Introduction
Inflammation is a vital defense mechanism for the
organism in response to pathogen stimuli. Monocytes and macrophages
are dominant at the locations of lipopolysaccharide (LPS) induced
inflammation. Macrophages become active upon LPS stimulation, and
several cellular mediators such as tumor necrosis factor-α (TNF-α),
IL-1β, nitric oxide (NO) and cyclooxygenase (COX)-2 are released to
regulate inflammation (1).
NO is also known as a short-lived free radical and
one of key cellular mediator for inflammatory responses. There are
three isoforms of NO synthases (NOS) in tissues to generate NO
(2). The neural NOS and endothelial
NOS isoforms are constitutively expressed in select tissues.
Inducible NOS (iNOS), a third member of the NOS family, is known to
have beneficial effects in response to inflammatory stimuli. The
expression level of iNOS is elevated in response to LPS via a
variety of transcription factors, particularly nuclear
factor-kappaB (NF-κB) (3).
Cyclooxygenase (COX)-2, another cellular inflammatory mediator, is
undetectable in most normal tissues (4). Upon LPS stimulation, COX-2 expression
is induced rapidly and transiently at inflammatory sites (5). Furthermore, NF-κB plays vital roles in
the coordination of the expression of pro-inflammatory mediators
and cytokines, including iNOS (6)
and COX-2 (7). Once cells are
stimulated by LPS, NF-κB becomes activated, dissociates with IκB
and then translocates from cytosol to nucleus, which leads to
induction of NF-κB downstream genes via binding to the NF-κB
response element (8). MicroRNAs
function as non-coding RNA molecules that regulate the expression
of target genes involved in a wide range of biological processes.
It has been shown that microRNAs are found in almost all eukaryotic
cells. Mature microRNAs bind to the 3′ untranslated regions of
target genes and inhibit protein synthesis (9). Recently, microRNAs have been found to
be involved in the growth and differentiation of immune cells
(10). Previous studies showed that
miR-146a and miR-155 were up-regulated in response to LPS
stimulation. MiR-146 has been considered as a negative regulator in
the innate immunity, while miR-155 regulated mature T cells and B
cells (11). Moreover, miR-424 was
found to be involved in the differentiation of macrophages
(12).
Over-reactive and uncontrolled inflammatory
responses can cause the tissues to remain in a chronic inflammatory
status, which leads to a variety of diseases including rheumatoid
arthritis, pulmonary fibrosis and even cancer (13). Previous studies have been shown that
excessive NO production causes inflammation and carcinogenesis
(14,15). Natural products have been used in
drug discovery and development to regulate inflammation.
Calophyllum inophyllum Linn. (Guttiferae) is
a medium to large tree distributed throughout Taiwan, India, and
Australia (16–19). The active constituents of C.
inophyllum is well known for containing xanthone, flavone, and
terpene derivatives, some of which exhibit antitumor (20,21),
and anti-HIV activities (22,23).
The oil of C. inophyllum enhanced the healing of Ocular burn
(24). The present study was
undertaken to evaluate the anti-inflammatory effects of C.
inophyllum in macrophage cells under LPS exposure. Given that
NO and COX-2 are specific to inflamed tissue, the inhibition of NO
over-production and COX-2 expression is important for evaluating
the effects of anti-inflammatory drugs. However, the effect of
C. inophyllum L. leaf (CIL) extract on LPS-induced RAW264.7
cells is still unclear. The purpose of this study was to
investigate the anti-inflammatory actions of CIL extract on NO
production, COX-2 expression and translocation of NF-κB. In
addition, we also provide evidence suggesting that CIL extract also
suppressed LPS-induced miR-146a expression.
Materials and methods
Chemicals and reagents
Celebrex, acetone, dimethylsulfoxide (DMSO),
lipopolysaccride (LPS), and
N-(3-aminomethyl)-benzylacetamidine (1400W) were purchased
from Sigma-Aldrich Corp. (St. Louis, MO, USA).
Preparation of the Calophyllum inophyllum
Linn extracts
The leaves of C. inophyllum L. (Guttiferae)
were collected in Ping Tung Hsieng, Taiwan, in October, 2008, and a
voucher specimen (2008) has been deposited in the Department of
Biological Science and Technology, China Medical University. The
dried leaves of C. inophyllum (1 kg) was ground, extracted
with acetone at room temperature, and concentrated under reduced
pressure to afford a brown residue (90 g). The series
concentrations of CIL extract were further diluted with DMSO.
Quantitative analysis of active compounds
of C. inophyllum by HPLC
Before analysis by HPLC, CIL extract was filtered
through a 0.2 μm Millipore filter, and then total volume of 20 μl
was loaded into the HPLC column. External standards were prepared
as concentration of 100 μg/ml in HPLC grade-methanol and used to
calculate the concentration of examined compounds. Reverse phase
HPLC was performed on a Perkin-Elmer HPLC system (Perkin-Elmer,
Waltham, MA, USA) equipped with Perki-Elmer Series 200 pump,
Perki-Elmer 785A UV/VIS detector and Perk-Elmer Series 200
autosampler. Separations were accomplished on LiChroCART 250-4 C18
HPLC-cartridge (5 μm; Merck, Whitehouse Station, NJ, USA). The
separation conditions of HPLC analysis of examined compounds are
described in Table I.
 | Table IHPLC separation conditions for
identifying marked components within CIL extract.a |
Table I
HPLC separation conditions for
identifying marked components within CIL extract.a
| Compounds | Mobile phase | Wavelength
(nm) | RT (min) | Contents (mg/g of
CIL) |
|---|
| Amentoflavone |
MeOH:H3PO4 (0.11%,
pH 2.2) = 25:75 | 330 | 10.39 | 84.72±1.46 |
| Oleanolic acid |
ACN:H3PO4 (0.11%, pH
2.2) = 28:72 | 215 | 10.15 | 1.05±0.01 |
Cell culture and treatment
The murine macrophage RAW 264.7 cells were obtained
from the Bioresource Collection and Research Center (BCRC, Hsinchu,
Taiwan) and cultured in Dulbecco’s modified Eagle’s medium
(Invitrogen, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal bovine serum, 100 U/ml of penicillin, and
100 μg/ml of streptomycin. Cells were incubated with CIL extract as
indicated for 1 h and then stimulated with 1 μg/ml lipopolysaccride
(LPS) (Escherichia coli 011:B4, Sigma Chemical Co., St.
Louis, MO, USA) for 24 h.
Cell viability assay
Cell viability of RAW 264.7 cells was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
as described elsewhere (25,26).
Briefly, 1×104 RAW 264.7 cells/well were seeded in
96-well plates and incubated with various concentrations of CIL
extract (0–20 μg/ml) at 37°C for 24 h and medium was completely
removed. MTT was added to the cells, followed by incubation for 4 h
at 37°C. After incubation, the medium was discarded and the
formazan crystals in viable cells were dissolved in 100 μl of fresh
DMSO for 10 min. The absorbance was calculated at 590 nm using a
microplate autoreader (Molecular Devices, Sunnyvale, CA, USA).
Relative cell viability was calculated by comparing the absorbance
of the treated group to the LPS stimulated control group. All
experiments were performed in triplicate.
Measurement of nitrite production
Nitric oxide (NO) production was determined by
measurement of the accumulation of nitrite, the stable metabolite
of NO in the culture medium. Nitrite was assayed colorimetrically
after reaction with the Griess reagent as described previously
(27). Briefly, 2×105
cells per well were seeded onto 96-well plates and then treated
with CIL extract (0, 1, 2.5, 5, and 10 μg/ml) at 37°C for 1 h
before stimulation with 1 μg/ml of LPS for 24 h in a final volume
of 0.2 ml. The supernatant of LPS-induced RAW 264.7 cell cultures
was mixed with an equal volume of Griess reagent (1% sulfanilamide
and 0.1% naphthylenediamine in 1 N hydrochloric acid) in a 96-well
plate. Nitrite concentrations were calculated by comparison with
OD550 of standard solutions of sodium nitrite in culture medium.
All determinations were performed in triplicate.
Quantification of mRNA and miRNA
expression level by quantitative real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). Total RNA (1 μg) was heated at 70°C for 10 min and
reversely transcribed using reverse transcriptase 200 U (Promega,
Madison, WI, USA). The mixture was then incubated at 37°C for 60
min, heated at 95°C for 10 min and stored at −20°C until use.
Real-time PCR was performed 40 cycles with primers for iNOS and
β-actin as an internal control using the ABI PRISM 7300 Sequence
Detector System (Applied Biosystems, Foster City, CA, USA). Cycles
consisted of 30 sec of denaturation at 95°C, 30 sec of annealing at
60°C, 1 min of extension at 72°C, followed by 10 min of elongation
at 72°C. Data were collected by the Sequence Detection Software
(SDS; Version 1.3.1, Applied Biosystems) and analyzed using the
threshold cycle relative quantification method. Primers were
designed with computer assistance according to the gene bank. The
sequence of the primers are as follows; iNOS sense primer 5′-CAT
CAA CCA GTA TTA TGG CTC CT-3′, and iNOS anti-sense 5′-TCC TGT TGT
TTC TAT TTC CTT TGT T-3′; β-actin sense 5′-CTA AGG CCA ACC GTG AAA
AG-3′, and β-actin antisense 5′-ACC AGA GGC ATA CAG GGA CA-3′. The
cycle threshold (Ct) values were determined in at least three
independent experiments for each sample. Results were normalized to
the endogenous gene β-actin.
MicroRNA quantification
TaqMan miRNA assays (Applied Biosystems) were used
to quantify mature miRNA of miR-146a, miR-155 and miR-424. RNU6B
was used as a reference gene control. Quantitative assays were
performed in the 7900HT system according to the manufacturer’s
instructions (Applied Biosystems). Briefly, 10 ng of RNA samples
extracted either normal parts or tumor of same oral patient were
mixed and reacted with reverse transcription master mix reagents
(Applied Biosystems) at 16°C for 30 min, 42°C for 30 min, and 85°C
for 5 min. Each of PCR cycle contained the denaturation step at
95°C for 15 sec and the extension step at 60°C for 1 min for a
total of 40 cycles. miRNA expression values were normalized using
RNU6B following the 2−ΔΔCt method and analyzed by using
ABI 7900HT SDS 2.2 software. All reactions were run in
triplicate.
Transient transfection
Transfection followed the manufacturer’s protocol
(Thermo Scientific Open Biosystems, Huntsville, AL, USA). Briefly,
3×105 RAW 264.7 cells/well were incubated in 6-well
plates overnight. Medium was changed into the DMEM without 10%
heat-inactivated fetal bovine serum, 100 U/ml of penicillin, and
100 μg/ml of streptomycin before transfection. Solution A (2 μg of
DNA and 50 μl of DMEM) was mixed with solution B (10 μl of
Arrest-In transfection reagent and 50 μl of DMEM) at room
temperature for 20 min. Transfection reagents were equally added
into cells. Cells were harvested after 48-h transfection.
Luciferase reporter gene assays
pCOX-2-LUC plasmid was used to quantify COX-2
promoter activity. pRL-CMV, a renilla luciferase reporter plasmid
under the control of the cytomegavirus promoter (Promega), was used
as an internal control to normalize the reporter gene activity.
Plasmids were transfected into RAW264.7 cells. After 24-h
transfection, cells were treated with CIL extract for 1 h followed
by stimulation with LPS and analyzed the luciferase activity after
48-h transfection. The luciferase activity was determined by a
luminometer using a dual-luciferase reporter assay system (Promega)
according to the instructions of the manufacturer. Briefly, cells
were lysed with 1× PLB (passive lysis buffer) for 15 min. PLB
lysate (20 μl) was added into a 96-well plate and mixed with 80 μl
of LAR II (luciferase assay substrate in luciferase assay buffer
II) to measure luciferase activity by using the SpectraMax L
spectrometer (Molecular Devices, Sunnyvale, CA, USA). The values
were normalized with the measurement of renilla luciferase
activity.
Preparation of nuclear and cytosolic
extract
The nuclear extract was prepared as described
previously (28). Briefly,
5×105 RAW 264.7 cells were incubated with or without
concentrations of CIL extract for 1 h, and then treated with LPS
(100 ng/ml) for 30 min. After LPS treatment for 24 h, cells were
harvested, washed with ice-cold PBS, and then centrifuged at 2500 g
for 5 min at 4°C. Cell pellets were added to 100 μl lysis buffer
(10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.5% Nonidet-P 40, 1
mM dithiothreitol, 0.5 mM PMSF) and vortexed mildly. Samples were
incubated for 10 min on ice and centrifuged at 2500 g for 5 min at
4°C. The supernatant was collected as a cytosolic fraction. Pellets
containing crude nuclei were resuspended in 100 μl extraction
buffer (20 mM HEPES pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM
dithiothreitol, 1 mM PMSF) and incubated for 30 min on ice, and
centrifuged at 15,000 g for 10 min. The supernatant containing
nuclear extracts was collected and stored at −80°C until
required.
Western blot analysis
Proteins were separated by SDS-PAGE and transferred
onto PVDF (Millipore, Billerica, MA, USA) as described previously
(29–31). Nonspecific binding on the
nitrocellulose filter paper was minimized with a blocking buffer
containing 5% non-fat dry milk and 0.1% Tween-20 in PBS. The
membrane was incubated with specific primary antibodies to COX-2 or
NF-κB (Abcam, Cambridge, UK) followed by incubation with
horseradish peroxidase-conjugated goat anti-rabbit antibody (1:7000
dilution, Abcam). For internal controls, the same membranes were
incubated with mouse anti-β-actin and anti-PCNA for 1 h followed by
incubation with horseradish-peroxidase-linked goat anti-mouse IgG
for 1 h. Reactive bands were visualized with an enhanced
chemiluminescence system (Amersham Biosciences, Arlington Heights,
IL). The intensity of the bands was scanned and quantified with
Adobe Photoshop software.
Statistical analysis
Data are expressed as mean ± standard deviations
(SD) from three different experiments. Statistical analysis was
carried out using the Student’s t-test. It was considered
statistically significant at *p<0.05, and
**p<0.01.
Results and Discussion
Effect of CIL extract on cell
proliferation of LPS-induced RAW264.7 cells
After 24-h treatment with CIL extract at the
indicated concentrations (0–25 μg/ml) in RAW264.7 cells, CIL
extract was found to inhibit RAW264.7 cell proliferation in a
dose-dependent manner, and the IC50 value of CIL extract
for 24 h was 14 μg/ml (Fig. 1).
Therefore, sample treatments between 1 and 10 μg/ml were used in
the subsequent experiments.
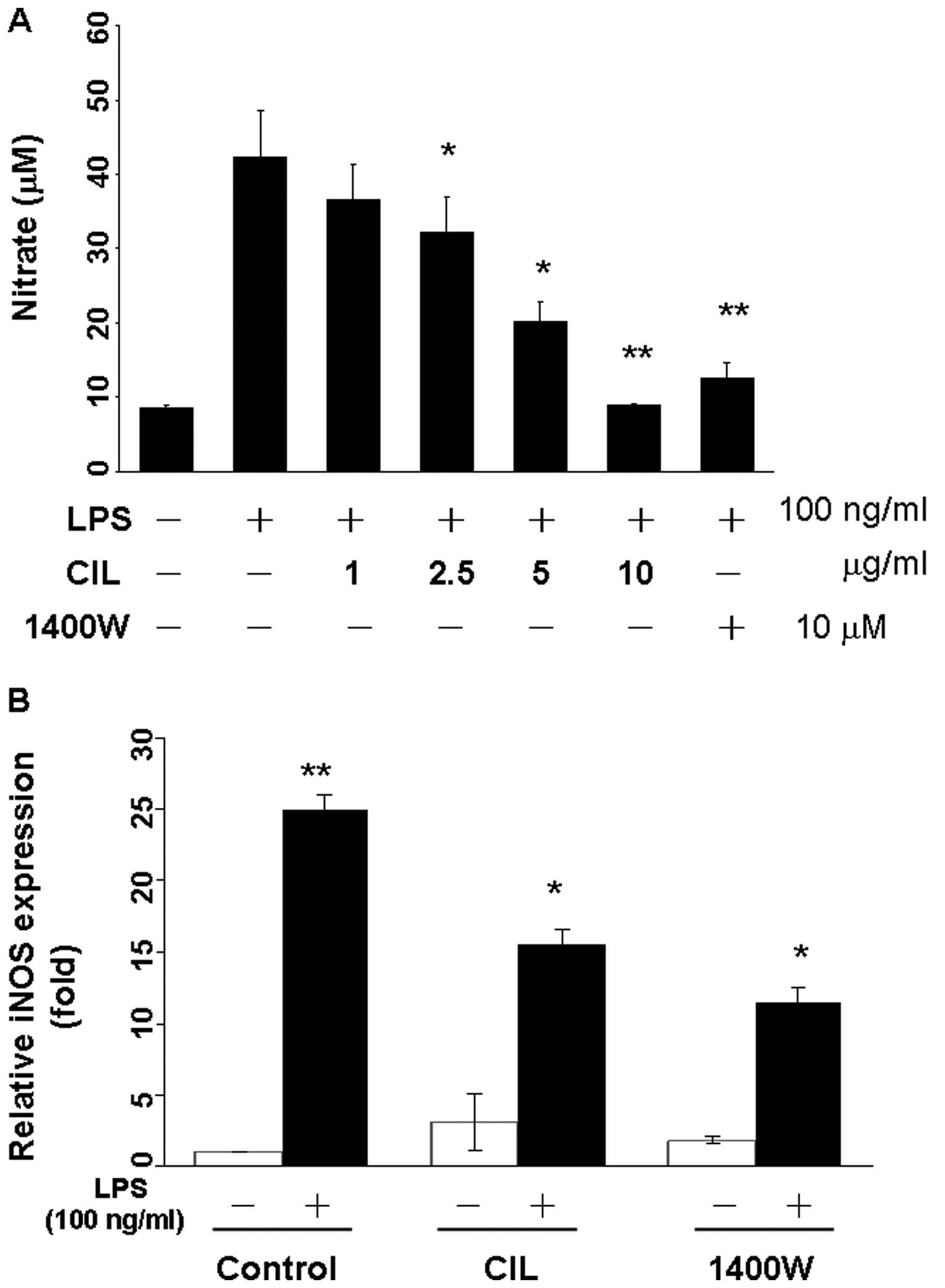
CIL extract inhibits LPS-induced NO
production and iNOS gene expression in RAW 264.7 macrophage
cells
We investigated the inhibitory effect of CIL extract
on LPS-induced NO production in RAW264.7 cells. Cells were
preincubated with CIL extract at the indicated concentrations for 1
h and then stimulated with LPS for 23 h. Supernatants were
collected for determination of nitrite production. CIL extract
inhibited NO production in RAW264.7 cells in a dose-dependent
manner as compared to controls, and the anti-inflammatory agent
N-(3-aminomethyl)-benzylacetamidine (1400W) as the positive
control (Fig. 2A). Quantitative
RT-PCR showed that the iNOS mRNA expression was almost undetectable
in unstimulated cells but were markedly augmented by LPS. Upon LPS
stimulation, iNOS expression was induced ~25-fold, and CIL extract
blocked iNOS expression which was induced by LPS 7.6-fold in
RAW264.7 cells (Fig. 2B). The
LPS-induced iNOS expression was inhibited 11.5-fold by the iNOS
inhibitor 1400W at 10 μM (Fig.
2B).
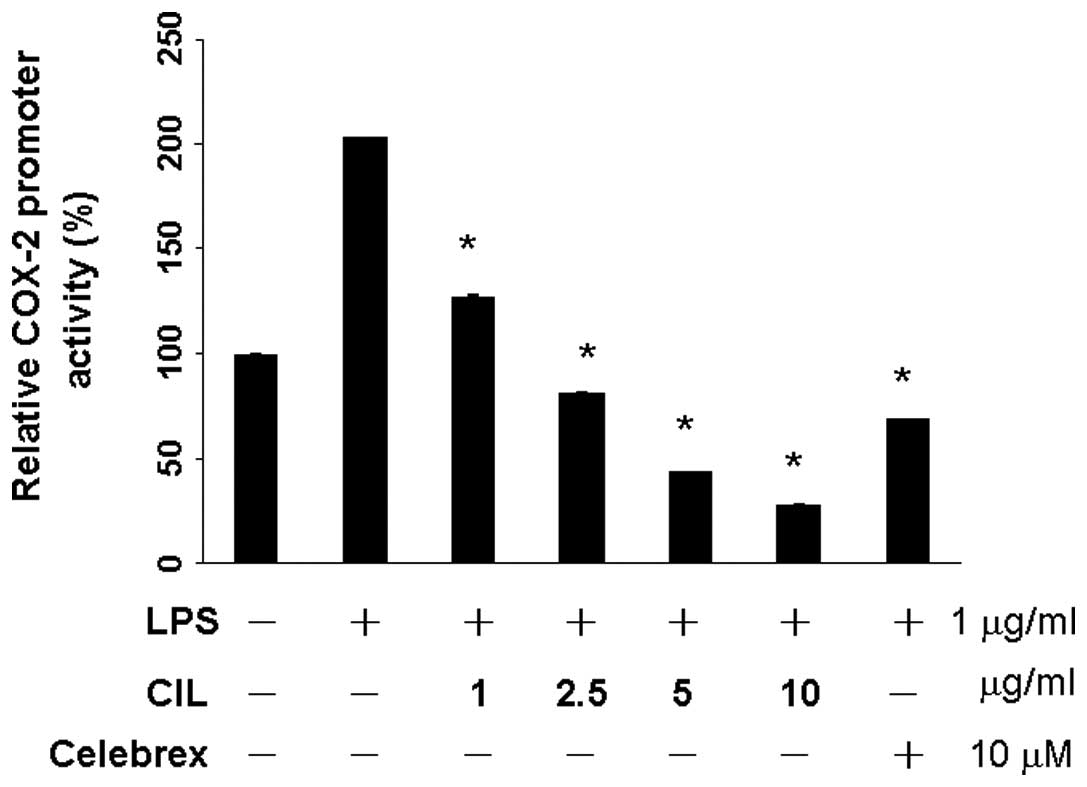
CIL extract suppresses mRNA levels of
COX-2
QPCR was performed to determine whether the
inhibitory effects of the CIL extract on the pro-inflammatory
mediators (NO) were related to the modulation of the expression of
iNOS and COX-2. After the transient transfection of RAW 264.7 cells
with pCOX-2-LUC and pRL-CMV, the expressions of firefly luciferase
and Renilla luciferase, respectively, were used to quantify the
COX-2 promoter activity. RAW264.7 cells were treated with the
various concentrations of CIL extract (0, 1, 2.5, 5 and 10 μg/ml).
As shown in Fig. 1, CIL extract did
not exhibit cytotoxicity against RAW264.7 cells <2.5 μg/ml. The
basal level of COX-2 promoter activity was set in the absence of
LPS stimulation. LPS significantly induced up to 203% in the
production of COX-2. CIL extract significantly reduced to 128% at 1
μg/ml, and 82% at 2.5 μg/ml in the production of LPS-induced COX-2
promoter activity (Fig. 3).
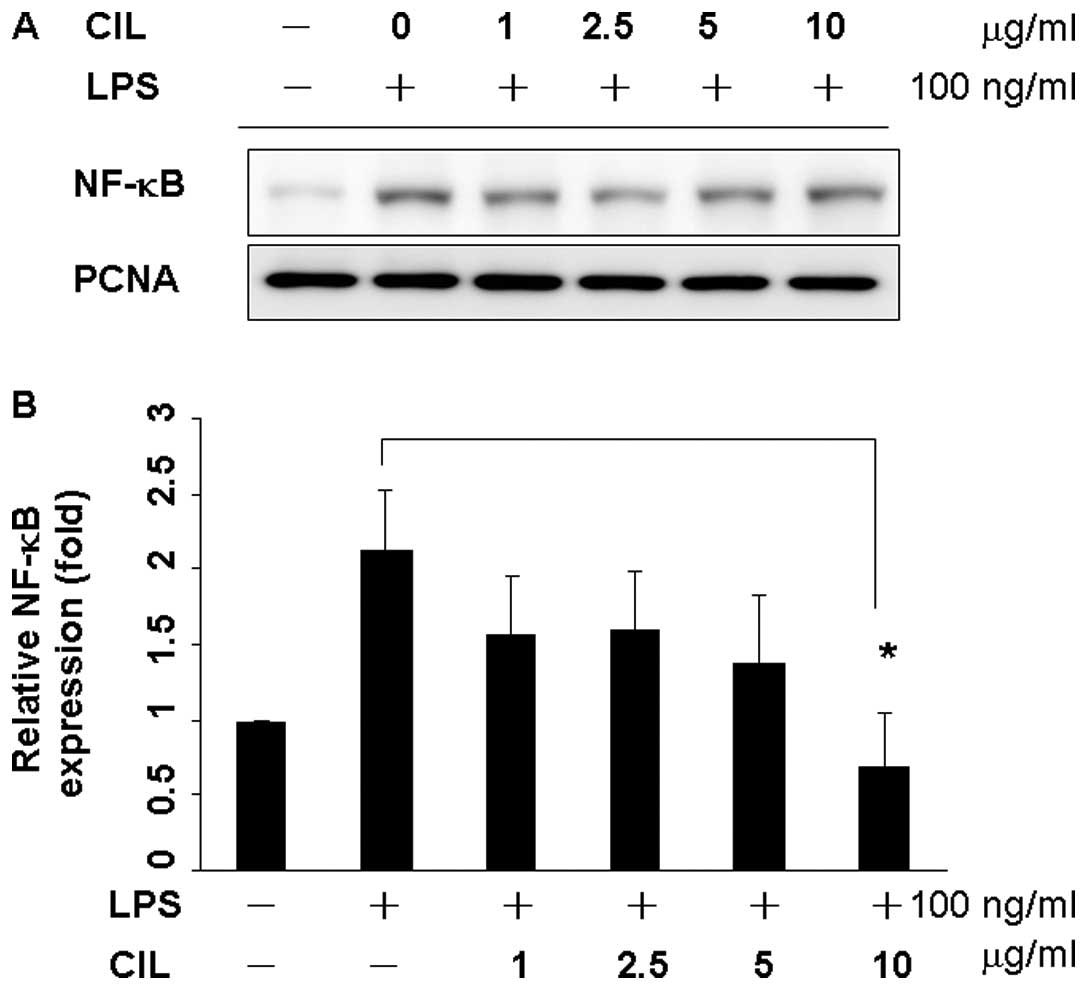
CIL extract inhibits LPS-induced NF-κB
activation
Since p65 and p50 are the major components of NF-κB
activated by LPS in the macrophage (32,33),
the levels of NF-κB/p65 in the nuclear extract were determined by
western blot analysis (Fig. 4A).
RAW 264.7 cells were incubated with LPS in the presence or absence
of the CIL extract with various concentrations for 24 h. The
expression of NF-κB/p65 in the nucleus was markedly increased upon
exposure to LPS alone 2.3-fold, but the extract or CIL extract
inhibited LPS-mediated nuclear translocation of NF-κB/p65 in a
dose-dependent manner (Fig. 4B)
(p<0.05), indicating that CIL extract could inhibit the nuclear
translocation of NF-κB/p65.
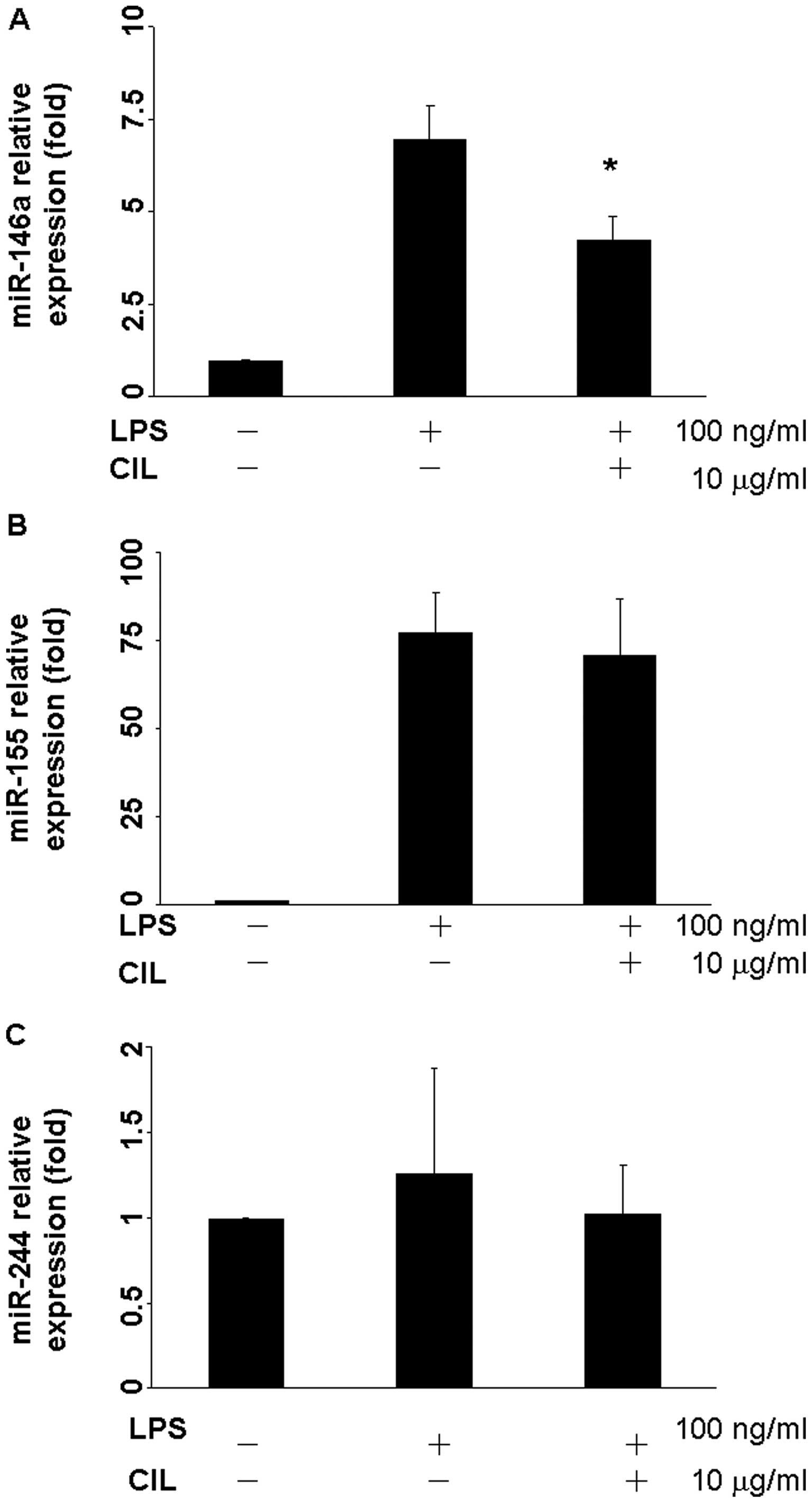
CIL extract attenuates LPS-induced
microRNA-146a, 155, and 424 expressions
When RAW264.7 cells were exposed to LPS, expressions
of microRNA-146a and miR-155 were up-regulated 6.97- and
77.23-fold, respectively (Fig. 5A and
B). After treatment with 10 μg/ml CIL extract for 24 h, the
expression of microRNA-146a was reduced to 4.26-fold (Fig. 5A). However, there was no significant
change for LPS or CIL extract in the miR-424 expression level
(Fig. 5C).
Quality of extraction procedure of CIL
extract by HPLC analysis
Two of the marked components of CIL extract,
including amentoflavone and oleanolic acid, were identified by HPLC
analysis to be indicator compounds for quality check of extraction
procedure of each batch (Fig. 6).
Using HPLC, the contents of amentoflavone and oleanolic acid were
calculated to be 84.72 and 1.05 mg/g of CIL extract, respectively
(Table I).
Macrophage activation is important to the
progression of multiple diseases through the release of
inflammatory mediators. Lipopolysaccharide (LPS)-induced RAW264.7
macrophages are widely used in vitro, because LPS is a
pathogen that triggers toll-like receptor 4 (TLR4) and activates
various inflammatory signals (34).
Natural products have played a significant role in drug discovery
and development, especially agents against several diseases that
have existed from antiquity to the present. Since inflammation is
closely linked to the promotion of certain tumors, substances with
potent anti-inflammatory activities are anticipated to exert
chemopreventive effects on carcinogenesis (35).
In the present study, we prepared acetone extract
from CIL extract and examined its effects on the LPS-induced
inflammation in a murine macrophage cell line RAW 264.7 model.
First, the cytotoxicity of CIL extract in RAW 264.7 cells were
evaluated by MTT assay, and it was observed that CIL extract did
not affect cell viability <2.5 μg/ml. Many lines of evidence
have indicated that NO is a potent proinflammatory mediator and may
have a multi-faceted role in mutagenesis and carcinogenesis
(35). The massive amounts of NO
produced in response to bacterial LPS or cytokines play an
important role in the inflammatory condition. Improper activation
or upregulation of iNOS or COX-2 has been shown to be associated
with the pathophysiologies of certain types of human cancer as well
as inflammatory disorders. Therefore, aberrant or excessive
expression of iNOS is often implicated in the oncogenesis and
pathogenesis of cancer. Indeed, we found that CIL extract
demonstrated the inhibition of the NO production in LPS-stimulated
RAW 264.7 macrophages. The mRNA expression levels of iNOS in
LPS-stimulated cells were examined by western blot analyses and
quantitative RT-PCR compared with the specific iNOS inhibitor
1400W. Our results suggest that CIL extract blocks the
transcription level of iNOS. Moreover, CIL extract at 1 μg/ml also
suppressed the LPS-induced COX2 promoter activity in RAW 264.7
cells. Taken together, CIL extract suppressed the production of NO
and downregulated the expression of iNOS and COX-2 in LPS-induced
RAW 264.7 cells.
NF-κB is a transcription factor that plays a
critical role in inflammatory and immune responses. It is present
in the cytoplasm, binding to the inhibitory protein IκB in
unstimulated cells (36,37). When the cells are exposed to the
stimulants such as LPS, IκB is phosphorylated and liberates NF-κB,
resulting in NF-κB translocation into the nucleus. Nuclear NF-κB
then binds to the promoters of pro-inflammatory mediators,
resulting in the induction of their gene expression (38). Here, we have elucidated that CIL
extract diminished the LPS-induced NF-κB nuclear translocation in
RAW 264.7 cells by western blot analysis. The nuclear translocation
and DNA binding of NF-κB is essential for the LPS-mediated NO
production and COX2 expression (39). These findings suggest that CIL
extract may prevent inflammation by suppressing the NF-κB-mediated
inflammatory gene.
Up to date, miRNAs have been demonstrated to be
dysregulated in cancer (40) and
aberrantly expressed in such inflammatory diseases as rheumatoid
arthritis (41). Thus, miRNAs may
form a key link between inflammation and cancer; however, the
induction of specific miRNAs, including miR-146a and miR-155, as a
key step in tumor progression is still unclear. While miR-146a has
previously been reported in response to various microbial
components and cytokines, much is still not known about its
biological significance. In this study, miR-146a expression was
induced by LPS stimulation in RAW 264.7 cells. Consistent with
previous findings, exposure of THP-1 monocytes to various bacterial
inflammatory insults, such as LPS and endotoxin, resulted in rapid
and continuous expression of mature miR-146a (42). Our results show that LPS-induced
miR-146a expression was inhibited by the CIL extract. Notably,
during LPS stimulation, several miRNAs, including miR-146a, miR-155
and miR-424 are upregulated. The biological significance of miR-155
and miR-424 to LPS-induced inflammation has been widely
investigated. The new data are focused on the expression of
miR-146a, miR-155 and miR-424 in LPS-induced inflammatory
responses. CIL extract reduced miR-146a expression, instead of
miR-155 and miR-424 expression. This finding highlights the
importance of further studies on whether miR-146a and miR-155 can
be used as a therapeutic intervention for controlling immune
response and defining the role of miR-146a, miR-155 and miR-424 in
LPS-induced inflammatory responses.
Furthermore, amentoflavone and oleanolic acid are
two major components isolated from CIL extract (20,40,43).
Flavonoids are naturally occurring polyphenolic compounds that have
many biological properties, including antioxidative,
anti-inflammatory and neuroprotective effects (44). Oleanolic acid is a triterpenoid
compound that is widely found in vegetables, medicinal herbs, and
other plants. It has been shown that oleanolic acid has potent
antioxidant and anti-inflammatory effects (45,46).
Collectively, we have demonstrated that CIL extract
inhibits LPS-induced NO production and iNOS expression which
mediated through the inhibition of NF-κB activation in RAW 264.7
macrophages. CIL extract exerts its potent anti-inflammatory
activity by suppressing COX-2 promoter activity and expression. Our
results demonstrate the strong anti-inflammatory properties of CIL
extract by inhibition of iNOS and COX-2 expression as well as
miR-146a expression.
Acknowledgements
This study was supported by grants from the China
Medical University and National Science Council
(NSC98-2815-C-039-080-B) (CMU96-116).
Abbreviations:
|
CIL
|
Calophyllum inophyllum L.
leaves
|
|
NF-κB
|
nuclear factor kappaB
|
|
NO
|
nitric oxide
|
|
DMSO
|
dimetylsulfoxide
|
|
miRNA
|
microRNA
|
|
1400W
|
N-(3-aminomethyl)-benzylacetamidine
|
|
COX-2
|
cyclooxygenase-2
|
|
LPS
|
lipopolysaccride
|
References
|
1
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleinert H, Schwarz PM and Forstermann U:
Regulation of the expression of inducible nitric oxide synthase.
Biol Chem. 384:1343–1364. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldring CE, Reveneau S, Pinard D and
Jeannin JF: Hyporesponsiveness to lipopolysaccharide alters the
composition of NF-kappaB binding to the regulatory regions of
inducible nitric oxide synthase gene. Eur J Immunol. 28:2960–2970.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turini ME and DuBois RN: Cyclooxygenase-2:
a therapeutic target. Annu Rev Med. 53:35–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams JA and Shacter E: Regulation of
macrophage cytokine production by prostaglandin E2. Distinct roles
of cyclooxygenase-1 and -2. J Biol Chem. 272:25693–25699. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Gao X, Fukumoto S, Tademoto S,
Sato K and Hirai K: Post-isolation inducible nitric oxide synthase
gene expression due to collagenase buffer perfusion and
characterization of the gene regulation in primary cultured murine
hepatocytes. J Biochem. 124:892–899. 1998. View Article : Google Scholar
|
|
7
|
Baeuerle PA and Baltimore D: NF-kappa B:
ten years after. Cell. 87:13–20. 1996.PubMed/NCBI
|
|
8
|
Rothwarf DM and Karin M: The NF-kappa B
activation pathway: a paradigm in information transfer from
membrane to nucleus. Sci STKE. 1999:RE11999.PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pedersen I and David M: MicroRNAs in the
immune response. Cytokine. 43:391–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosa A, Ballarino M, Sorrentino A, et al:
The interplay between the master transcription factor PU. 1 and
miR-424 regulates human monocyte/macrophage differentiation. Proc
Natl Acad Sci USA. 104:19849–19854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doggrell SA: Inflammation, the key to much
pathology. Drug News Perspect. 18:531–539. 2005.PubMed/NCBI
|
|
14
|
Moon TC, Kim JC, Song SE, et al:
Saucerneol D, a naturally occurring sesquilignan, inhibits
LPS-induced iNOS expression in RAW264.7 cells by blocking NF-kappaB
and MAPK activation. Int Immunopharmacol. 8:1395–1400. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vodovotz Y, Lucia MS, Flanders KC, et al:
Inducible nitric oxide synthase in tangle-bearing neurons of
patients with Alzheimer’s disease. J Exp Med. 184:1425–1433.
1996.PubMed/NCBI
|
|
16
|
Venkanna BK and Venkataramana Reddy C:
Biodiesel production and optimization from Calophyllum inophyllum
linn oil (honne oil) - a three stage method. Bioresour Technol.
100:5122–5125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawazu K, Ohigashi H and Mitsui T: The
piscicidal constituents of Calophyllum inophyllum Linn. Tetrahedron
Lett. 19:2383–2385. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Govindachari TR, Viswanathan N, Pai BR,
Rao R and Srinivasan M: Triterpenes of Calophyllum inophyllum Linn.
Tetrahedron. 23:1901–1910. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arora RB, Mathur CN and Seth SD:
Calophyllolide, a complex coumarin anticoagulant from Calophyllum
inophyllum Linn. J Pharm Pharmacol. 14:534–535. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YZ, Li ZL, Yin SL, et al: Triterpenoids
from Calophyllum inophyllum and their growth inhibitory effects on
human leukemia HL-60 cells. Fitoterapia. 81:586–589. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai HF, Zeng YB, Xiao Q, Han Z, Zhao YX
and Mei WL: Caloxanthones O and P: two new prenylated xanthones
from Calophyllum inophyllum. Molecules. 15:606–612. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spino C, Dodier M and Sotheeswaran S:
Anti-HIV coumarins from Calophyllum seed oil. Bioorg Med Chem Lett.
8:3475–3478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Clercq E: Current lead natural products
for the chemotherapy of human immunodeficiency virus (HIV)
infection. Med Res Rev. 20:323–349. 2000.PubMed/NCBI
|
|
24
|
Said T, Dutot M, Labbe A, Warnet JM and
Rat P: Ocular burn: rinsing and healing with ionic marine solutions
and vegetable oils. Ophthalmologica. 223:52–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
26
|
Wu PP, Liu KC, Huang WW, et al: Triptolide
induces apoptosis in human adrenal cancer NCI-H295 cells through a
mitochondrial-dependent pathway. Oncol Rep. 25:551–557.
2011.PubMed/NCBI
|
|
27
|
Lin MW, Tsao LT, Huang LJ, et al:
Inhibition of lipopolysaccharide-stimulated NO production by
crotafuran B in RAW 264.7 macrophages involves the blockade of
NF-kappaB activation through the increase in IkappaBalpha
synthesis. Toxicol Appl Pharmacol. 210:108–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andrews NC and Faller DV: A rapid
micropreparation technique for extraction of DNA-binding proteins
from limiting numbers of mammalian cells. Nucleic Acids Res.
19:24991991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsai SC and Seto E: Regulation of histone
deacetylase 2 by protein kinase CK2. J Biol Chem. 277:31826–31833.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
31
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeon YJ, Han SB, Ahn KS and Kim HM:
Differential activation of murine macrophages by angelan and LPS.
Immunopharmacology. 49:275–284. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo CT, Chiang LL, Lee CN, et al:
Induction of nitric oxide synthase in RAW 264.7 macrophages by
lipoteichoic acid from Staphylococcus aureus: involvement of
protein kinase C- and nuclear factor-κB-dependent mechanisms. J
Biomed Sci. 10:136–145. 2003.PubMed/NCBI
|
|
34
|
Denlinger LC, Fisette PL, Garis KA, et al:
Regulation of inducible nitric oxide synthase expression by
macrophage purinoreceptors and calcium. J Biol Chem. 271:337–342.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Surh YJ, Chun KS, Cha HH, et al: Molecular
mechanisms underlying chemopreventive activities of
anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS
through suppression of NF-kappa B activation. Mutat Res.
480–481:243–268. 2001.
|
|
36
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Simeonidis S, Liang S, Chen G and Thanos
D: Cloning and functional characterization of mouse IkappaBepsilon.
Proc Natl Acad Sci USA. 94:14372–14377. 1997. View Article : Google Scholar
|
|
38
|
Gilroy DW, Lawrence T, Perretti M and
Rossi AG: Inflammatory resolution: new opportunities for drug
discovery. Nat Rev Drug Discov. 3:401–416. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Vandenboom TG II, Wang Z, et al:
miR-146a suppresses invasion of pancreatic cancer cells. Cancer
Res. 70:1486–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan EK, Satoh M and Pauley KM: Contrast
in aberrant microRNA expression in systemic lupus erythematosus and
rheumatoid arthritis: is microRNA-146 all we need? Arthritis Rheum.
60:912–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nahid MA, Pauley KM, Satoh M and Chan EK:
miR-146a is critical for endotoxin-induced tolerance: implication
in innate immunity. J Biol Chem. 284:34590–34599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li YZ, Li ZL, Hua HM, Li ZG and Liu MS:
Studies on flavonoids from stems and leaves of Calophyllum
inophyllum. Zhongguo Zhong Yao Za Zhi. 32:692–694. 2007.PubMed/NCBI
|
|
44
|
Shin DH, Bae YC, Kim-Han JS, et al:
Polyphenol amentoflavone affords neuroprotection against neonatal
hypoxic-ischemic brain damage via multiple mechanisms. J Neurochem.
96:561–572. 2006. View Article : Google Scholar
|
|
45
|
Pereira DA, Dalmarco JB, Wisniewski A Jr,
Simionatto EL, Pizzolatti MG and Frode TS: Lotus corniculatus
regulates the inflammation induced by bradykinin in a murine model
of pleurisy. J Agric Food Chem. 59:2291–2298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sung HY, Kang SW, Kim JL, et al: Oleanolic
acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr
Res. 30:831–839. 2010. View Article : Google Scholar : PubMed/NCBI
|