Introduction
Ovarian cancer is the most deadly malignancy of the
female reproductive system with more than 70% of cases diagnosed at
an advanced stage. Current treatment of this cancer includes
cytoreductive surgery (tumor debulking) followed by platinum- and
taxane-based therapy (1). However,
despite a high rate of initial remissions, patients usually relapse
and subsequently require additional second- or third-line therapy
(2,3). Unfortunately, the patients eventually
develop drug resistance, causing limitations of further treatment
options and decreasing overall survival (1). Thus, intervention with chemopreventive
agents or new adjuvant therapy may offer a desirable option for
ovarian cancer (4).
Experimental studies, animal tumor models and many
in vitro experiments have all demonstrated that
non-steroidal anti-inflammatory drugs (NSAIDs) appear to be
effective in chemoprevention and possible treatment of various
types of cancer (5–10). The rationale of their use is their
cyclooxygenases (COXs)-blocking activity. Overexpression of COX
genes is a frequent phenomenon in preneoplastic and tumor tissues,
including ovarian cancer (9), and
is recognized as a bad prognostic factor. Upregulation of the COX
enzymes in ovarian tumor cells has been implicated in platinum drug
resistance and promotion of tumor progression (11,12).
However, some studies suggest that overexpression of COXs is not
obligatory for anticancer effect, at least in therapeutic approach,
and NSAIDs can directly kill tumor cells via different
intracellular pathways including NF-κB inhibition (10,13,14).
One of the most promising pharmaceutical agent from
the group of NSAIDs, reported to inhibit carcinogenesis and acting
directly against tumor cells in vitro and in experimental
tumor models, is sulindac. This agent itself does not inhibit
cyclooxygenases but is metabolized to COX-inhibiting sulindac
sulfide and inactive sulindac sulfone. Sulindac and its
derivatives, alone or in combination with some chemotherapeutics,
have been found to induce growth suppression and apoptosis in
cultures of tumor cells (15–22),
including ovarian cancer (23). At
present, sulindac is being evaluated as a chemopreventive or
therapeutic agent in several clinical trials (NCT00755976,
NCT00299195 and NCT00118365 available at http:/www.clinicaltrials.gov). There are also attempts
to use sulindac sulfone, known as exisulind, in combination
treatments of various types of cancer (24,25).
Pyrrolidine dithiocarbamate (PDTC) is a
thiol-containing synthetic compound, which is known for its
antioxidant, metal-chelating and strong NF-κB inhibitory properties
(26,27). Occasionally, it exerts paradoxical
prooxidant activity (28).
Recently, PDTC has attracted the attention of investigators as a
potential anticancer agent. In in vitro studies this agent
exerted cytotoxic effects against many types of cancer cells
(28–30). Interestingly from the therapeutic
point of view, PDTC has been shown to inhibit blood vessel
formation and tumor angiogenesis in ex vivo studies and in
animal models (31).
In a previous study, we have demonstrated that
sulindac and sulindac sulfide but not other NSAIDs such as
acetylsalicylic acid and rofecoxib inhibited the growth of various
ovarian cancer cells (32). We
supposed that this effect could result from NF-κB targeting. The
aim of the present study was: i) to assess the effect of sulindac
or sulindac sulfide in combination with PDTC on the growth of cells
of different ovarian cancer lines; and ii) to identify possible
mechanisms of their action.
Materials and methods
Cell cultures
The following ovarian cancer cell lines were used in
the study: OVA-14 (established in our laboratory from solid
epithelial (serous) tumor, CAOV-1 (obtained from Dr M. Siedlar,
Jagiellonian University Collegium Medicum, Krakow), OVP-10
(obtained from Dr B. Szaniawska, Institute of Oncology, Warsaw),
MDAH 2774 (ATCC no. CRL-10303), SKOV-3 (ATCC no. HTB-77). The cells
were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco-BRL,
Invitrogen) (OVA-14, CAOV-1, MDAH 2774, SKOV-4) or RPMI-1640
(Gibco-BRL, Invitrogen) (OVP-10) supplemented with 10%
heat-inactivated fetal calf serum (FCS, Gibco-BRL, Invitrogen) and
antibiotic-antimycotic (Sigma). The cells were maintained in
25-cm2 tissue flasks (Nunc, Roskdile, Denmark) at 37°C
in a humidified atmosphere of 5% CO2 and were passaged
two to three times weekly.
Drugs
Sulindac was from Sigma and sulindac sulfide was
purchased from Biomol Research Laboratories (Plymouth Meeting, PA,
USA). The drugs were dissolved in dimethylsulfoxide (DMSO; Sigma)
and stock solutions were prepared (200 mM) for further preparation.
The cells in control cultures were incubated either without diluent
or with 0.1% DMSO; no significant differences in cell growth were
observed. Pyrrolidine dithiocarbamate (PDTC) was purchased from
Sigma, with the stock solution (200 mM) prepared in distilled
water.
MTT assay
The cytotoxic effects of sulindac and/or PDTC on
ovarian cancer cells was tested in a standard
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. This assay relies on the ability of viable cells to reduce a
yellow MTT to a purple formazan product. Cells were incubated in
96-well plates (2×104/200 μl/well) with PDTC (final
concentrations: 1, 2, 4, 8 and 16 μM) and sulindac or sulindac
sulfide (final concentrations: 50, 100 and 200 μM), alone or in
combination, for 24 h. At the end of the incubation, 25 μl of MTT
(2.5 mg/ml) was added to each well for the last 4 h. Then the cells
were centrifuged (350 × g, 10 min), supernatants were removed and
the formazan product was dissolved in acid DMSO. The plates were
read on an ELISA reader (SLT-Labinstruments, Salzburg, Austria)
using a 550 nm filter. The means and standard deviations were
determined for triplicate samples. The cytotoxic effect was
expressed as the relative viability and was calculated as follows:
relative viability = [(experimental absorbance - background
absorbance)/(absorbance of vehicle-treated cells - background
absorbance) × 100.
Western blot analysis
At the end of incubation with sulindac ± PDTC (1 or
4 h), OVA-14 cells were washed three times in PBS and were lysed
with RIPA buffer containing protease inhibitor cocktail (PMSF 0.1
mg/ml, aprotinin 1.7 mg/ml, pepstatin 5 μg/ml, leupeptin 5 mg/ml,
sodium orthovanadate 1 mM) (protease inhibitor cocktail:RIPA
buffer, 1:100). The protein concentration in the lysates was
determined by Lowry’s method. The cell extract was separated by 10%
gel (NF-κB p50, NF-κB p65, GAPDH) or by 12% gel (Bcl-2, Bax,
procaspase-9) and transferred onto a nitrocellulose membrane
(Bio-Rad Laboratories). The membrane was blocked with 5% non-fat
milk in TBST (25 mM Tris, pH 7.6, 138 mM NaCl and 0.05% Tween-20)
for 1 h and probed with primary antibodies against: NF-κB p50,
NF-κB p65, Bcl-2, Bax, procaspase-9 p35 (Santa Cruz Biotechnology,
Santa Cruz, CA), and GAPDH (Chemicon International). After 3
washes, the membrane was further incubated with secondary antibody
conjugated with horseradish peroxidase (HRP). The expression of
targeted proteins was detected with the enhanced chemiluminescent
(ECL) detection system (Amersham, Buckinghamshire, UK).
Apoptosis assay
Analysis of apoptosis was performed using Annexin
V-FITC Apoptosis Detection KIT I (BD Pharmingen). Briefly, OVA-14
cells were incubated with sulindac (100 μM) and PDTC (16 μM),
either alone or in combination, for 4 or 24 h. At the end of the
incubation the cells were trypsinized, washed twice with cold PBS
and then resuspended in binding buffer at a concentration of
1×106 cells/ml. Cell suspensions (1×105 cells
in 100 μl) were transferred to 5-ml tubes, mixed with 5 μl FITC
Annexin-V and 5 μl propidium iodide (PI), vortexed, and incubated
for 15 min at room temperature in the dark. Next, 400 μl of binding
buffer was added to each tube and the samples were measured using a
flow cytometer (FACSCalibur; Becton-Dickinson, Mountain View, CA)
and CellQuest software.
Flow cytometric analysis of cell
cycle
OVA-14 cells were cultured with sulindac (100 μM)
and PDTC (16 μM), either alone or in combination, for 24 h. The
cells were then trypsinized, washed twice in PBS and fixed
(~1×106) in 3 ml of 70% ethanol (−20°C). After
incubation at −20°C for 48 h, cells were washed three times in PBS
and stained with 50 μg/ml propidium iodide (PI) and 25 mg/ml RNase
in PBS for 30 min in dark at room temperature. The samples were
analyzed using a flow cytometer (FACSCalibur) and the CellQuest
software.
Statistical analysis
Data are presented as mean ± standard deviation
(SD). Results of MTT assay were analyzed by the Student’s t-test.
Additionally, the nature of the interaction between tested drug
combinations was analyzed by the method described by Chou and
Talalay (33). The combination
index (CI) method is a mathematical and quantitative evaluation of
a two-drugs pharmacological interaction. Using data from the
cytotoxicity experiments and CalcuSyn ver. 2.0 software (Biosoft,
Cambridge, UK), CI values were generated. CIs of <1 indicate
synergism, CIs=1 indicate additivity, and CIs >1 indicate
antagonism.
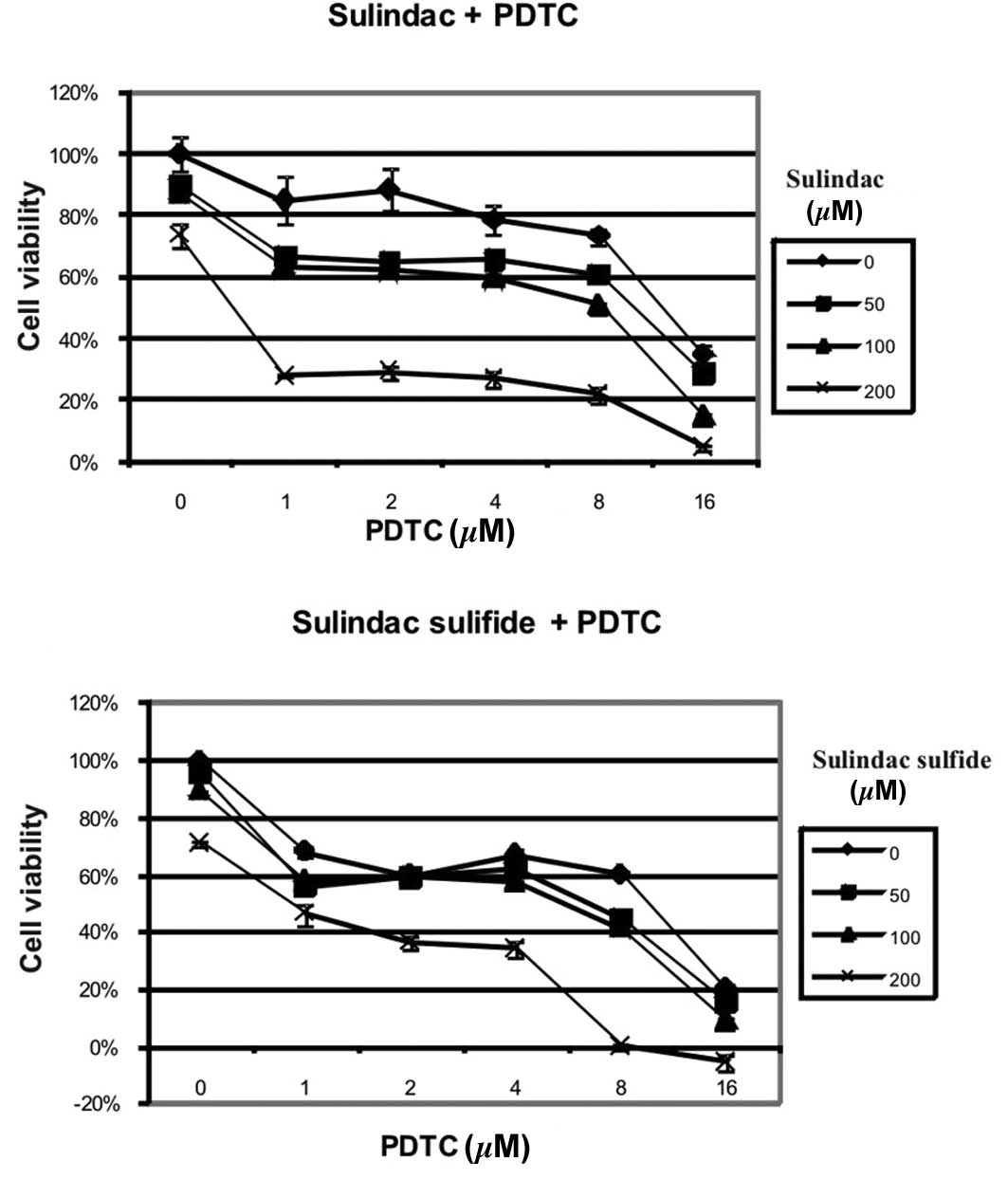
Results
Combinations of sulindac or sulindac
sulfide with PDTC synergistically inhibit the growth of OVA-14
cells in vitro
In our preliminary experiments, sulindac and
sulindac sulfide were the most effective agents, from amongst other
COX-1/COX-2 inhibitors (including acetylsalicylic acid, rofecoxib,
and sulindac sulfone), in inhibition of the viability of various
ovarian cancer cell lines (32). To
determine if effectiveness of these two agents could be enhanced by
PDTC we incubated OVA-14 ovarian cancer cells with different
concentrations of these compounds for 24 h. As measured in the MTT
assay, both sulindac and sulindac sulfide synergized in the
cytotoxic effect with PDTC (Fig.
1). For example, incubation with 200 μM sulindac resulted in
72% viability, 16 μM PDTC resulted in 37% viability, and the
combination of these two doses of drugs reduced viability of tumor
cells below 5% (Fig. 1A) (CI
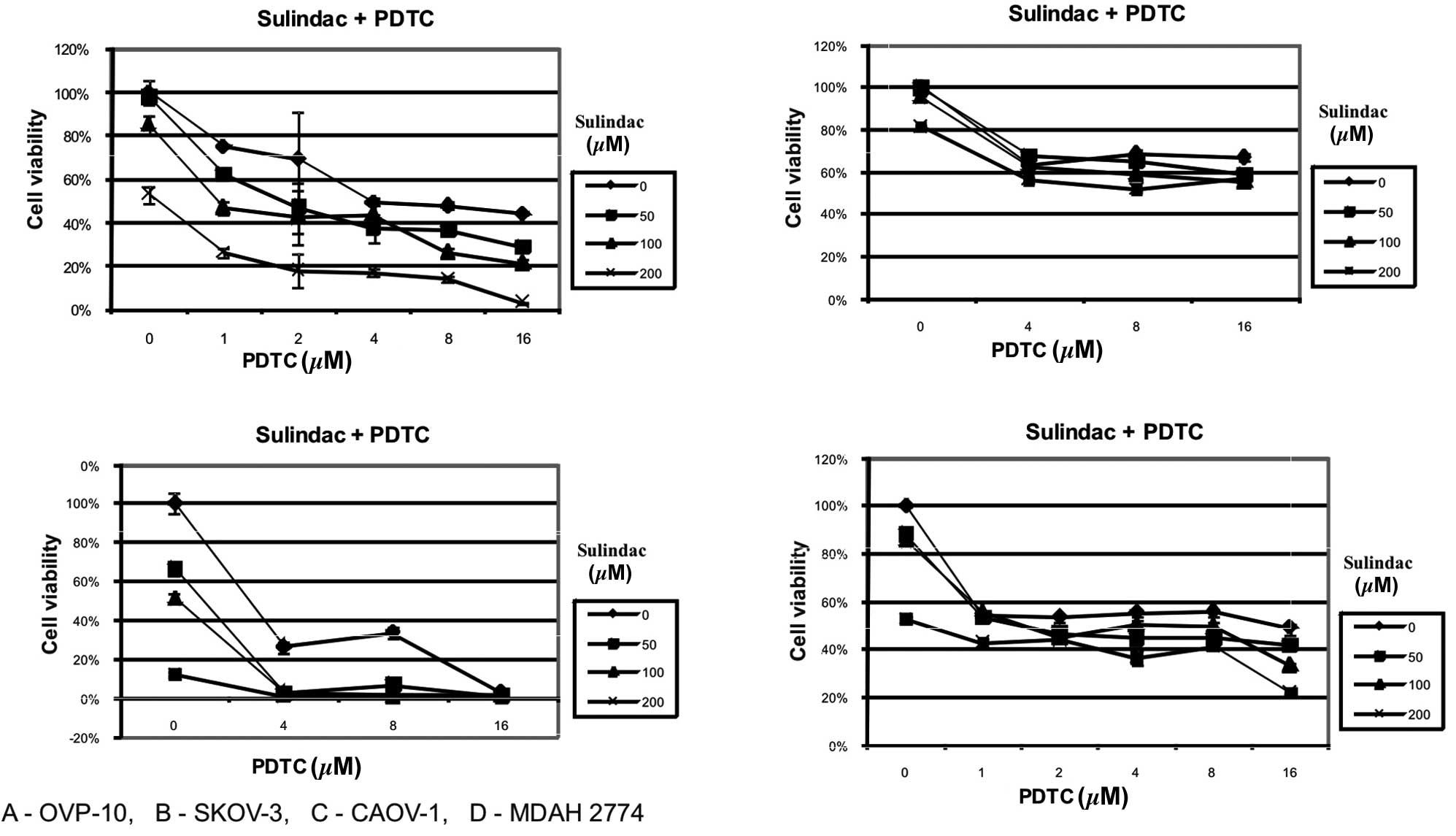
<0.003). The synergistic effect of sulindac and PDTC was also
manifested in CAOV-1 and OVP-10 cell cultures. MDAH 2774 and SKOV-3
cells were found to be susceptible to sulindac or PDTC but the
combination of these two agents did not result in synergy (Fig. 2).
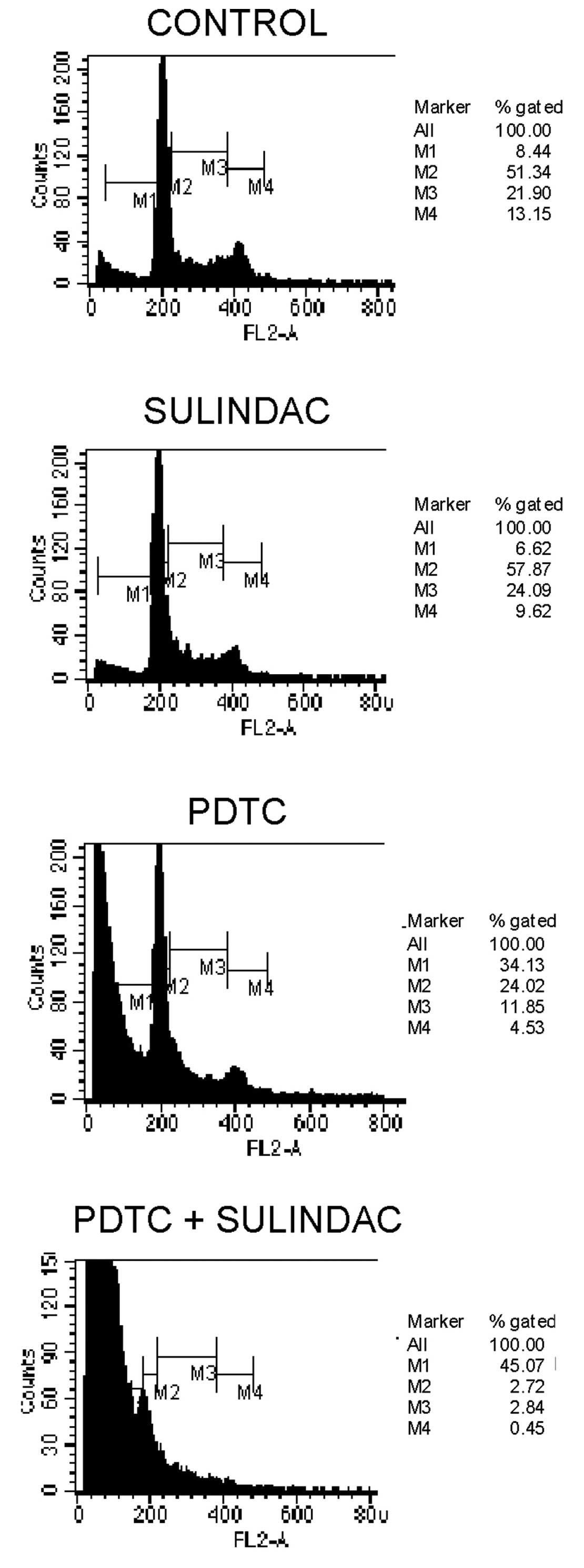
The combination of sulindac with PDTC
results in cell cycle arrest
To evaluate the effects of sulindac and PDTC on cell
cycle behavior, cell cycle analysis by FACS was performed. We found
that incubation of OVA-14 cells with 100 μM sulindac did not
influence cell cycle progression (Fig.
3). On the other hand, 16 μM PDTC induced inhibition of a cell
cycle in the sub-G1 and G0/G1 phases. Combination treatment with
sulindac and PDTC, however, resulted in the strongest inhibitory
effect and a dramatic increase of cell number in a sub-G1 phase;
only 5% of the cells progressed to the G0/G1 + M + G2 phases
(Fig. 3).
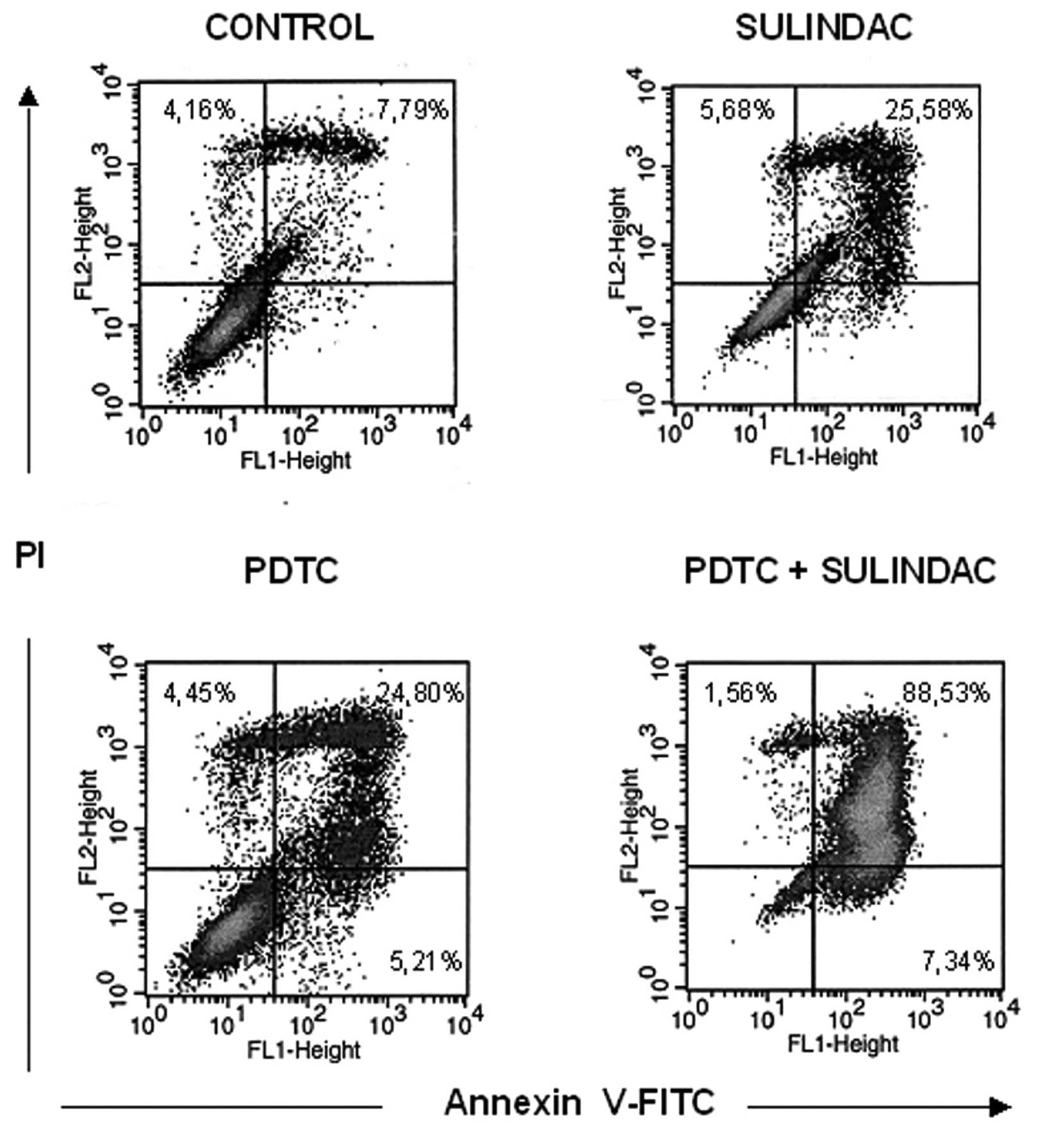
The synergistic cytotoxic effect of
sulindac and PDTC can be attributed to apoptosis
To determine if decreased viability of OVA-14 cells
in cultures with sulindac and PDTC result from apoptosis or other
mechanisms, the cells were incubated with 100 μM sulindac and 16 μM
PDTC, either alone or in combination for 4 or 24 h, with subsequent
Annexin-V flow cytometric analysis. There was a slight increase in
the percentage of cells in early apoptosis [Annexin-V(+), propidium
iodide(−) cells] in double-treated cultures in comparison with
cells incubated with sulindac alone, PDTC alone or untreated
cultures (2.14%, in comparison with 1.49, 1.42, and 1.44%,
respectively) at the 4-h incubation timepoint (data not shown). In
24-h cultures, the process of apoptosis progressed and was
especially exhibited in OVA-14 cells co-treated with sulindac and
PDTC: 89% cells were found to be fully apoptotic, in comparison
with 26% in sulindac cultures, 25% in PDTC cultures, and 8% in
control cultures (Fig. 4). To shed
some light on the intracellular mechanisms responsible for
apoptosis progression in the cells co-incubated with sulindac and
PDTC we examined, by Western blotting, the expression of various
proteins that are characteristic of the apoptosis pathway.
Cytoplasimc cell lysate proteins did not show any significant
changes in the expression levels of Bax, NF-κB p50, and NF-κB p65,
either in cells treated with sulindac and PDTC alone or in
combination (Fig. 5). No effects of
these two agents on NF-κB and no NF-κB DNA-binding activity were
confirmed in an EMSA (data not shown). Western blot analysis
revealed a decrease of antiapoptotic Bcl-2 protein after 1 h of
incubation of OVA-14 cells with both agents and a drop of
procaspase-9 in single- and double-agent-treated cultures (Fig. 5). The latter observation argues for
activation of caspase-9, which is typical for the downstream
activation of the mitochondrial pathway of apoptosis.
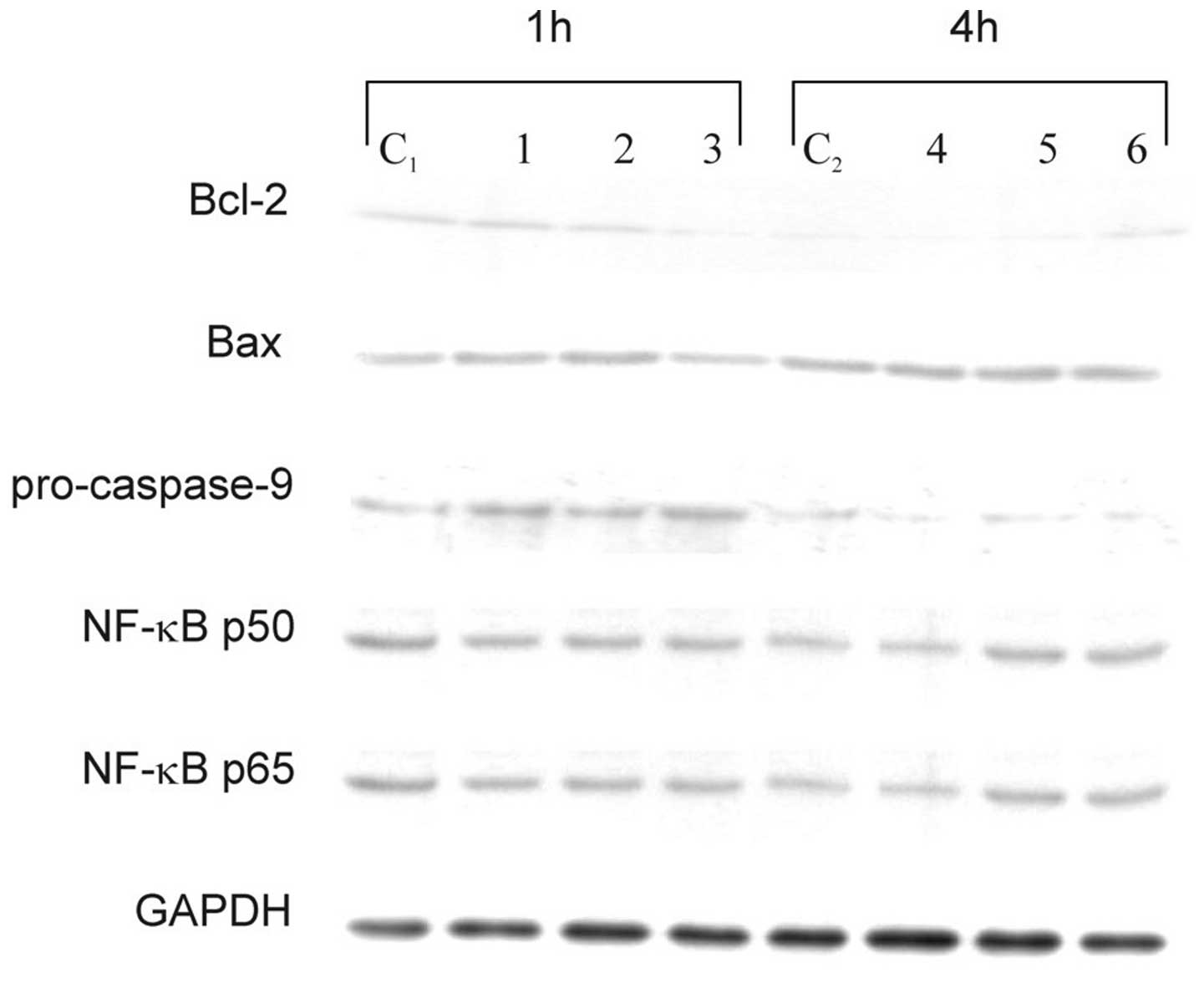 | Figure 5Effect of sulindac and PDTC on
expression of Bcl-2, Bax, procaspase-9, NF-κB p50 and NF-κB p65 in
OVA-14 cells incubated with 100 μM sulindac and/or 16 μM PDTC for 1
or 4 h. Cytoplasmic cell lysates were immunoblotted with antibodies
against each of the above-mentioned proteins, as described in
Materials and methods. GAPDH was used as control. C1, C2, controls;
lanes 1 and 4, cells incubated with sulindac alone; lanes 2, 5,
cells incubated with PDTC alone; lanes 3 and 6, cells incubated
with both sulindac and PDTC. |
Discussion
A combination approach of several therapeutic agents
has become the standard treatment of ovarian cancer. In the present
study, we demonstrated that the combined use of sulindac or
sulindac sulfide and PDTC in vitro resulted in a synergistic
cytotoxic effect against OVA-14 ovarian cancer cells (Fig. 1). This effect was also observed in
our studies on other ovarian cancer cell lines (OVP-10, CAOV-1)
(Fig. 2). The primary mechanism
responsible for the cytotoxic effect of sulindac + PDTC was
apoptosis (Fig. 4). Increased level
of Bcl-2 and decreased expression of procaspase-9 (reciprocally
proportional to the active form of caspase-9) argues for the
mitochondrial apoptotic pathway and is in agreement with results of
other investigators showing correlation of caspase-9 activation and
cancer cell death following incubation with sulindac or sulindac
sulfide (16) or PDTC (26). In our model, susceptibility of
OVA-14 cells to the cytotoxic effect of sulindac and PDTC was not
related to the expression of COX-1/COX-2 since both sulindac
sulfide (an active inhibitor of cyclooxygenases in vitro)
and sulindac (inactive compound) synergized with PDTC. Moreover,
OVA-14 cells did not express COX-1 and COX-2 enzymes in our
preliminary experiments (data not shown).
Both sulindac and PDTC have been shown to inhibit
the pro-survival NF-κB signaling pathway in different tumor models
(29,34–36).
However, interfering with the NF-κB pathway did not seem to play a
role in the cytotoxic effect of sulindac and PDTC in our studies
(Fig. 5). This observation does not
exclude the possibility of NF-κB inhibition by sulindac and/or PDTC
in case of activation of NF-κB in some circumstances, e.g.
following standard chemotherapy (30) or in tumor cells with high
constitutive expression of NF-κB. Recent results of Nai et
al (37) argue for the latter
scenario; they reported that application of PDTC in vivo led
to a decreased level of NF-κB (and also prevention of cachexia) in
tumor tissue in a mouse model of colon cancer.
COX inhibitors, including sulindac and its
metabolites, have been recognized as promising agents in the
prevention of neoplasia and in cancer treatment, especially when
combined with standard therapy [(38) and clinical trial NCT00755976).
Several in vitro studies have shown their antitumor efficacy
in ovarian cancer models (10,13,19,23).
On the other hand, PDTC has been reported to inhibit the growth of
SKOV-3 ovarian cancer cells and to increase chemosensitivity of
these cells to paclitaxel (30).
Combination of sulindac and PDTC has not been tested before, to our
knowledge, in any tumor model but, as shown in our investigation,
their joined application seems very promising.
Apart from the direct pro-apoptotic activity of
sulindac and PDTC, which we and others observed in vitro,
these agents exert pleiotropic biological effects in vivo,
beneficial from the therapeutic point of view. Upregulation of COX
enzymes is a frequent feature of neoplasia and in ovarian cancers
COXs have been implicated in platinum resistance and promotion of
tumor progression (11,12). Furthermore, taxanes, that are
standard drugs used in first- and second-line therapy of ovarian
cancer, have been shown to induce COX-2 expression (39). Therefore, one may expect that
sulindac may increase the efficacy of these drug classes. Sulindac,
alone or in combination with thalidomide, inhibited growth of
carcinoma in rabbits (8) and was
also effective in suppressing pancreatic tumor growth in a
xenograft model in mice (14).
Recently, sulindac in combination with epirubicin has been tested
in patients with advanced cancers and stabilization of the disease
in a patient with ovarian carcinoma was observed (38). Since PDTC, apart from potent NF-κB
inhibitory properties and direct cytotoxic effect against tumor
cells, manifests strong anti-angiogenic effects (31,40),
its addition to sulindac-based therapy could reveal stronger
antitumor activity in vivo than those observed in ovarian
cancer cell cultures in vitro. Thus, preclinical in
vivo investigations of potential antitumor effect of PDTC and
sulindac against ovarian cancers are worth continuing.
References
|
1
|
Gubbels JAA, Claussen N, Kapur AK, Connor
JP and Patankar MS: The detection, treatment, and biology of
epithelial ovarian cancer. J Ovarian Res. 3:8–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pautier P, Joly F, Kerbrat P, Bougnoux P,
Fumoleau P, Petit T, Rixe O, Ringeisen F, Tisseron Carrasco A and
Lhomme C: Phase II study of gefitinib in combination with
paclitaxel (P) and carboplatin (C) as second-line therapy for
ovarian, tubal or peritoneal adenocarcinoma (1839IL/0074). Gynecol
Oncol. 116:157–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pectasides D, Pectasides E, Papaxoinis G,
Psyrri A, Pliarchopoulou K, Koumarianou A, Macheras A, Athanasas G,
Xiros N and Economopoulos T: Carboplatin/gemcitabine alternating
with carboplatin/pegylated liposomal doxorubicin and
carboplatin/cyclophosphamide in platinum-refractory/resistant
paclitaxel-pretreated ovarian carcinoma. Gynecol Oncol. 118:52–57.
2010. View Article : Google Scholar
|
|
4
|
Ledermann JA and Raja FA: Targeted trials
in ovarian cancer. Gynecol Oncol. 119:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cuzick J, Otto F, Baron JA, Brown PH, Burn
J, Greenwald P, Jankowski J, La Vecchia C and Meyskens F: Aspirin
and non-steroidal anti-inflammatory drugs for cancer prevention: an
international consensus statement. Lancet Oncol. 10:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akhmedkhanov A, Toniolo P,
Zeleniuch-Jacquotte A, Kato I, Koenig KL and Shore RE: Aspirin and
epithelial ovarian cancer. Prev Med. 33:682–687. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sporn MB and Hong WK: Concomitant DFMO and
sulindac chemoprevention of colorectal adenomas: a major clinical
advance. Nat Clin Pract Oncol. 5:628–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verheul HMW, Panigrahy D, Yuan J and
D’Amato RJ: Combination oral antiangiogenic therapy with
thalidomide and sulindac inhibits tumour growth in rabbits. Br J
Cancer. 79:114–118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uddin S, Ahmed M, Hussain A, Assad L,
Al-Dayel F, Bavi P, Al-Kuraya KS and Munkarah A: Cyclooxygenase-2
inhibition inhibits PI3K/AKT kinase activity in epithelial ovarian
cancer. Int J Cancer. 126:382–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez-Burford C, Barnes MN,
Oelschlager DK, Myers RB, Talley LI, Partridge EE and Grizzle WE:
Effects of non-steroidal anti-inflammatory agents (NSAIDs) on
ovarian carcinoma cell lines: preclinical evaluation of NSAIDs as
chemopreventive agents. Clin Cancer Res. 8:202–209. 2002.
|
|
11
|
Gupta RA, Tejada LV, Tong BJ, Das SK,
Morrow JD, Dey SK and DuBois RN: Cyclooxygenase-1 is overexpressed
and promotes angiogenic factor production in ovarian cancer. Cancer
Res. 63:906–911. 2003.PubMed/NCBI
|
|
12
|
Munkarah A and Ali-Fehmi R: COX-2: a
protein with an active role in gynecological cancers. Curr Opin
Obstet Gynecol. 17:49–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andrews P, Zhao X, Allen J, Li F and Chang
M: A comparison of the effectiveness of selected non-steroidal
anti-inflammatory drugs and their derivatives against cancer cells
in vitro. Cancer Chemother Pharmacol. 61:203–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yip-Schneider MT, Wu H, Ralstin M,
Yiannoutsos C, Crooks PA, Neelakantan S, Noble S, Nakshatri H,
Sweeney CJ and Schmidt CM: Suppression of pancreatic tumor growth
by combination chemotherapy with sulindac and LC-1 is associated
with cyclin D1 inhibition in vivo. Mol Cancer Ther. 6:1736–1744.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunte DP, Wali RK, Koetsier JL and Roy HK:
Antiproliferative effect of sulindac in colonic neoplasia
prevention: role of COOH-terminal Src kinase. Mol Cancer Ther.
7:1797–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marotta A, Parhar K, Hundal R, Duronio V
and Salh B: Differential targeting of protein kinase B in cell
death induced by sulindac and its metabolite sulindac sulfide. Int
J Oncol. 28:1471–1479. 2006.PubMed/NCBI
|
|
17
|
Karl T, Seibert N, Stohr M, Osswald H,
Rosl F and Finzer P: Sulindac induces specific degradation of the
HPV oncoprotein E7 and causes growth arrest and apoptosis in
cervical carcinoma cells. Cancer Lett. 245:103–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song Z, Tong C, Liang J, Dockendorff A,
Huang C, Augenlicht LH and Yang W: JNK1 is required for
sulindac-mediated inhibition of cell proliferation and induction of
apoptosis in vitro and in vivo. Eur J Pharmacol. 560:95–100. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JS, Baek SJ, Sali T and Eling TE: The
conventional non-steroidal anti-inflammatory drug sulindac sulfide
arrests ovarian cancer cell growth via the expression of
NAG-1/MIC-1/GDF-15. Mol Cancer Ther. 4:487–493. 2005.PubMed/NCBI
|
|
20
|
Nikitakis NG, Hebert C, Lopes MA, Reynolds
MA and Sauk JJ: PPARγ-mediated antineoplastic effect of NSAID
sulindac on human oral squamous carcinoma cells. Int J Cancer.
98:817–823. 2002.
|
|
21
|
Park JH, Kim EJ, Jang HY, Shim H, Lee KK,
Jo HJ, Kim HJ, Yang SH, Jeong ET and Kim HR: Combination treatment
with arsenic trioxide and sulindac enhances apoptotic cell death in
lung cancer cells via activation of oxidative stress and
mitogen-activated protein kinases. Oncol Rep. 20:379–384.
2008.PubMed/NCBI
|
|
22
|
Flis S and Spławiński J: Inhibitory
effects of 5-fluorouracil and oxaliplatin on human colorectal
cancer cell survival are synergistically enhanced by sulindac
sulfide. Anticancer Res. 29:435–442. 2009.PubMed/NCBI
|
|
23
|
Barnes AP, Miller BE and Kucera GL:
Cyclooxygenase inhibition and hyperthermia for the potentiation of
the cytotoxic response in ovarian cancer cells. Gynecol Oncol.
104:443–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Govindan R, Wang X, Baggstrom MQ,
Burdette-Radoux S, Hodgson L, Vokes EE and Green MR; Cancer and
Leukemia Group B. A phase II study of carboplatin, etoposide, and
exisulind in patients with extensive small cell lung cancer: CALGB
3014. J Thorac Oncol. 4:220–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sinibaldi VJ, Elza-Brown K, Schmidt J,
Eisenberger MA, Rosenbaum E, Denmeade SR, Pili R, Walczak J, Baker
SD and Zahurak M: Phase II evaluation of docetaxel plus exisulind
in patients with androgen independent prostate carcinoma. Am J Clin
Oncol. 29:395–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oh DH, Bang JS, Choi HM, Yang HI, Yoo MC
and Kim KS: Fetal bovine serum requirement for pyrrolidine
dithiocarbamate-induced apoptotic cell death of MCF-7 breast tumor
cells. Eur J Pharmacol. 649:135–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen D, Peng F, Cui QC, Danie KG, Orlu S,
Liu J and Dou QP: Inhibition of prostate cancer cellular proteasome
activity by a pyrrolidine dithiocarbamate-copper complex is
associated with suppression of proliferation and induction of
apoptosis. Front Biosc. 10:2932–2939. 2005. View Article : Google Scholar
|
|
28
|
Chinery R, Brockman JA, Peeler MO, Shyr Y,
Beauchamp RD and Coffey RJ: Antioxidants enhance the cytotoxicity
of chemotherapeutic agents in colorectal cancer: a p53-independent
induction of p21WAF1/CIP1 via C/EBPβ. Nat Med.
3:1233–1241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morais C, Gobe G, Johnson DW and Healy H:
Inhibition of nuclear factor kappa B transcription activity drives
a synergistic effect of pyrrolidine dithiocarbamate and cisplatin
for treatment of renal cell carcinoma. Apoptosis. 14:412–425. 2010.
View Article : Google Scholar
|
|
30
|
Liu GH, Wang SR, Wang B and Kong BH:
Inhibition of nuclear factor-κB by an antioxidant enhances
paclitaxel sensitivity in ovarian carcinoma cell line. Int J
Gynecol Cancer. 16:1777–1782. 2006.
|
|
31
|
Gu JW, Young E, Busby B, Covington J, Tan
W and Johnson JW: Oral administration of pyrrolidine
dithiocarbamate (PDTC) inhibits VEGF expression, tumor angiogenesis
and growth of breast cancer in female mice. Cancer Biol Ther.
8:514–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jakubowska-Mucka A, Sienko J, Switaj T,
Golab J and Lasek W: Antitumor effects of sulindac in ovarian
cancer cell cultures. Ginekol Pol. 82:195–199. 2011.(In
Polish).
|
|
33
|
Chou TC and Talalay P: Quantitive analysis
of dose-effect relationships: the combined effects of multiple
drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seo AM, Hong SW, Shin JS, Park IC, Hong
NJ, Kim DJ, Lee WK, Lee WJ, Jin DH and Lee MS: Sulindac induces
apoptotic cell death in susceptible human breast cancer cells
through, at least in part, inhibition of IKKβ. Apoptosis.
14:913–922. 2009.PubMed/NCBI
|
|
35
|
Yamamoto Y, Yin MJ, Lin KM and Gaynor RB:
Sulindac inhibition activation of the NF-κB pathway. J Biol Chem.
274:27307–27314. 1999.
|
|
36
|
Nakanishi C and Toi M: Nuclear factor κB
inhibitors as sensitizers to anticancer drugs. Nature Rev Cancer.
5:297–309. 2005.
|
|
37
|
Nai YJ, Jiang ZW, Wang ZM, Li N and Li JS:
Prevention of cancer cachexia by pyrrolidine dithiocarbamate (PDTC)
in colon 26 tumor-bearing mice. JPEN J Parenter Enteral Nutr.
31:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O’Connor R, O’Leary M, Ballot J, Collins
CD, Kinsella P, Mager DE, Arnold RD, O’Driscoll L, Larkin A,
Kennedy S, Fennelly D, Clynes M and Crown J: A phase I clinical and
pharmacokinetic study of the multi-drug resistance protein-1
(MRP-1) inhibitor sulindac, in combination with epirubicin in
patients with advanced cancer. Cancer Chemother Pharmacol.
59:79–87. 2007.PubMed/NCBI
|
|
39
|
Moos PJ, Muskardin DT and Fitzpatrick FA:
Effect of taxol and taxotere on gene expression in macrophages:
induction of the prostaglandin H synthase-2 isoenzyme. J Immunol.
162:467–473. 1999.PubMed/NCBI
|
|
40
|
Morais C, Gobe G, Johnson DW and Healy H:
Anti-angiogenic actions of pyrrolidine dithiocarbamate, a nuclear
factor kappa B inhibitor. Angiogenesis. 12:365–379. 2009.
View Article : Google Scholar : PubMed/NCBI
|