0.58 to 0.41 in AGS, from 0.40 to 0.22
in MKN45, from 0.63 to 0.34 in MKN74, from 0.66 to 0.39 in NCI-N87,
from 1.2 to 0.63 in SNU1, from 0.44 to 0.21 in SNU16, from 0.42 to
0.19 in Kato III, from 0.45 to 0.19 in MKN28, and from 0.99 to 0.45
in SNU638 (all P<0.05) as determined by the cell proliferation
assay (Fig. 1).
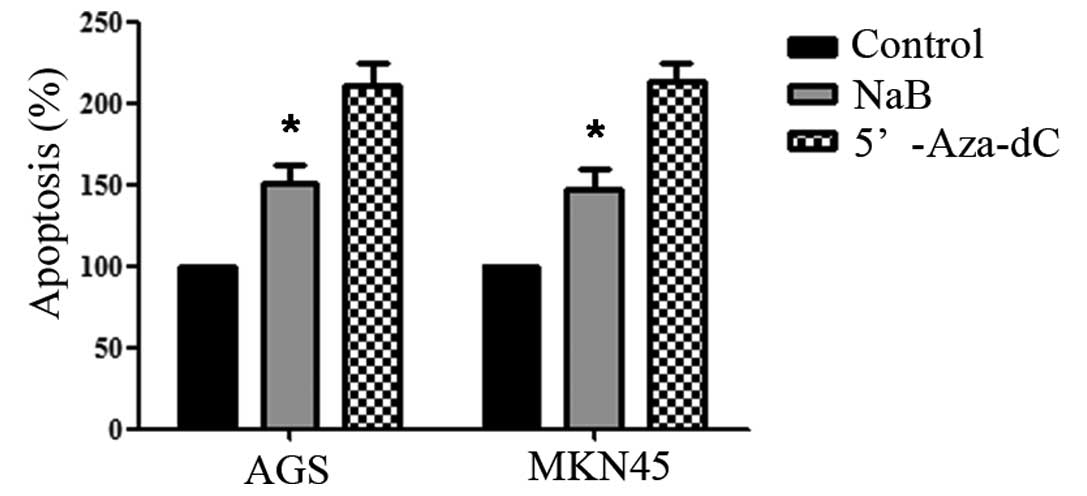
Sodium butyrate induced apoptosis of
human gastric cancer cells
Cell apoptosis was determined by counting sub-G1
phase cells with flow cytometry analysis. After 48 h of NaB
treatment, cell apoptosis significantly increased from 0.05 to 7.2%
in AGS and from 0.07 to 5.83% in MKN45 (both P<0.05). These
findings suggested that NaB induced cell apoptosis (Fig. 2).
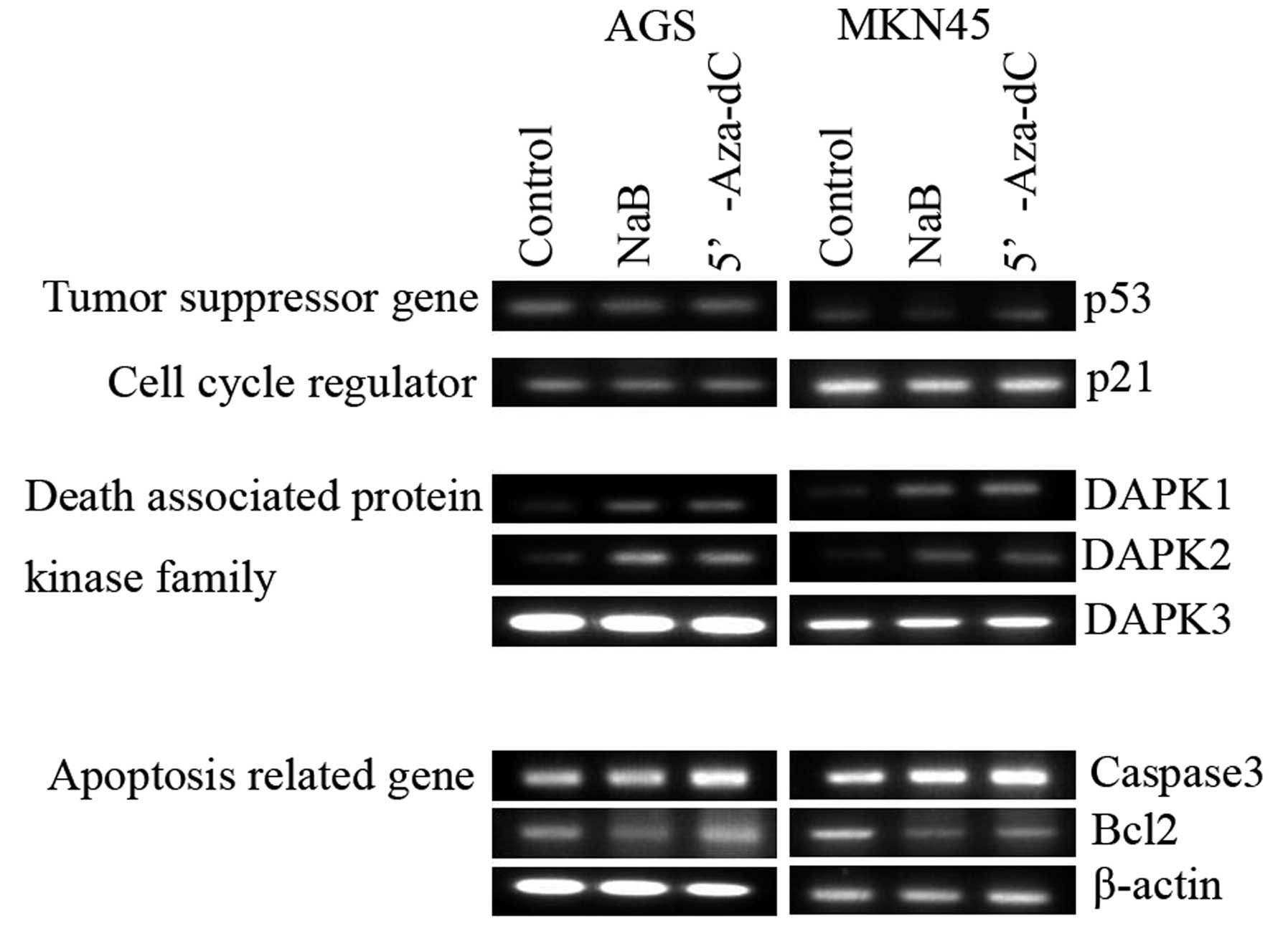
Effect of sodium butyrate on
apoptosis-regulated protein expression
Sodium butyrate increased the expression of
caspase-3, DAPK1, and DAPK2 but decreased the
expression of Bcl-2 in both AGS and MKN45 cells. The
expression of DAPK3, p53 and p21 genes was not
altered by NaB treatment (Fig. 3).
These finding suggests that NaB induces DAPK1/2 expression
in human gastric cancer cells.
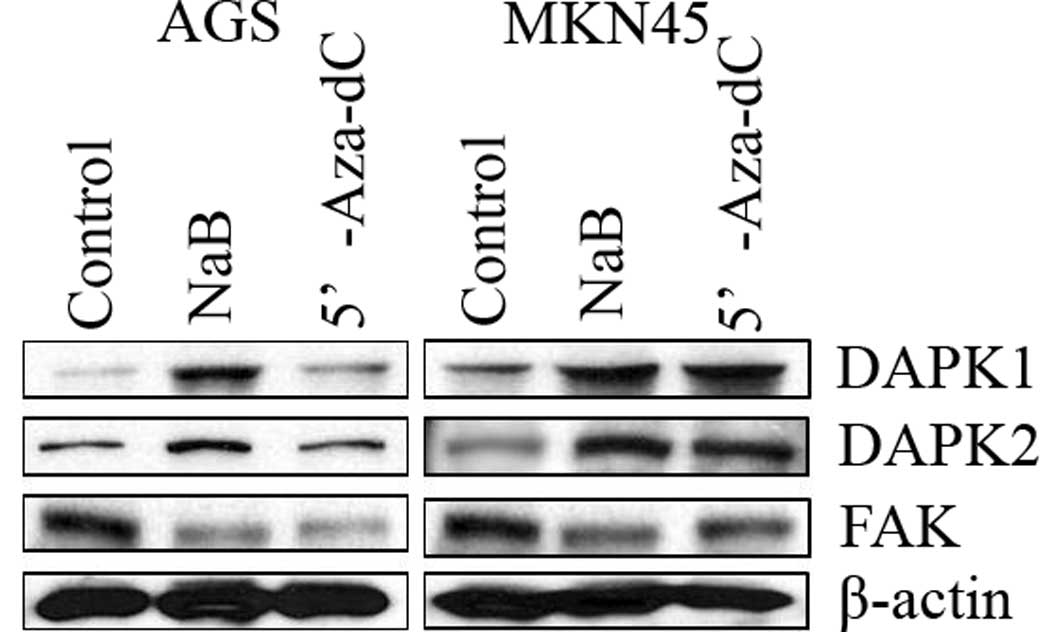
Sodium butyrate increased DAPKs
expression and decreased the expression of FAK in human gastric
cancer cells
To confirm DAPKs expression induced by NaB
treatment, Western blot analysis was performed 48 h after treatment
with NaB. Expression of DAPK1/2 was increased, while that of FAK
was decreased in human gastric cancer cells (Fig. 4). These finding suggest that NaB
induced the expression of DAPK1/2, leading to decreased protein
levels of FAK, prompting cell apoptosis.
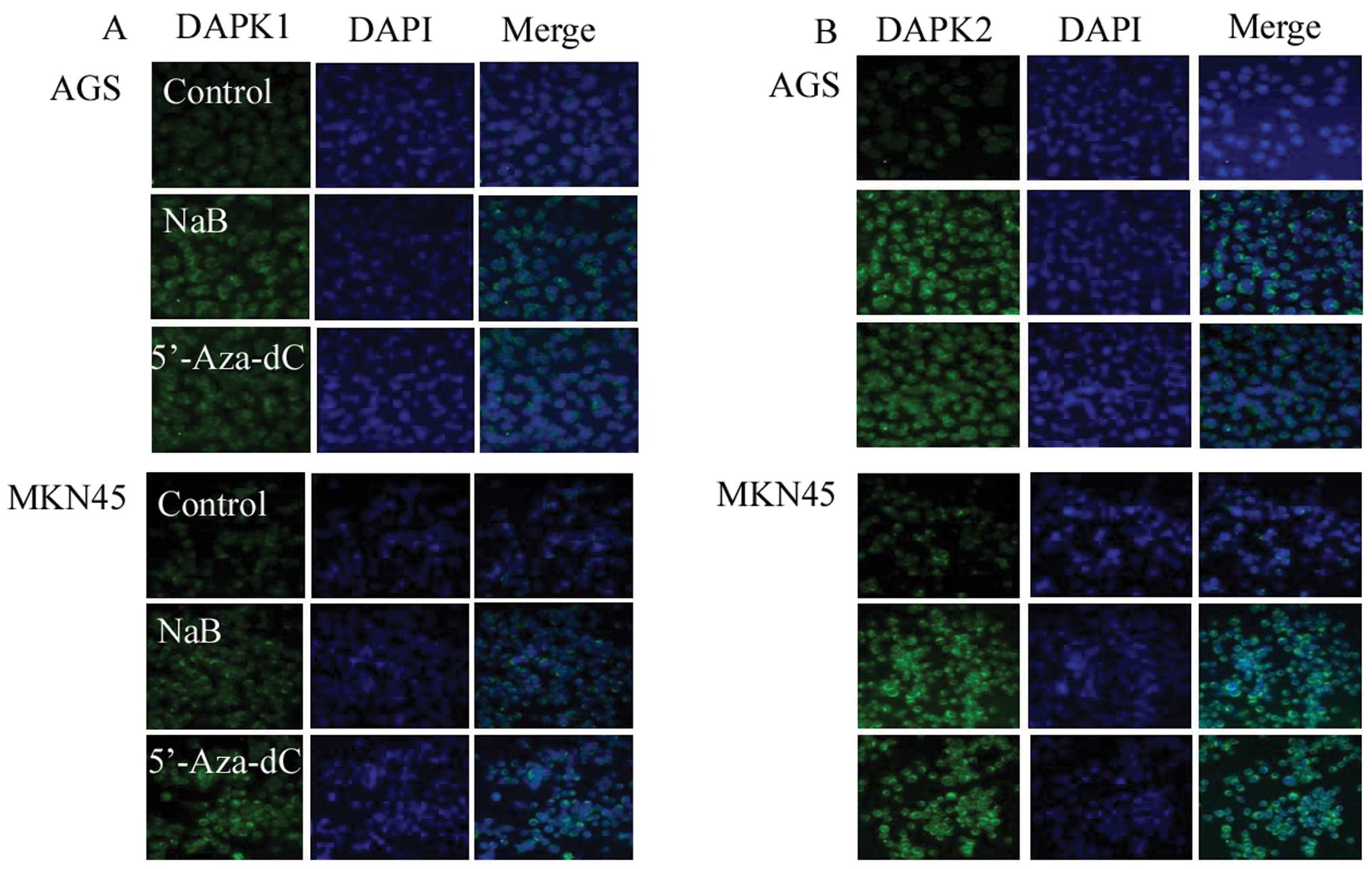
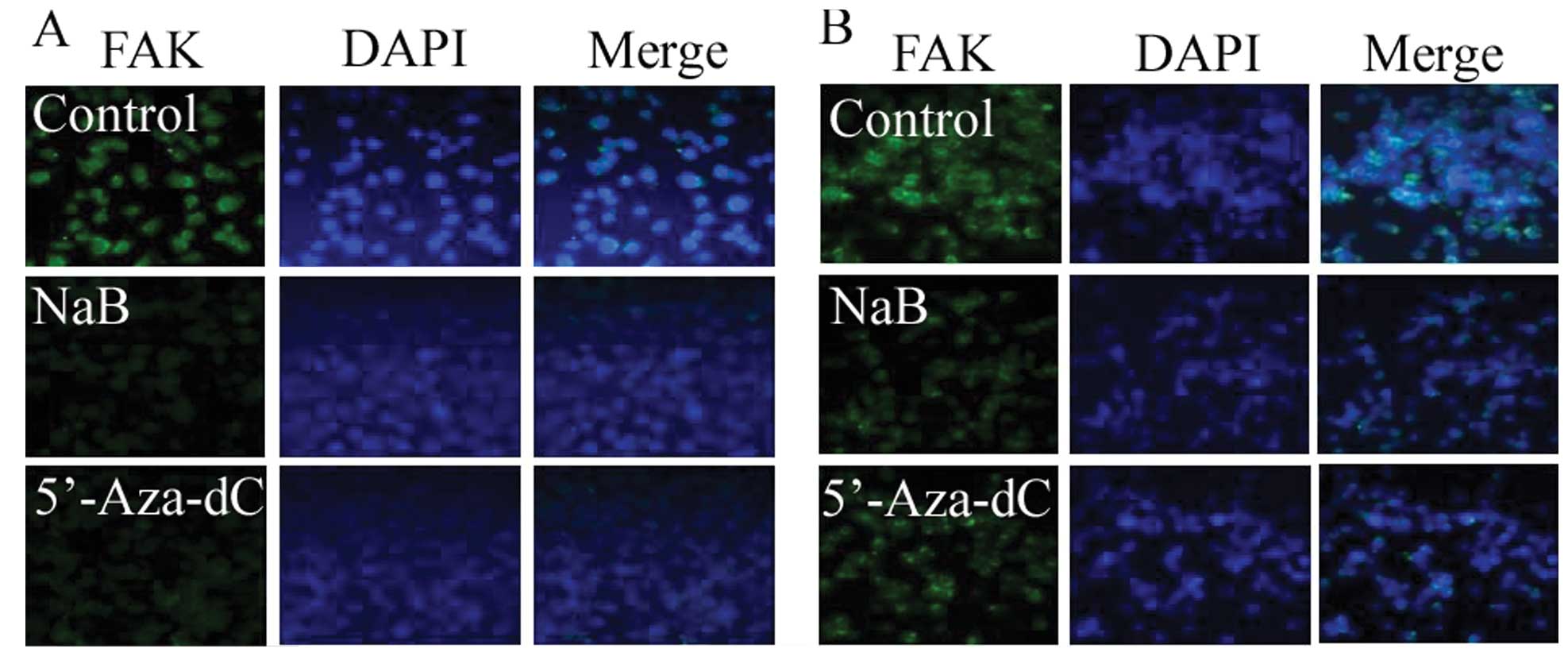
Increased DAPKs expression and decreased
FAKs expression induced by sodium butyrate treatment demonstrated
by immunofluorescence
To confirm DAPKs expression induced by NaB, an
immunofluorescence assay was analyzed 48 h after treatment of cells
with NaB. Sodium butyrate increased the expression of the DAPK1/2
protein but decreased the expression of FAK. These findings suggest
that NaB induced the expression of DAPK1/2, leading to
decreased protein levels of FAK (Figs.
5 and 6).
Discussion
Sodium butyrate enhances the response of
chemotherapeutic agents in human gastric cancer cells (13–15).
Consistent with previous studies, we have confirmed that sodium
butyrate can induce demethylation of the SFRP gene promoter. HDAC
inhibitors are capable of inducing apoptosis and cell cycle arrest
at the G1/G2 phase by altering gene expression (16–23).
In the current study, we aimed to assess sodium
butyrate-induced death-associated protein kinase expression, which
promotes human gastric cancer cell apoptosis. We first sought to
assess the role of sodium butyrate in human gastric cancer cells
using several independent approaches. Our results suggest that
sodium butyrate inhibits cell proliferation and induces cell
apoptosis in human gastric cancer cells.
The level of caspase-3 mRNA expression
increased in human gastric cancer cells following treatment with
sodium butyrate. Although no studies support the fact that the
transcription of caspase-3 can be upregulated directly by
histone acetylation, recent evidence suggests that HDAC inhibitors
also enhance the acetylation of non-histone proteins (24,25).
Down-regulation of Bcl-2 was present after 48 h of exposure
to sodium butyrate. These findings suggest that caspase-3
was increased by sodium butyrate in human gastric cancer cells, but
not Bcl-2. The p53 gene is not involved in
butyrate-induced growth inhibition of breast cancer cells (26). The current study also demonstrates
that sodium butyrate did not induce p53 or p21
expression. Recently, it has been reported that the histone
deacetylase inhibitor, TSA, could induce cells to express
DAPK and promote cell apoptosis (24). DAPK expression causes tumor
cells to lose sensitivity to anoikis, enabling anchor-independent
survival of tumor cells (25). Our
results found that DAPK1/2, but not DAPK3, expression
was increased by sodium butyrate treatment in human gastric cancer
cells. DAPK1/2 is responsible for the induction of
apoptosis, while DAPK3 usually induces morphological changes
in apoptosis.
FAK is involved in adhesion between cells and the
extra-cellular matrix, and joins cytoskeletal proteins. FAK is a
survival protein that suppresses apoptosis (23). Our experiments demonstrate that
sodium butyrate induced DAPK1/2 expression but
down-regulated FAK expression in human gastric cancer cells. These
findings suggest that a sodium butyrate-induced
DAPK-dependent decrease in FAK via the caspase-dependent
pathway leads to apoptosis in human gastric cancer cells. In
conclusion, sodium butyrate induced DAPK expression in human
gastric cancer cells and this expression prompted apoptosis by
decreasing FAK levels.
Acknowledgements
This study was supported by the research grant
(7-2011-0016) from Yonsei University College of Medicine, Seoul,
Republic of Korea, and the authors wish to thank Yonsei-Carl Zeiss
Advanced Imaging Center, Yonsei University College of Medicine, for
technical assistance.
References
|
1
|
Lehninger AL, Nelson DL and Cox MM:
Principles of Biochemistry. 2nd edition. Worth; New York: 1993
|
|
2
|
Widmer J, Fassihi KS, Schlichter SC,
Wheeler KS, Crute BE, King N, Nutile-Mcmenemy N, Noll WW, Daniel S,
Ha J, Kim KH and Witters LA: Identification of a second human
acetyl-CoA carboxylase gene. Biochem J. 316:915–922.
1996.PubMed/NCBI
|
|
3
|
Medina V, Edmonds B, Young GP, James R,
Appleton S and Zalewski PD: Induction of caspase-3 protease
activity and apoptosis by butyrate and trichostatin A (inhibitors
of histone deacetylase): dependence on protein synthesis and
synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer
Res. 57:3697–3707. 1997.
|
|
4
|
Richon VM, Emiliani S, Verdin E, Webb Y,
Breslow R, Rifkind RA and Marks PA: A class of hybrid polar
inducers of transformed cell differentiation inhibits histone
deacetylases. Proc Natl Acad Sci USA. 95:3003–3007. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Saunthararajah Y, Redner RL and
Liu JM: Inhibitors of histone deacetylase relieve ETO-mediated
repression and induce differentiation of AML1-ETO leukemia cells.
Cancer Res. 59:2766–2769. 1999.PubMed/NCBI
|
|
6
|
Butler LM, Agus DB, Scher HI, Higgins B,
Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA and Richon
VM: Suberoylanilide hydroxamic acid, an inhibitor of histone
deacetylase, suppresses the growth of prostate cancer cells in
vitro and in vivo. Cancer Res. 60:5165–5170. 2000.PubMed/NCBI
|
|
7
|
Roy S, Packman K, Jeffrey R and Tenniswood
M: Histone deacetylase inhibitors differentially stabilize
acetylated p53 and induce cell cycle arrest or apoptosis in
prostate cancer cells. Cell Death Differ. 12:482–491. 2005.
View Article : Google Scholar
|
|
8
|
Heerdt BG, Houston MA, Anthony GM and
Augenlicht LH: Initiation of growth arrest and apoptosis of MCF-7
mammary carcinoma cells by tributyrin, a triglyceride analogue of
the shortchain fatty acid butyrate, is associated with
mitochondrial activity. Cancer Res. 59:1584–1591. 1999.PubMed/NCBI
|
|
9
|
Benjamin D and Jost JP: Reversal of
methylation-mediated repression with short-chain fatty acids:
evidence for an additional mechanism to histone deacetylation.
Nucleic Acids Res. 29:3603–3610. 2001. View Article : Google Scholar
|
|
10
|
Lee MG, Wynder C, Bochar DA, Hakimi MA,
Cooch N and Shiekhattar R: Functional interplay between histone
demethylase and deacetylase enzymes. Mol Cell Biol. 26:6395–6402.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan H, Heckman CA and Boxer LM: Histone
deacetylase inhibitors down-regulate bcl-2 expression and induce
apoptosis in lymphomas. Mol Cell Biol. 25:1608–1619. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Earel JK Jr, VanOosten RL and Griffith TS:
Histone deacetylase inhibitors modulate the sensitivity of tumor
necrosis factor-related apoptosis-inducing ligand-resistant bladder
tumor cells. Cancer Res. 66:499–507. 2006. View Article : Google Scholar
|
|
13
|
Neuzil J, Swettenham E and Gellert N:
Sensitization of mesothelioma to TRAIL apoptosis by inhibition of
histone deacetylase: role of Bcl-xL down-regulation. Biochem
Biophys Res Commun. 314:186–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi YH: Induction of apoptosis by
trichostatin A, a histone deacetylase inhibitor, is associated with
inhibition of cyclooxygenase-2 activity in human non-small cell
lung cancer cells. Int J Oncol. 27:473–479. 2005.PubMed/NCBI
|
|
15
|
Donadelli M, Costanzo C, Faggioli L,
Scupoli MT, Moore PS, Bassi C, Scarpa A and Palmieri M:
Trichostatin A, an inhibitor of histone deacetylases, strongly
suppresses growth of pancreatic adenocarcinoma cells. Mol Carcinog.
38:59–69. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wetzel M, Premkumar DR, Arnold B and
Pollack IF: Effect of trichostatin A, a histone deacetylase
inhibitor, on glioma proliferation in vitro by inducing cell cycle
arrest and apoptosis. J Neurosurg. 103:549–556. 2005.PubMed/NCBI
|
|
17
|
Archer SY, Johnson J, Kim HJ, Ma Q, Mou H,
Daesety V, Meng S and Hodin RA: The histone deacetylase inhibitor
butyrate down-regulates cyclin B1 gene expression via a
p21/WAF-1-dependent mechanism in human colon cancer cells. Am J
Physiol Gastrointest Liver Physiol. 289:G696–G703. 2005.
|
|
18
|
Sakimura R, Tanaka K, Nakatani F,
Matsunobu T, Li X, Hanada M, Okada T, Nakamura T, Matsumoto Y and
Iwamoto Y: Antitumor effects of histone deacetylase inhibitor on
Ewing’s family tumors. Int J Cancer. 116:784–792. 2005.
|
|
19
|
Terui T, Murakami K, Takimoto R, Takahashi
M, Takada K, Murakami T, Minami S, Matsunaga T, Takayama T, Kato J
and Niitsu Y: Induction of PIG3 and NOXA through acetylation of p53
at 320 and 373 lysine residues as a mechanism for apoptotic cell
death by histone deacetylase inhibitors. Cancer Res. 63:8948–8954.
2003.PubMed/NCBI
|
|
20
|
Sathyanarayana UG, Padar A, Tockman MS,
Lam S, Shivapurkar N and Gazdar AF: Epigenetic down-regulation of
death-associated protein kinase in lung cancers. Clin Cancer Res.
9:3034–3041. 2003.PubMed/NCBI
|
|
21
|
Zhang X, Yashiro M, Ren J and Hirakawa K:
Histone deacetylase inhibitor, trichostatin A, increases the
chemosensitivity of anticancer drugs in gastric cancer cell lines.
Oncol Rep. 16:563–568. 2006.PubMed/NCBI
|
|
22
|
Inbal B, Cohen O, Polak-Charcon S,
Kopolovic J, Vadai E and Eisenbach L: DAP-kinase links the control
of apoptosis to metastasis. Nature. 390:180–184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chopin V, Toillon RA, Jouy N and Le
Bourhis X: Sodium butyrate induces p53-independent Fas-mediated
apoptosis in MCF-7 human breast cancer cells. Br J Pharmacol.
135:79–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen
DM, Chen GA and Schrump DS: Modulation of p53, ErbB1, ErbB2 and
Raf-1 expression in lung cancer cells by depsipeptide FR901228. J
Natl Cancer Inst. 94:504–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blagosklonny MV, Robey R, Sackett DL, Du
L, Traganos F, Darzynkiewicz Z, Fojo T and Bates SE: Histone
deacetylase inhibitors all induce p21 but differentially cause
tubulin acetylation, mitotic arrest and cytotoxicity. Mol Cancer
Ther. 1:937–941. 2002.
|
|
26
|
Xia H, Nho RS, Kahm J, Kleidon J and Henke
CA: Focal adhesion kinase is upstream of phosphatidylinositol
3-kinase/akt in regulating fibroblast survival in response to
contraction of type I collagen matrices via a beta 1 integrin
viability signaling pathway. J Biol Chem. 279:33024–33034. 2004.
View Article : Google Scholar
|