Introduction
Without neovascularization tumors are typically
restricted in growth to a small mass not more than approximately
0.4 mm in diameter (1). In order to
ensure the oxygen and nutrients essential for tumor growth and
spread, tumor cells secrete pro-angiogenic factors designed to
induce the angiogenesis and vasculogenesis by binding their related
tyrosine kinase receptor (2).
Vascular endothelial growth factor (VEGF) is one of the most well
studied pro-angiogenic factors overexpressed in many types of
cancer cells (3). Vascular
endothelial growth factor receptor 2 (VEGFR2), which is primarily
expressed in the endothelial cells (ECs) of the neovasculature
(4), is the major tyrosine kinase
receptor of VEGF. During angiogenesis, VEGF combines with its
cognate receptor tyrosine kinase, VEGFR2 (also known as KDR and
FLK1), and activates multiple downstream pathways via signaling
intermediates, such as mitogen-activated protein kinases (MAPKs),
phosphoinositide 3-kinase (PI3K), AKT, phospholipase Cγ and small
GTPases (5).
Epidermal growth factor receptor (EGFR) and its
ligand, epidermal growth factor (EGF), also play critical roles in
the development of cancer through their effects on cellular
proliferation, apoptosis, angiogenesis and metastasis (6). As one type of pro-angiogenic factor,
EGFR is overexpressed in epithelial cells of many tumors and
provides an essential survival signal to tumor cells by activating
the expression of anti-apoptotic Akt, MAPKs, Jak/Stat and protein
kinase C (7,8). In addition, knockdown of the EGFR
expression in the tumor cell has shown direct or indirect
anti-angiogenesis and tumor inhibitory effects (9).
The pivotal roles in the progression of cancer and
angiogenesis that EGFR and VEGFR2 play make them attractive targets
for anticancer therapy (10).
Treatment strategies, by blocking the pathways of either
VEGF/VEGFR2 or EGF/EGFR, have produced modest objective responses
in clinical trials, and have failed to improve long-term patient
survival (11,12). Furthermore, monotherapy approaches,
including anti-angiogenesis monoclonal antibodies, or tyrosine
kinase inhibitors (TKIs), did not show any survival advantage over
chemotherapy alone in patients (13). Markedly, prolonged survival of
patients up to five months was only achieved when bevacizumab was
combined with traditional chemotherapy (14). This success indicated that the
co-administration of cytotoxic drugs (chemotherapy agents) and
anti-angiogenesis therapy yields maximal benefits, due to the
dual-inhibition of the growth of both tumor and endothelial cells
(15). Another presumption of this
synergetic antitumor effect was that the normalization of the
vascular conducted by anti-angiogenesis therapy subsequently
lowered the pressure inside the tumor, which increased the delivery
of cytotoxic drugs to the tumor more effectively (15).
Therefore, siRNA, having the ability to knock down
or ‘silence’ overexpressed or mutated genes involved in the
development of cancer, is a powerful antitumor drug candidate.
Several RNAi based therapies are under evaluation in the clinical
trials (16). The first clinical
trial of human cancer therapy based on systemically delivered siRNA
by transferrin-tagged, cyclodextrin-based polymeric nanoparticles,
has recently been launched (CALAA-01; Calando Pharmaceuticals,
Pasadena, CA, USA; phase I) (17).
However, the delivery system for siRNA therapy remains the major
hurdle in this type of approach (18). Commercially available branched
cationic polymers polyethylenimines (PEI) have been utilized to
improve the efficiency of in vivo siRNA delivery by
complexing the negatively charged siRNA to form non-covalent
nanoparticles (19). The
positively-charged PEI-siRNA complexes protect the siRNA from the
degradation, enable effective uptake by cellular endocytosis
mechanisms and facilitate subsequent siRNA released from endosomes
based on the proton-sponge effect (20).
To explore a potentially novel therapeutic cancer
regimen and minimize the side-effects of cytotoxic drugs, we
examined the in vivo antitumor effects of cisplatin combined
with siRNA aimed at silencing the expression of VEGFR2 or EGFR and
mediated by PEI complexes. Both receptor tyrosine kinases (VEGFR2
or EGFR) were found to be overexpressed in human non-small cell
lung cancer (NSCLC) and closely correlated with the malignant
disease progression (21). Herein,
siRNA targeting murine VEGFR2 was designed, validated and found to
be robust in silencing the expression of VEGFR2 mRNA in
vitro. Next, the antitumor effects of cisplatin alone, or
combined with siRNAs targeting VEGFR2 and EGFR, were investigated
and compared in murine A549 NSCLC tumor xenograft models. Finally,
siRNA targeting both VEGFR2 and EGFR when combined with cytotoxic
chemotherapy resulted in profound antitumor effects and prolonged
the survival of mice bearing the tumor xenografts and may,
therefore, provide new insight into future clinical anticancer
treatments.
Materials and methods
Cell lines and culture
A549 cells (from ATCC) were grown in RPMI-1640
(Gibco, China) medium supplemented with 10% fetal calf serum (FCS;
Gibco). MS1 cells (MILE SVEN 1 cells; ATCC) were maintained in high
glucose DMEM (Gibco) supplemented with 10% FCS. Cell cultures were
maintained at 37°C in a humidified atmosphere containing 5%
CO2. Cells were propagated by passage of 1 mM EDTA and
0.25% trypsin-dispersed cells.
siRNA sequence
Based on the design principles of siRNA made by
Tuschl et al(22), three
pairs of VEGFR2/Flk-1 siRNA sequences were designed according to
the mouse mRNA sequence (NM_010612). The sequences of VEGFR2 siRNA4
and EGFR siRNA were from Schiffelers et al and Weihua et
al, respectively (23,24). A scrambled siRNA was used as a
control. All the sequences were synthesized by RiboBio Co., Ltd.,
(Guangzhou, China) and are shown in Table I.
 | Table ISequences of siRNAs used in the
study. |
Table I
Sequences of siRNAs used in the
study.
| Name | Sequence
(5′–3′) |
|---|
| VEGFR2-siRNA1 | Sense strand:
5′-CCGGAAAUCUGGAGAAUCAdTdT-3′ |
| Antisense strand:
3′-dTdTGGCCUUUAGACCUCUUAGU-5′ |
| VEGFR2-siRNA2 | Sense strand:
5′-CGGAGAAGAAUGUGGUUAAdTdT-3′ |
| Antisense strand:
3′-dTdTGCCUCUUCUUACACCAAUU-5′ |
| VEGFR2-siRNA3 | Sense strand:
5′-CGGAUGAUCAAGAGAAAUAdTdT-3′ |
| Antisense strand:
3′-dTdTGCCUACUAGUUCUCUUUAU-5′ |
| VEGFR2-siRNA4 | Sense strand:
5′-GCUCAGCACACAGAAAGACdTdT-3′ |
| Antisense strand:
3′-dTdTCGAGUCGUGUGUCUUUCUG-5′ |
| EGFR siRNA | Sense strand:
5′-CUGACUCCGUCCAGUAUUGAUdTdT-3′ |
| Antisense strand:
3′-dTdTGACUGAGGCAGGUCAUAACUA-5′ |
| Control siRNA | Sense strand:
5′-CGUGAUUGCGAGACUCUGAdTdT-3′ |
| Antisense strand:
3′-dTdTGCACUAACGCUCUGAGACU-5′ |
Transfection experiments
For in vitro tests, siRNAs were delivered to
MS1 cells using Lipofectamine™ 2000 (Invitrogen) according to the
manufacturer’s instructions. Briefly, cells were seeded in a 6-well
plate at a density of 5×104 cells/well 24 h before
transfection. siRNA complexes were added to cells when cultures
reached 50% confluence at a final concentration of 50 nM in the
absence of serum. Following incubation at 37°C for 4 h, the culture
medium was replaced with 2 ml of fresh medium supplemented with 10%
fetal bovine serum. Cells were cultured under standard conditions
for a further 48 h before being examined by semi-quantitative
RT-PCR or quantitative RT-PCR. For western blot analysis, cells
were cultured for a further 72 h.
siRNA/PEI complex
For in vivo experiments, siRNAs were
encapsulated by branched PEI (Mw 25 kDa, Sigma Aldrich) with an N/P
ratio =8 (the molar ratio of nitrogens in the PEI to the ratio of
phosphates in the siRNA). Theoretically, 1 μg of siRNA (Mw 13,300
g/mol) contained 3 nmol of phosphate, and 1 μg PEI contained 10
nmol of amine nitrogen. Briefly, PEI was diluted in HEPES buffer at
pH 7.4 to make stock solution with a concentration of 1.02 mg/ml.
Then, 0.5 nmol (6.5 μg) of siRNA was dissolved in 0.05 ml of HEPES
buffer and incubated for 10 min, and appropriate amounts of PEI
solution (15.65 μg) were pipetted to the siRNA solution resulting
in the desired N/P ratio (N/P ratio=8). The mixtures were vortexed
for 5 sec and incubated at room temperature for at least 20 min to
allow complex formation. The final complex was prepared immediately
before intratumoral injection.
Semi-quantitative RT-PCR
Total-RNA was extracted from cells 48 h after
transfection by the TRIzol reagent (Invitrogen). Total-RNA (1 μg)
was reverse-transcribed into cDNA by ReverTra Ace-α-®
cDNA Synthesis kit (Toyobo). The RT products were then amplified
using Takara Taq™ DNA polymerase (Takara). For normalization of RNA
loading, β-actin was also amplified from each sample. The
expression of VEGFR2 mRNA in MS1 cells was analyzed by PCR using
the following primers: forward, 5′-AGAACACCAAAAGAGAGGAACG-3′;
reverse, 5′-GCACACAGGCAGAAAGTAG-3′. Primers for β-actin mRNA
amplification were: forward, 5′-CAACT GGGACGATATGGAGAAG-3′, reverse
5′-TCTCCTTCTGC ATCCTGTCAG-3′. PCR was carried out at 95°C for 2
min, followed by 20 cycles of 30 sec at 94°C, 30 sec at 54°C and
ended with 1 min at 72°C. The PCR products were analyzed by 1.2%
agarose gel (containing 0.05% ethidium bromide), photographed under
UV light and quantified by ImageJ software. The data are presented
as the ratio of the expression level of interest protein to that of
β-actin expression.
Quantitative real-time PCR
The mRNA expression levels of VEGFR2 and EGFR in
tumors were determined by two-step quantitative real-time reverse
transcription PCR. Total-RNA was isolated from tumor tissue using
the TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions. For cDNA synthesis, 1 μg of extracted RNA was
transcribed using the ReverTra Ace-α-® cDNA Synthesis
kit (Toyobo). Real-time monitoring of PCR amplification of the cDNA
was performed in a Real-Time PCR Detection System (Bio-Rad
Laboratories) using Real Master Mix (SYBR-Green) (Tiangen, Beijing,
China). Primers used for VEGFR2 amplification were the same as
previously mentioned. Primers used for EGFR amplification were:
forward, 5′-AGGACCAAGCA ACATGGTCA-3′; reverse, 5′-CCTTGCAGCTGTTTTCA
CCT-3′. Levels of the GAPDH housekeeping gene mRNA were determined
as control with the following primers: forward,
5′-GAACGGGAAGCTCACTGG-3′; reverse, 5′-GC CTGCTTCACCACCTTCT-3′. Each
reaction was carried out at 95°C for 2 min, followed by 40 cycles
of 95°C for 10 sec and 60°C for 30 sec. The levels of VEGFR2 or
EGFR mRNA were normalized to that of GAPDH mRNA after the
confirmation that the amplification efficiency of the two genes was
comparable.
Western blot analysis
Cell suspensions were homogenized in lysis buffer
with protease inhibitors (Nanjing KeyGen Biotech. Co., Ltd.,
Nanjing, China). The samples were subjected to electrophoresis on
10% SDS-polyacrylamide gels and transferred to a polyvinylidene
difluoride membrane (Millipore). After blocking with 5% non-fat
milk at 4°C overnight and washing three times with a 0.1% Tween-20
solution in Tris-buffered saline (TBST), the membranes were
incubated with a primary monoclonal anti-mouse VEGFR2/Flk-1
antibody (R&D Systems) at 4°C overnight. The membranes were
then washed and incubated with HRP-conjugated secondary donkey
anti-rat IgG (H+L) antibody (Proteintech Group, Inc.), and detected
with Novex® ECL Chemiluminescent Substrate Reagent
(Invitrogen). Then, the membranes were stripped and reprobed for
detection of β-actin with rabbit anti-β-actin antibody (Abmart,
Shanghai, China) and developed as above following incubation with
the goat anti-rabbit secondary antibody (Boster, Wuhan, China). For
the detection of VEGFR2 protein expression levels, tumor tissues
were homogenized then centrifuged and the supernatants were
collected. Equal amounts of protein were subjected to western blot
analysis as described above. Briefly, membranes were incubated with
primary monoclonal anti-mouse VEGFR2/Flk-1 (Abcam) antibody at 4°C
overnight. The membranes were washed three times in TBST and
incubated with the corresponding HRP-conjugated secondary antibody,
as mentioned above, and then developed for the blots. Next, the
membranes were stripped for the detection of β-actin and developed
as above. Finally, the density of bands were scanned and the ratio
of band intensities for VEGFR2, which were normalized to that of
β-actin, were calculated using ImageJ software.
NSCLC A549 tumor xenograft model and
growth inhibition
In vivo experiments were carried out at the
Center of Experimental Animals of Sun Yat-sen University
(Guangdong, China), according to standardized animal care
guidelines, and the mice were housed in pathogen-free conditions.
This study was approved by the Center of Experimental Animals of
Sun Yat-sen University (permit no. SYXK2007-0081). A549 cells were
harvested by trypsinization, washed twice in PBS, centrifuged and
re-suspended in PBS. A total of 5×106 cells in 0.2 ml of
buffered saline were injected s.c. into the flank region of athymic
nude mice (BALB/C, nu/nu, 4–6 weeks) and tumors were allowed to
grow for 10 days. The tumor volume was measured with a caliper
every three days and calculated by the formula: Volume = 1/2 ×
length × width2, where length represented the longest
tumor diameter and width represented the shortest tumor diameter
(25). When tumor volume reached
30–100 mm3, the animals were randomized into different
groups for therapy testing. Each treatment group consisted of four
to five mice. Mice were injected with: i) saline (intratumorally,
twice a week); ii) PEI alone (intratumorally, twice a week); iii)
0.5 nmol VEGFR2 siRNA-PEI complexes (intratumorally, twice a week);
iv) 0.5 nmol EGFR siRNA-PEI complexes (intratumorally, twice a
week); v) 0.5 nmol siControl-PEI complexes (intratumorally, twice a
week); vi) (0.25 nmol VEGFR2 siRNA + 0.25 nmol EGFR siRNA)-PEI
complexes (intratumorally, twice/week); vii) 5 mg/kg cisplatin
(intraperitoneally, once a week); and viii) (0.25 nmol VEGFR2 siRNA
+ 0.25 nmol EGFR siRNA)-PEI complexes + 3 mg/kg cisplatin (siRNA,
intratumorally, twice a week; cisplatin, intraperitoneally, once a
week, one day after the first delivery of siRNA). siRNAs were
complexed with PEI prior to injection as previously described. The
animals were sacrificed when they became moribund or when the
experiment was terminated and the tumors were carefully dissected
and snap frozen for RNA, protein and immunohistochemical
analysis.
Statistical analysis
All descriptive statistics, including the means ±
SD, were performed. For parametric variables in the experiments,
ANOVA was used along with Fisher’s least-significant difference
(LSD) or Dunnett’s T3 method, depending on the homogeneity of
variance using the software program SPSS 13.0. Kaplan-Meier curves
were used for in vivo experiments and the log-rank test was
used for statistical comparisons. All of the analyses were
two-tailed. A P-value of <0.05 was considered to indicate
statistically significant difference and a P-value of <0.01 was
considered to indicate very significant difference. Each experiment
was repeated three times.
Results
Reduction of both mRNA and protein
expression of VEGFR2 in MS1 cells mediated by siRNA
Four siRNA sequences targeting VEGFR2 were assessed
for silencing efficiency in vitro. Three of the siRNA
sequences (siRNA1–3) were designed according to the mouse mRNA
sequence (NM_010612) and based on the siRNA design made by Tuschl
et al(22) and one siRNA
(sequence 4) was published previously (27). To evaluate gene silencing, VEGFR2
siRNA (100 pmol) and a scrambled siRNA control were transfected
into MS1 cells overexpressing VEGFR2 and the VEGFR2 mRNA expression
level was determined 48 h later by semi-quantitative reverse
transcription PCR. Agarose gel electrophoresis showed that the
expression of VEGFR2 mRNA was markedly dowregulated in response to
the siRNA treatment. The analysis of semi-quantitative reverse
transcription PCR revealed an approximate 60–75% reduced level of
VEGFR2 mRNA mediated by siRNA2, compared to that of the 50–60%
reduction by siRNA1 or 3, and ~40% reduction by siRNA4,
respectively. However, the cells treated with control siRNA showed
a high level of VGFR2 mRNA compared to that in untreated MS1 cells
(Fig. 1). In addition, all the
VEGFR2 mRNA expression levels were compared to expression levels in
control siRNA-treated cells (Fig.
1B) to confirm siRNA specificity.
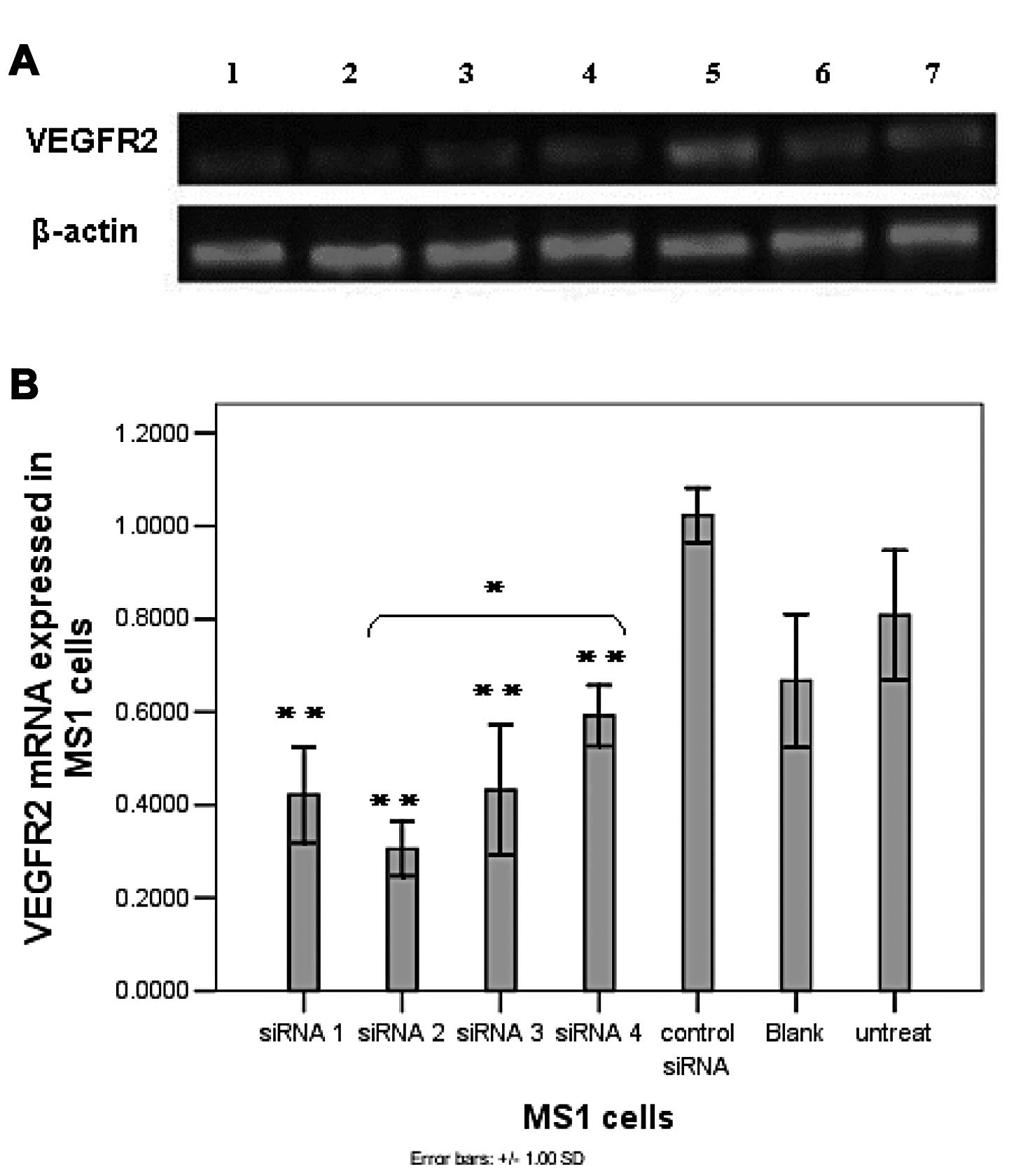 | Figure 1siRNA significantly reduces VEGFR2
expression of both mRNA and protein in MS1 cells. (A) Agarose gel
electrophoresis of semi-quantitative RT-PCR. Lane 1, siRNA 1; lane
2, siRNA 2; lane 3, siRNA 3; lane 4, siRNA 4; lane 5, control
siRNA; lane 6, blank (Lipofectamine™ 2000 only); lane 7, untreated
(normal MS1 cells). Representative bands are shown from 3
experiments. (B) Downregulation of VEGFR2 mRNA in MS1 cells
determined by semi-quantitative RT-PCR. (C and D) Western blot
analysis of VEGFR2 in MS1 cells transfected with siRNA. β-actin was
also amplified as an internal control. Columns, mean; bars, SD.
*P<0.05; **P<0.01, vs. the control
siRNA-treated group. |
The downregulation of mRNA expression was translated
into reduced VEGFR2 protein levels, as determined by western blot
analysis 72 h after transfection of siRNA (Fig. 1C and D). Compared to control siRNA,
siRNA2 could significantly silence VEGFR2 protein expression by 85%
in vitro. The reduced expression of the VEGFR2 protein in
MS1 cells mediated by siRNA1 and 3 was 55 and 42%, respectively.
However, no significant decline of VEGFR2 protein expression was
observed in siRNA 4-transfected cells compared to that in control
siRNA-transfected cells. Furthermore, the reduced VEGFR2 protein
expression results directly reflected the mRNA knockdown data in a
siRNA sequence-dependent manner, with siRNA2 standing out as most
effective. Therefore, siRNA2 was chosen for the subsequent tumor
inhibitory experiments in vivo based on its robust capacity
to decrease both the mRNA and protein expression levels of VEGFR2
in MS1 cells.
Low dose of cisplatin combined with siRNA
markedly reduce tumor growth on the well-established human NSCLC
xenografts
To test in vivo tumor growth inhibition
mediated by siRNA combined with low dose chemotherapy, A549 NSCLC
xenograft mouse models were established. VEGFR2 siRNA2 (siVEGFR2)
(0.5 nmol) (6.5 μg) and PEI (15.65 μg) was freshly complexed as
described above with an N/P ratio of 8, and as one dose for each
injection. Mice (n=5) were treated twice a week via intratumoral
injection with siVEGFR2/PEI complex and received a total of nine
administrations (Fig. 2C). As shown
in Fig. 2A and B, siVEGFR2 could
significantly inhibit the growth of s.c. tumor xenografts with the
reduction of tumor volume by 77% compared to that in the mice
treated by PEI alone (P<0.01). The mean survival time of the
siVEGFR2-treated animals was 17 days and only one in five mice
survived the experiment (40 days), which may result from the high
incidence of side-effects caused by the siRNA (Fig. 2F). In addition, marked weight loss,
up to 20%, was another factor contributing to the higher mortality
of tumor-bearing mice.
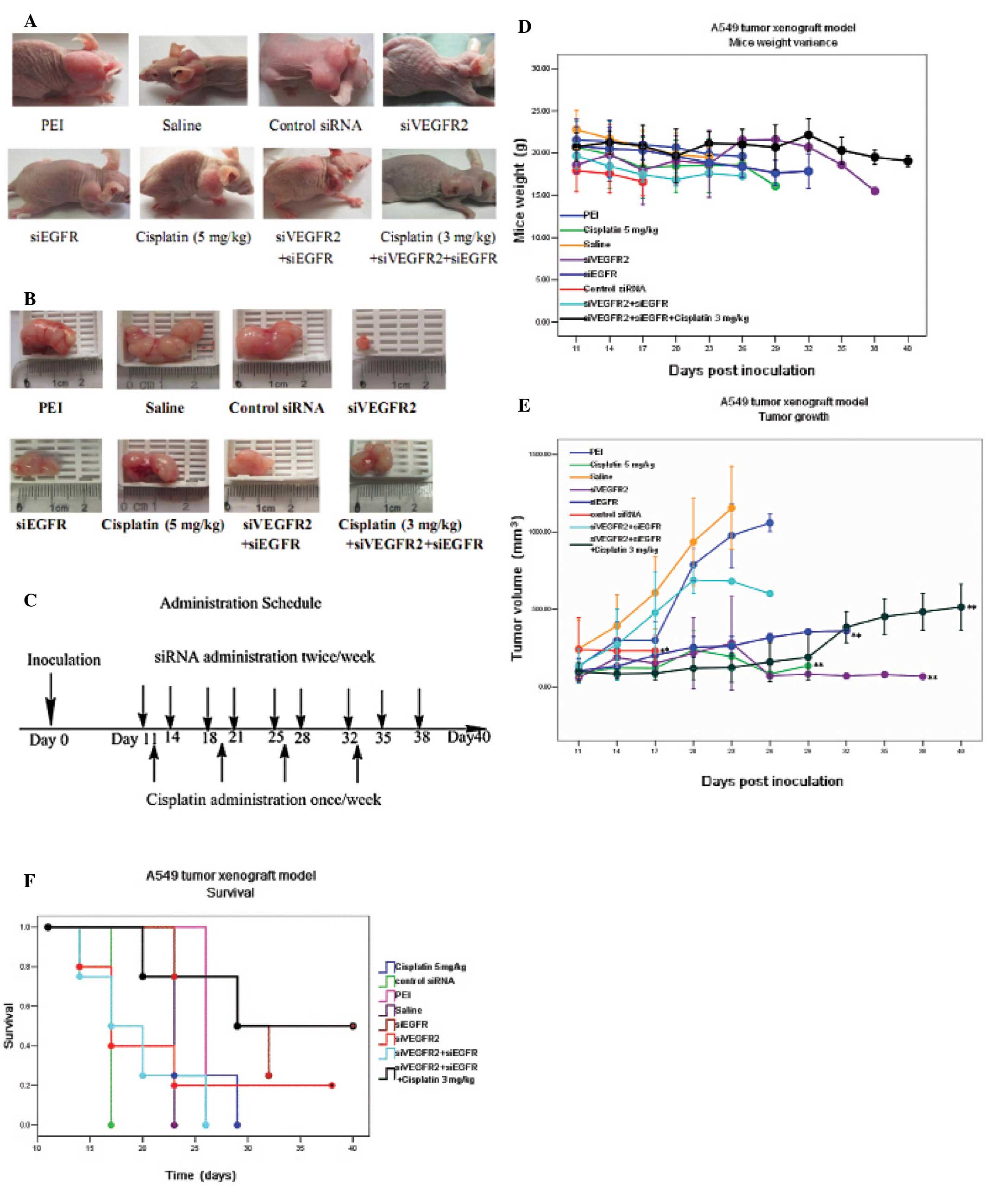 | Figure 2Survival and tumor growth of mice
bearing A549 tumor xenograft following treatment with
mono/combining therapies. When the tumor reached 30–100
mm3, mice were randomly distributed into 8 groups (n=4
or 5) and treated with: i) saline (intratumoral injection,
twice/week). ii) PEI (intratumoral injection, twice/week). iii) 0.5
nmol siVEGFR2 (intratumoral injection, twice/week). iv) 0.5 nmol
siEGFR (intratumoral injection, twice/week). v) 0.5 nmol control
siRNA (intratumoral injection, twice/week). vi) 0.25 nmol siVEGFR2
+ 0.25 nmol siEGFR (intratumoral injection, twice/week). vii) 5
mg/kg cisplatin (intraperitoneal injection, once/week). viii) 0.25
nmol siVEGFR2 + 0.25 nmol siEGFR + 3 mg/kg cisplatin (siRNA,
intratumoral injection, twice/week; cisplatin, intraperitoneal
injection, once a week, the day after the first shot of siRNA).
siVEGFR2, VEGFR2 siRNA; siEGFR, EGFR siRNA. (A) Representative
examples of mice bearing A549 tumor xenograft after treatments
finished. (B) Representative examples of A549 tumor xenografts. (C)
Time course of administration schedule. Day 0 is the day of tumor
implantation. The arrows indicate time points of drug
administration. (D) Mouse weight variance. Mice were weighed every
three days to monitor the change. Bars, SD. (E) Tumor growth was
inhibited by siRNA and chemotherapy. Bars, SD;
*P<0.05; **P<0.01, compared to that of
the PEI-treated group. (F) Kaplan Meier survival curves of A549
tumor-bearing mice. *P<0.05; **P<0.01,
compared to that of the PEI-treated group. |
To test whether or not siEGFR could inhibit the
tumor growth, freshly prepared siEGFR/PEI complex, containing 0.5
nmol (6.5 μg) siEGFR and PEI (15.65 μg) per injection dose was
intratumorally injected twice a week in A549 tumor bearing mice.
Similar tumor growth inhibition to that mediated by siVEGFR2 was
observed in the siEGFR-treated animals and was significantly
different to results found in the PEI-treated animals (P<0.01)
(Fig. 2A, B and E). However, the
tumor growth inhibitory effect was not found to be significantly
different between the siEGFR and siVEGFR2-treated mice. Of note,
siEGFR treatment alone resulted in increased survival with a median
survival of 29 days. In addition, siEGFR treatment was well
tolerated and was associated with fewer adverse events, such as
decreased weight loss, in the tumor bearing mice (Fig. 2D and F).
We also examined the in vivo antitumor effect
of combined therapy with both siVEGFR2 and siEGFR. Due to the drug
toxicity, a half dose of 0.5 nmol siRNA was adopted. For the
combined siRNA therapy, 0.25 nmol siVEGFR2 and 0.25 nmol siEGFR
were mixed with 15.65 μg PEI (N/P=8) for each injection. The
siRNA/PEI complex was prepared and intratumorally injected twice a
week. At this dosage no adverse effects or notable weight loss was
observed in the siVEGFR2+siEGFR treated mice (Fig. 2D), although no signification
reduction in the tumor volume or improvement in median survival was
found either (Fig. 2E and F). In
fact, the median survival time in this group was 17 days which was
shorter than that of the siEGFR treated mice (Fig. 2F).
In another cohort of mice bearing A549 tumors,
standard cisplatin chemotherapy (5 mg/kg, i.p) (26) was administered once a week (Fig. 2C) and tumor growth was well
inhibited by this dosage regimen when compared to PEI-or
saline-treated mice (P<0.01) (Fig.
2E). In spite of the positive effect on tumor inhibition,
severe side-effects of the drug were evident in the mice, leading
to marked body weight loss and a median survival time of 23 days
(Fig. 2D and F). Furthermore, no
significant difference in tumor growth inhibition or survival time
was observed between the cisplatin-treated mice and the
siVEGFR2-treated mice, or between the cisplatin- and the
siEGFR-treated mice.
To investigate whether dual-blockade of EGFR and
VEGFR2 expression could enhance the antitumor effect of
chemotherapy, cisplatin was administrated in combination with
siEGFR and siVEGFR2. For this group of mice
(siVEGFR2+siEGFR+cisplatin), 0.25 nmol of siVEGFR2 plus 0.25 nmol
of siEGFR were complexed with 15.65 μg PEI (N/P=8) as one injection
dose followed the next day by an i.p. dose of 3 mg/kg cisplatin
(Fig. 2C). Treatment was
discontinued two days before mice were sacrificed and resulted in
no obvious body weight changes (Fig.
2D) or adverse events compared to cisplatin alone (Table II). In addition, tumor growth was
significantly inhibited at the end of the experiment compared to
that in the PEI- or saline-treated mice (P<0.01) but no
significant difference between this group and siVEGFR2-treated,
siEGFR-treated, siVEGFR2+siEGFR-treated, or 5 mg/kg cisplatin
alone-treated groups was observed (Fig.
2E). Furthermore, a median survival time of 29 days was
observed in this group, which was longer than that of the
siVEGFR2+siEGFR-treated animals (P<0.05) (Fig. 2F).
 | Table IIAdverse effects observed in the
experiment in vivo. |
Table II
Adverse effects observed in the
experiment in vivo.
| Groups | Scurf | Purple skin | Itch | Wound | Diarrhea | Weakness |
|---|
| PEI-treated
n=4 | 75% | None | None | 25% | None | None |
| Saline-treated
n=4 | 75% | None | None | None | None | None |
| siControl-treated
n=4 | None | 75% | None | None | 100% | 100% |
| siVEGFR2-treated
n=5 | 100% | 60% | 40% | 20% | 100% | 60% |
| siEGFR-treated
n=5 | None | None | None | 20% | None | None |
| Cisplatin-treated
n=5 | 80% | 80% | None | None | 100% | 100% |
| siVEGFR2+
siEGFR-treated n=4 | 100% | None | 25% | 50% | 75% | None |
| siVEGFR2+ siEGFR+
cisplatin(3 mg/kg)-treated n=4 | 100% | 25% | 25% | 50% | 50% | None |
Downregulation of the VEGFR2 expression
of both mRNA and protein on the treated tumors
Quantitative RT-PCR was used to analyze the
expression level of VEGFR2 mRNA in the tumors from A549 xenografts
and showed obvious downregulation of VEGFR2 mRNA in siVEGFR2-, and
siVEGFR2+siEGFR+cisplatin-treated tumors compared to that of
saline-treated tumors (Fig. 3A). In
addition, monotherapy with 0.50 nmol siVEGFR2 showed significantly
more downregulated VEGFR2 mRNA in the tumors than tumors that were
treated with 0.25 nmol siVEGFR2 + 0.25 nmol siEGFR + 3 mg/kg
cisplatin, which indicated a dose-dependent silencing. Notably, the
VEGFR2 mRNA level in 5 mg/kg cisplatin-treated tumors was also
clearly reduced compared to that in saline-treated tumors (Fig. 3A), which may have resulted from the
sharp reduction of tumor size. There was no significant difference
in the VEGFR2 mRNA level between cisplatin-, and
siVEGFR2+siEGFR+cisplatin-treated tumors, although there was a
significant difference in the VEGFR2 mRNA level found between
cisplatin-, and siVEGFR2-treated tumors (Fig. 3A).
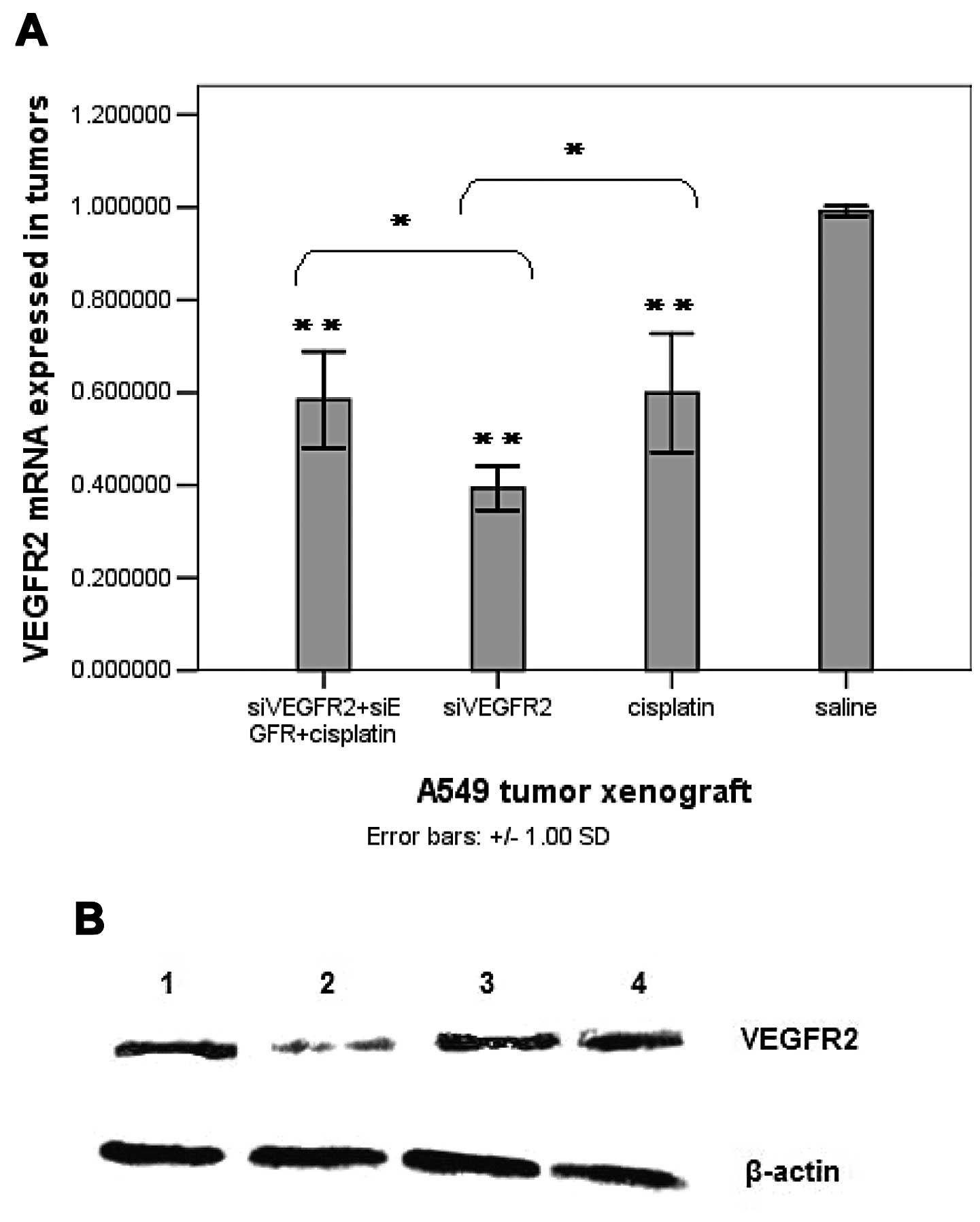 | Figure 3VEGFR2 expression in the tumor
tissue. (A) VEGFR2 mRNA expression in the tumors determined by
quantitative real-time PCR. (B) Western blot analysis of VEGFR2
protein expression in tumors implanted in nude mice. Representative
blots are shown from three experiments. β-actin served as a loading
control. Lane 1, 0.25 nmol siVEGFR2 + 0.25 nmol siEGFR+3 mg/kg
cisplatin (siRNA, intratumoral injection, twice/week; cisplatin,
intraperitoneal injection, once a week the day after the first shot
of siRNA). Lane 2, 0.5 nmol siVEGFR2 (intratumoral injection,
twice/week). Lane 3, 5 mg/kg cisplatin (intraperitoneal injection,
once/week). Lane 4, saline (intratumoral injection, twice/week).
(C) The expression level of VEGFR2 protein in the tumors following
treatments. All the experiments were performed in triplicates.
Columns, means; bars, SD. *P<0.05;
**P<0.01, vs. the saline-treated group. |
Western blot analysis further confirmed the
downregulation of VEGFR2 protein expression in siVEGFR2-,
siVEGFR2+siEGFR+cisplatin- (P<0.01), and cisplatin-treated
tumors (P<0.05) compared to that in saline-treated tumors
(Fig. 3B and C) which was parallel
with the reduced level of VEGFR2 mRNA. Furthermore, there was no
significant difference in the VEGFR2 protein levels among the
siVEGFR2-, siVEGFR2+siEGFR+cisplatin-, and cisplatin-treated tumors
(Fig. 3B and C).
Toxicity resulting from combination
therapy of cisplatin and siRNA in vivo
As an important parameter of the in vivo
studies, we assessed the toxicity of combination therapy of
cisplatin with siVEGFR2, siEGFR alone, or siVEGFR2+siEGFR (Table II). The dosage of 6.5 μg of siRNA
per tumor injection itself did not show any acute side-effects,
weight loss, or morbidity in the mice, although later in the study
some signs of toxicity, such as scurf, itching, wounds, purple
skin, diarrhea and weakness, became apparent. Among these, scurf
was a common but mild symptom, while the others were more severe
and may have contributed to animal death. Symptoms of diarrhea and
weakness were mainly found in the cisplatin (5 mg/kg)-, siVEGR2-,
and control siRNA-treated groups. Purple skin, which might be
credited to damage of the hematopoietic system, were common in the
groups of siVEGFR2-, cisplatin (5 mg/kg)-,
siVEGFR2+siEGFR+cisplatin (3 mg/kg)-, and control siRNA-treated
groups. Itching occurred in mice receiving siVEGFR2. There were few
side-effects found in the siEGFR-treated group, except for wounds
or tissue lesions manifested at the lateral skin of the tumor. In
addition, some mice developed wound lesions on the edge of the
ears. The adverse effects seen in our model most likely led to
discomfort or indirect impairment of the circulatory system of the
mice, and eventually may have contributed to morbidity of the
animals. The side-effects resulting from siRNA therapy in animal
models in our study, however, might provide a beneficial reference
for the future application of siRNA in the clinic.
Discussion
Cancer is a complex disease; and a leading cause of
human mortality, it has been described a ‘wound that does not heal’
(28). After many years of research
into cancer therapy, however, little progress has been made.
Extensive studies of the subtle network of tumorigenesis and the
relationship with vascularization have been carried out (29,30).
The formation of a new functional microvasculature derived from
pre-existing blood vessels, or angiogenesis, can be induced by the
tumor itself as a means to provide all the nutrients and oxygen the
tumor needs to grow and metastasize (29). Both endothelial cells and tumor
cells are responsible for this pathological process, in which
pro-angiogenic factors and the endogenous angiogenesis inhibitors
are secreted to play important roles in angiogenesis (31,32).
In normal physiological conditions, pro-angiogenic factors and the
endogenous angiogenesis inhibitors are in balance (32), however, during tumorigenesis the
‘angiogenic switch’ is turned on (30). Different pro-angiogenic factors
become overexpressed in different types of tumors. Non-small cell
lung cancer (NSCLC), one of the most malignant types of cancer, has
been found to upregulate the expression of EGFR and VEGF/VEGFR,
both of which are pro-angiogenic factors (33,34).
In the present study, we knocked down the expression
of EGFR and VEGFR2 to inhibit the growth of human NSCLC xenografts
with siVEGFR2 (siRNA2) and a previously published siEGFR. siRNA was
delivered by polyethylenimines (PEI), a type of cationic polymer,
which could encapsulate siRNA as nanoparticle complex. Our data
indicated that these two receptor tyrosine kinases acted
synergistically and both siRNA therapies, particularly the siEGFR,
could lead to the inhibition of tumor growth with few adverse
effects.
We also observed that a double dosage of siVEGFR2 or
siEGFR did not exert a dose-dependent effect, while the
siVEGFR2+siEGFR-treated animals receiving a 0.25 nmol (half dose)
of each siRNA did indeed display tumor inhibition, while the
adverse effects were reduced due to the lower dose of siVEGFR2. The
biphasic dose-efficacy curve offered an explanation that a higher
dose could not increase the silencing efficacy over a lower dose.
Of note, it has been reported that some angiogenesis inhibitors
follow a biphasic, U-shaped dose-efficacy curve, unlike
dose-dependent chemotherapy agents (35).
Cisplatin has been reported to inhibit tumor growth
by cytotoxicity and was found to hinder the DNA replication of
tumor cells by activating the transduction of DNA-damage signals
(36). Adverse effects caused by
cytotoxicity and subsequent drug resistance have restrained the
utilization of certain chemotherapy agents in the clinic. The
chemotherapy agent we applied in this study was cisplatin with one
injection of 5 mg/kg body weight per week. Western blot analysis
showed a significant reduction in expression of VEGFR2 in the
cisplatin-treated mice. The suppression of the bone marrow and
hematopoietic system by cisplatin might contribute to this
reduction of VEGFR2 protein expression, whereas killing both the
tumor cells and the tumor-associated endothelial cells by
destroying the DNA might also lead to the reduced level of VEGFR2
protein observed.
The combination of a chemotherapy agent with the
anti-angiogenesis therapy has been termed ‘metronomic therapy’.
This type of approach can exert an enhanced antitumor effect by
combining lower dose anti-angiogenesis inhibitors with standard
chemotherapy and has been shown to produce fewer side-effects than
seen in conventional dosing of chemotherapy agents (37). It has been hypothesized that
anti-angiogenesis inhibitors might lower intratumoral pressure by
decreasing vascular leakage to induce the ‘normalization’ of leaky
tumor vessels and thus increase the delivery of the cytotoxicity
drugs (38).
With longer survival, fewer adverse effects and,
most of all, significant tumor inhibition, the combination of
cisplatin with siRNA therapy showed some advantages over
monotherapy. Previously it was confirmed that VEGFR2 is expressed
not only in endothelial cells, but also in tumor cells, which
indicates that targeted inhibition of VEGFR2 expression can inhibit
tumor growth both directly and indirectly when mediated by VEGFR2
siRNA (39). Taking the therapeutic
effects and the side-effects observed in our study, an optimized
clinical dosage regimen should consider a lower dose of siVEGFR2
due to the current lack of an effective delivery system.
Intratumoral injection has been shown to be inefficient with only
0.1% of the given siRNA remaining in the tumor mass, while the
remainder might suffer to degradation or induce side-effects
(40). Moreover, since siRNA
activity is dose-independent, a lower dose of siVEGFR2 might be
more beneficial in practical application. Most importantly, the
therapeutic regimen with the lower dose of siRNA plus lower dose of
cisplatin exerted more benefits on the therapy of tumor than that
cisplatin alone (Fig. 2E) and
resulted in reduced side-effects (Table II).
In conclusion, targeted knockdown by siVEGFR2 and
siEGFR inhibited tumor growth effectively, and a combination of
this approach with low dose chemotherapy might offer a novel
strategy for the treatment of cancer.
Acknowledgements
The study was supported by the National Nature
Science Foundation of China (NSFC30672557), the Key Project of
Nature Science Foundation of Guangdong Province, China (grant
no.8251051501000008) and the Specialized Research Fund for the
Doctoral Program of Higher Education (20094433110013).
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
ECs
|
endothelial cells
|
|
EGFR
|
epidermal growth factor receptor
|
|
PEI
|
polyethylenimines
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Gimbrone MA, Leapman SB Jr, Cotran RS, et
al: Tumor dormancy in vivo by prevention of neovascularization. J
Exp Med. 136:261–276. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar
|
|
3
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown LF, Detmar M, Claffey K, et al:
Vascular permeability factor/vascular endothelial growth factor: a
multifunctional angiogenic cytokine. EXS. 79:233–269.
1997.PubMed/NCBI
|
|
5
|
Herbert SP and Stainier DY: Molecular
control of endothelial cell behaviour during blood vessel
morphogenesis. Nat Rev Mol Cell Biol. 1:551–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hynes NE and Lane HA: ERBB receptors and
cancer: the complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wells A: EGF receptor. Int J Biochem Cell
Biol. 31:637–643. 1999. View Article : Google Scholar
|
|
8
|
Ciardiello F and Tortora G: Epidermal
growth factor receptor (EGFR) as a target in cancer therapy:
understanding the role of receptor expression and other molecular
determinants that could influence the response to anti-EGFR drugs.
Eur J Cancer. 39:1348–1354. 2003. View Article : Google Scholar
|
|
9
|
Morelli MP, Cascone T, Troiani T, et al:
Anti-tumor activity of the combination of cetuximab, an anti-EGFR
blocking monoclonal antibody and ZD6474, an inhibitor of VEGFR and
EGFR tyrosine kinases. J Cell Physiol. 208:344–353. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar
|
|
11
|
Kerbel RS: Antiangiogenic therapy: a
universal chemosensitization strategy for cancer? Science.
312:1171–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dancey JE and Freidlin B: Targeting
epidermal growth factor receptor - are we missing the mark? Lancet.
362:62–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JC, Haworth L, Sherry RM, et al: A
randomized trial of bevacizumab, an anti-vascular endothelial
growth factor antibody, for metastatic renal cancer. N Engl J Med.
349:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Li Z, Han Y, et al:
Nanoparticle-based delivery system for application of siRNA in
vivo. Curr Drug Metab. 11:182–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh YK and Park TG: siRNA delivery systems
for cancer treatment. Adv Drug Deliv Rev. 6:850–862. 2009.
|
|
18
|
Castanotto D and Rossi JJ: The promises
and pitfalls of RNA-interference-based therapeutics. Nature.
457:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urban-Klein B, Werth S, Abuharbeid S, et
al: RNAi-mediated gene-targeting through systemic application of
polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther.
12:461–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boussif O, Lezoualc’h F, Zanta MA, et al:
A versatile vector for gene and oligonucleotide transfer into cells
in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA.
92:7297–7301. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franklin WA, Veve R, Hirsch FR, et al:
Epidermal growth factor receptor family in lung cancer and
premalignancy. Semin Oncol. 29:3–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tuschl T, Zamore PD, Lehmann R, et al:
Targeted mRNA degradation by double-stranded RNA in vitro. Genes
Dev. 13:3191–3197. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schiffelers RM, Ansari A, Xu J, et al:
Cancer siRNA therapy by tumor selective delivery with
ligand-targeted sterically stabilized nanoparticle. Nucleic Acids
Res. 32:e1492004. View Article : Google Scholar
|
|
24
|
Weihua Z, Tsan R, Huang WC, et al:
Survival of cancer cells is maintained by EGFR independent of its
kinase activity. Cancer Cell. 13:385–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan Y, Sun X, Xu M, et al: Efficacy of
recombinant methioninase in combination with cisplatin on human
colon tumors in nude mice. Clin Cancer Res. 5:2157–2163.
1999.PubMed/NCBI
|
|
26
|
Bruyere C, Lonez C, Duray A, et al:
Considering temozolomide as a novel potential treatment for
esophageal cancer. Cancer. 117:2004–2016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang H, Che O, Chen S, et al: Silence of
VEGFR2 expression mediated by PEI/siRNA complexes. Yao Xue Xue Bao.
45:576–581. 2010.(In Chinese).
|
|
28
|
Dvorak HF: Tumors: wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murukesh N, Dive C and Jayson GC:
Biomarkers of angiogenesis and their role in the development of
VEGF inhibitors. Br J Cancer. 102:8–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nyberg P, Xie L and Kalluri R: Endogenous
inhibitors of angiogenesis. Cancer Res. 65:3967–3979. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohsaki Y, Tanno S, Fujita Y, et al:
Epidermal growth factor receptor expression correlates with poor
prognosis in non-small cell lung cancer patients with p53
overexpression. Oncol Rep. 7:603–607. 2000.PubMed/NCBI
|
|
34
|
Wu W, Onn A, Isobe T, et al: Targeted
therapy of orthotopic human lung cancer by combined vascular
endothelial growth factor and epidermal growth factor receptor
signaling blockade. Mol Cancer Ther. 6:471–483. 2007. View Article : Google Scholar
|
|
35
|
Slaton JW, Perrotte P, Inoue K, et al:
Interferon-alpha-mediated down-regulation of angiogenesis-related
genes and therapy of bladder cancer are dependent on optimization
of biological dose and schedule. Clin Cancer Res. 5:2726–2734.
1999.PubMed/NCBI
|
|
36
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hanahan D, Bergers G and Bergsland E: Less
is more, regularly: metronomic dosing of cytotoxic drugs can target
tumor angiogenesis in mice. J Clin Invest. 105:1045–1047. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jain RK: Antiangiogenic therapy for
cancer: current and emerging concepts. Oncology (Williston Park).
19:7–16. 2005.PubMed/NCBI
|
|
39
|
Higgins KJ, Abdelrahim M, Liu S, et al:
Regulation of vascular endothelial growth factor receptor-2
expression in pancreatic cancer cells by Sp proteins. Biochem
Biophys Res Commun. 345:292–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hobel S, Koburger I, John M, et al:
Polyethylenimine/small interfering RNA-mediated knockdown of
vascular endothelial growth factor in vivo exerts anti-tumor
effects synergistically with Bevacizumab. J Gene Med. 12:287–300.
2010.
|

















