Introduction
D-limonene (1-methyl-4-isopropyl-cyclohexene) is a
cyclic monoterpene and a major constituent in essential oils of
orange, lemon, mandarin, lime, grapefruit and several other plants.
It is listed in the Code of Federal Regulations as generally
recognized as safe (GRAS) for a flavoring agent and can be found in
common food items such as fruit juices, soft drinks, baked goods,
ice cream and pudding (1). One
epidemiological study suggested that citrus peel consumption, the
major source of dietary D-limonene, might have a potential
preventive effect on squamous cell carcinoma (2). In chemically-induced rodent tumor
models, D-limonene has been reported to have chemopreventive
activity at both the initiation and promotion/progression phases
(3–8). Evidence from a phase I clinical trial
showed that D-limonene is well tolerated and may have clinical
activity in cancer patients (9).
While these observations are of interest, a better understanding of
the underlying action mechanisms of D-limonene is necessary to
confirm its effectiveness as a potential chemopreventive and
treatment agent.
Several mechanisms may account for the anticancer
effect of D-limonene. The chemopreventive activity of D-limonene
during the initiation phase of carcinogenesis might be attributed
to the induction of phase I and phase II carcinogen-detoxifying
enzymes, which are known to be responsible for detoxification of
carcinogen (10–12). A previous report indicated that the
topical treatment of D-limonene inhibits the Ras-ERK signaling
pathway, inflammation and oxidative stress as well as the induction
of pro-apoptotic state in the TPA-mediated promotion of
DMBA-induced skin cancer in a mouse model (13). In vivo and in vitro
studies demonstrated that the chemotherapeutic activity of
D-limonene might be attributed to the induction of apoptosis
(14–16). Ji et al found that the
limonene-induced apoptotic death of human leukemia cells involves
the increase in Bax protein expression, the release of cytochrome
c from mitochondria and the activation of caspase,
suggesting that the mitochondria-mediated intrinsic death pathway
may play a major role in limonene-induced death (16).
The serine/threonine protein kinase (Akt, a member
of the PI3K pathway) is involved in widely divergent cellular
processes including apoptosis and cell proliferation (17). The aberrant activation of
phosphoinositide 3-kinase (PI3K)/AKT has been documented as a
frequent occurrence in human cancer, including colorectal cancer
(18–20). Furthermore, abnormal activation of
the PI3K/AKT pathway rendered these cells less sensitive to
apoptosis stimuli (21,22) and inhibition of this pathway should
provide a therapeutic approach for cancer (23). Once activated, however, Akt promotes
the cell survival partially by phosphorylation and inactivation of
several pro-apoptotic proteins, including GSK-3 (24), BAD (25) and caspase-9 (26). Although it has been reported that
geraniol, an acyclic dietary monoterpene, potently induced
apoptosis and autophagy by inhibition of AKT signaling (27), the effect of D-limonene on Akt
signaling remains unclear.
One phase I study conducted by Vigushin et al
indicated that three individuals with colorectal carcinoma, while
on D-limonene at dose of 0.5–1 g/m2 per day, were able
to suspend progression of the disease for over six months (9). This clinical trial strongly suggested
that D-limonene could be an efficient therapeutic agent for
colorectal cancer. The mechanism by which D-limonene inhibits the
viability of colorectal cancer cells has yet to be established,
therefore, we addressed this in the present study.
Materials and methods
Materials
D-limonene was purchased from Sigma Chemical Co.
(St. Louis, MO, USA). Antibodies against total Akt, phospho-Akt
(Ser473), phospho-Akt (Thr308), phospho-GSK-3β (Ser9),
poly(ADP-ribose)polymerase (PARP), cleaved PARP, pro-caspase-3,
cleaved caspase-3, pro-caspase-9, cleaved caspase-9, pro-caspase-8,
cleaved caspase-8 and β-actin were purchased from Cell Signaling
Technology (Beverly, MA, USA). Antibodies against Bcl-2 and Bax
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Horseradish peroxidase (HRP)-conjugated secondary antibodies were
purchased from Cell Signaling Technology. Annexin V-fluorescein
isothiocyanate (FITC) labeled apoptosis detection kit was obtained
from Baosai Biological Technology Co., Ltd. (Beijing, China).
Cell culture
The LS174T human colon cancer cell line
(Heilongjiang Cancer Institute, China) was maintained in RPMI-1640
medium at 37°C in a 5% CO2 atmosphere. All cell samples
used were in the logarithmic growth phase.
Cell viability assay
Effect of D-limonene on the viability of LS174T
cells was performed using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
conversion assay. Briefly, LS174T cells were plated into a 24-well
plate and cultured in the presence of varying concentrations of
D-limonene for 48 h. Thereafter, 10 μl of MTT (5 mg/ml) was added
to each well and the cells were incubated at 37°C for another 4 h.
Following removal of the medium, the cells were lysed in 100 μl of
dimethylsulfoxide (DMSO). Then, the optical density (OD) was
measured at 490 nm by a microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA). The following formula was used: relative
percentage of cell viability = (OD of the experimental sample/OD of
the control group) × 100%.
Measurement of apoptosis by Annexin
V-FITC staining
Apoptosis was determined by staining cells with
Annexin V- FITC staining. Briefly, after various treatments, both
floating and trypsinized adherent LS174T cells were collected. Then
the cell pellets were incubated with 5 μl propidium iodide (PI), 10
μl Annexin V-FITC for 20 min in the dark at room temperature and
the samples were analyzed by flow cytometry.
Western blot analysis
Cytosolic extracts were prepared for cytochrome
c according to the instructions for the Nuclear and
Cytoplasmic Protein Extraction kit (active motif 40010). Total cell
extracts were prepared by resuspending cells in cold lysis buffer
(Beyotime, Jiangsu, China). The supernatants were then collected by
centrifugation at 12,000 × g for 5 min. Equal amounts of lysate
were loaded on a 10% SDS-polyacrylamide gel. After electrophoresis,
proteins were transferred to a PVDF membrane and the blots were
probed by corresponding primary antibodies, followed by incubation
with HRP-conjugated secondary antibodies. The positive bands
representing protein were visualized by chemiluminescent reagents
(EZ-ECL; Biological Industries, Kibbutz Beit Haemek, Israel).
Statistical analysis
Data are expressed as the means ± SD of 3 repeated
experiments. The one way analysis of variance (ANOVA) was used for
statistical analyses. P<0.05 was considered to indicate
statistically significant differences. All experiments were
performed at least 3 times independently.
Results
D-limonene decreases the cell viability
of LS174T cells
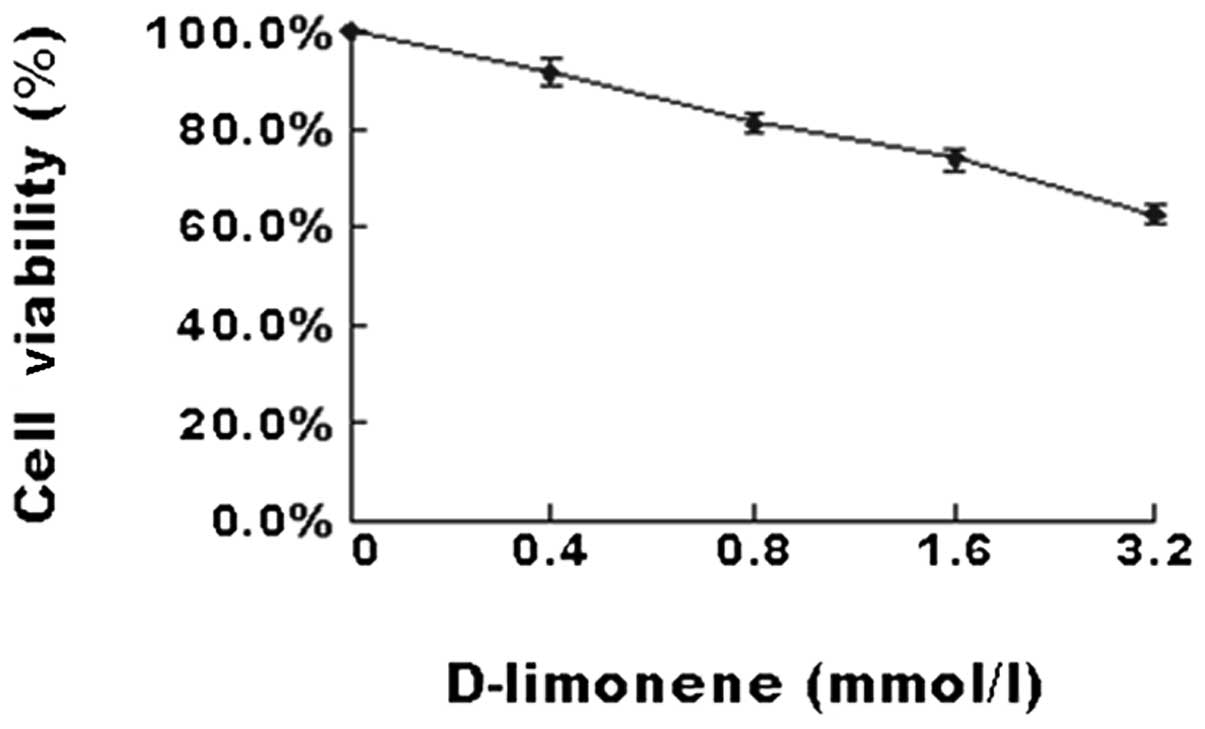
To investigate the effect of D-limonene on the
viability of colon cancer cells, LS174T cells were treated with
various concentrations of D-limonene for 48 h. The results of the
MTT assay (Fig. 1) demonstrated
that D-limonene significantly inhibited cell viability in a
dose-dependent manner.
D-limonene induces apoptosis of LS174T
cells
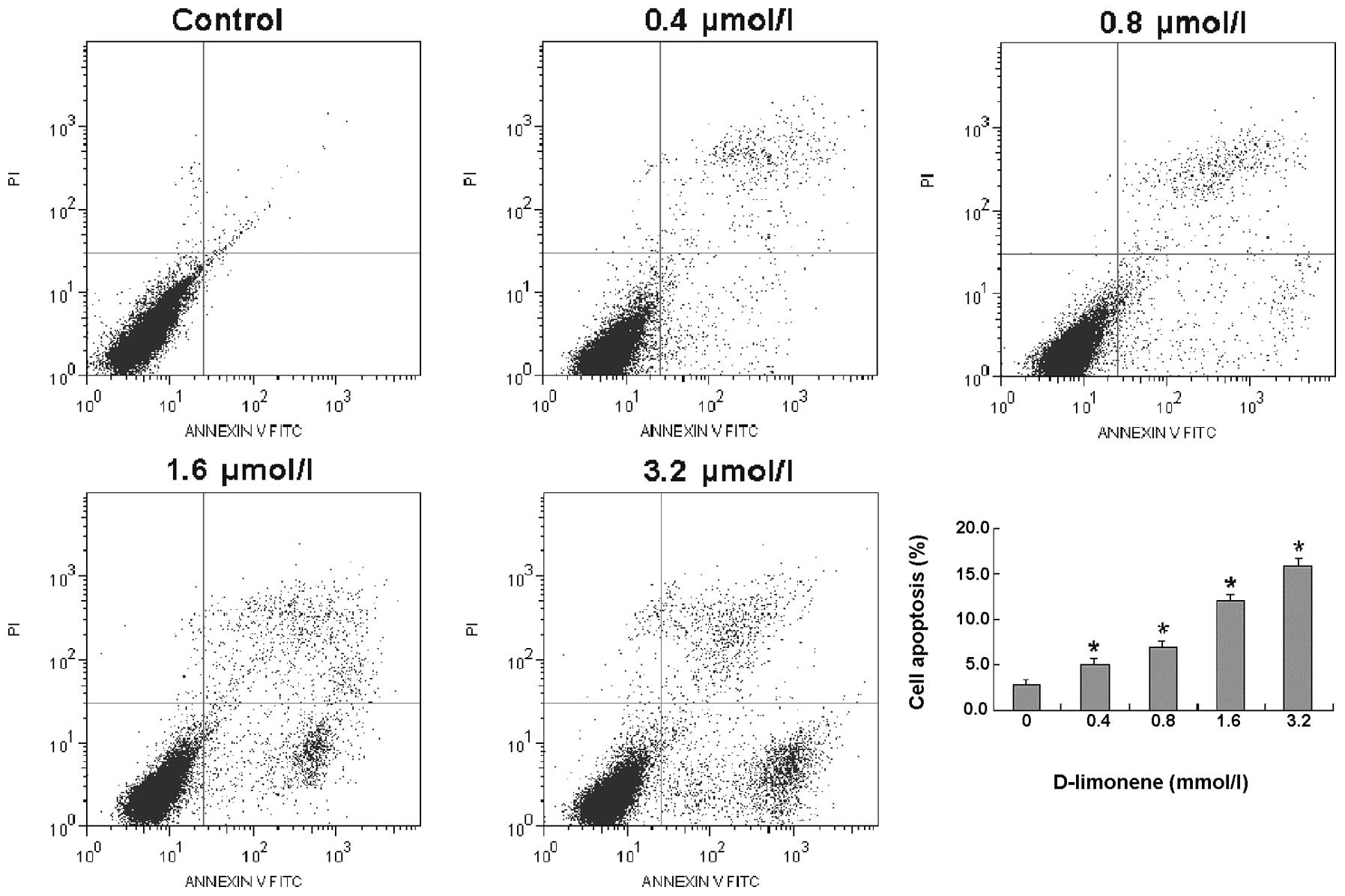
To determine whether D-limonene inhibits the
viability of LS174T cells via induction of apoptosis, LS174T cells
were treated with various concentrations of D-limonene for 48 h and
apoptosis was quantified using flow cytometry with Annexin V/PI
dual staining. The results revealed that D-limonene resulted in a
dose-dependent induction of apoptosis in LS174T cells (Fig. 2). These results suggest that
D-limonene inhibited the viability of LS174T cells by inducing
apoptosis in a dose-dependent manner.
D-limonene induces caspase activation in
LS174T cells
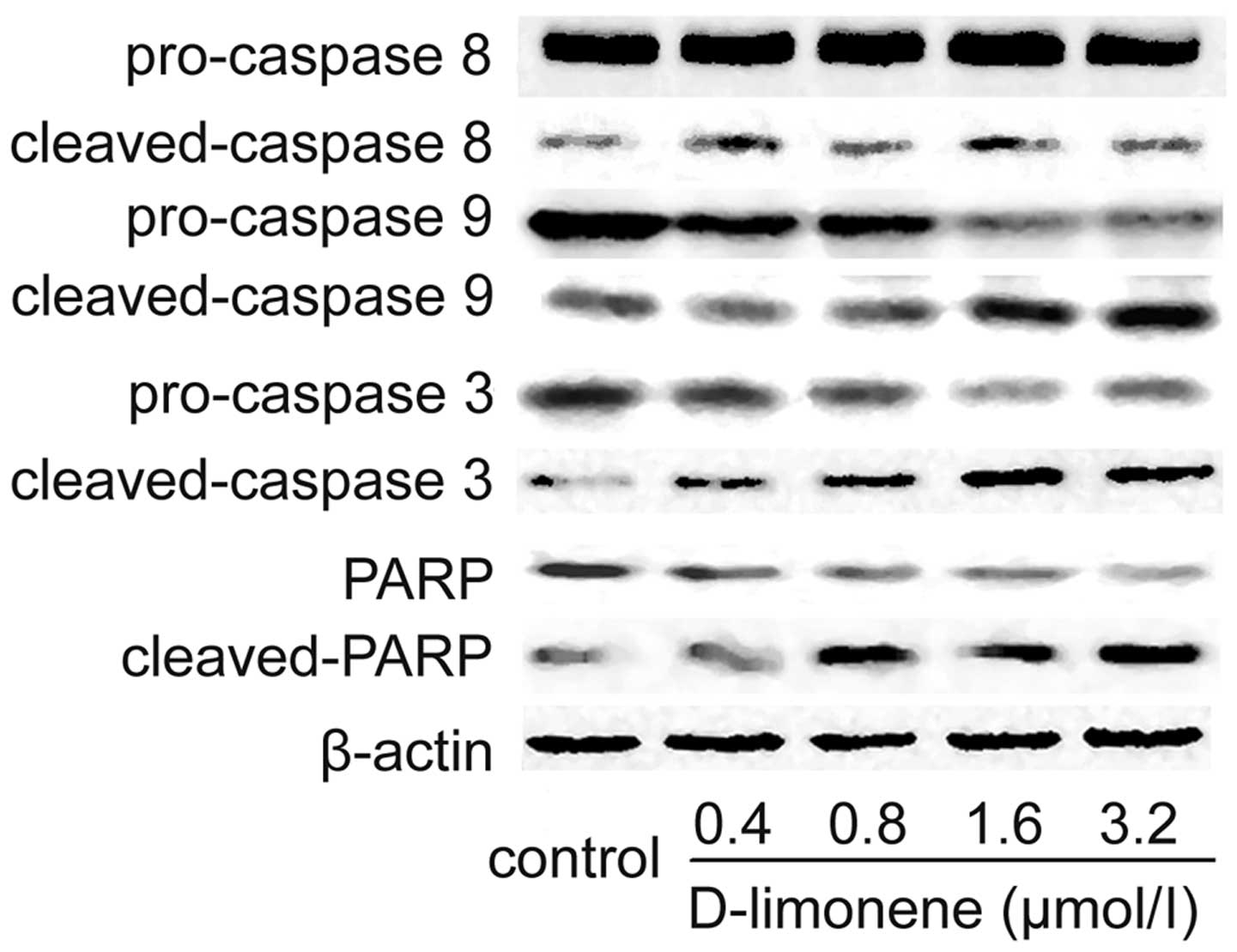
To investigate whether or not the observed apoptosis
was due to caspase activation, the cell extracts were obtained
after treatments and processed for western blot analysis. The
changes in protein levels in the LS174T cells treated with
D-limonene were determined using western blot analysis. After 48 h,
D-limonene treatment resulted in partial cleavage of pro-caspase-3,
and -9, whereas it had no significant effect on caspase-8
expression (Fig. 3), which
indicated that D-limonene could increase the cleavage maturation of
caspase-3 and caspase-9. Meanwhile, the cleavage of PARP, an
executioner caspase substrate, was also detected using western blot
analysis. Similarly, D-limonene treatment resulted in cleavage of
PARP from 116 to 89 kDa (Fig. 3).
Therefore, these results suggest that D-limonene-induced apoptosis
is partly mediated through the mitochondrial pathway.
D-limonene regulates the expression of
Bcl-2 and Bax protein
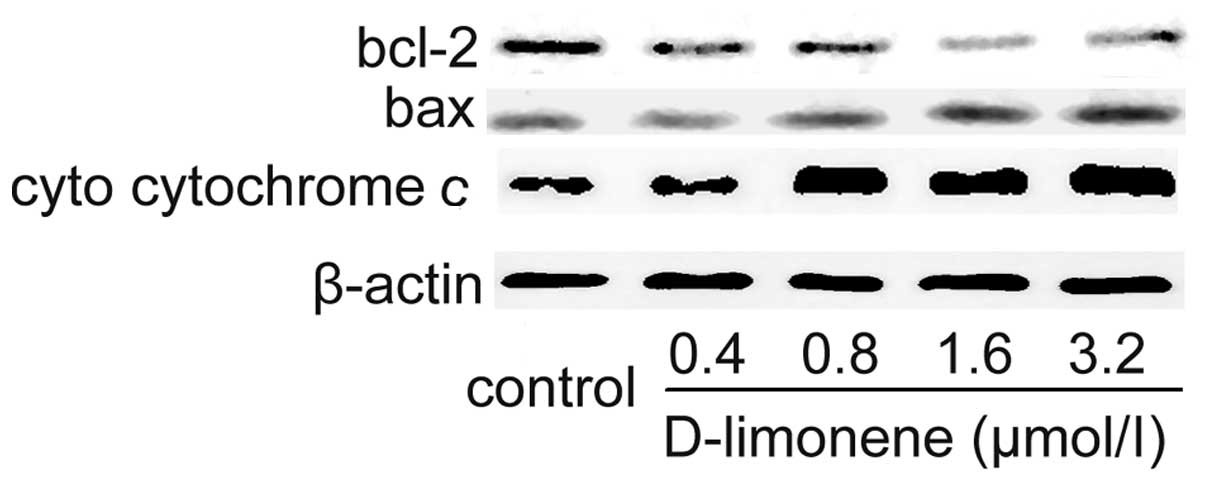
To confirm the involvement of the mitochondrial
pathway of apoptosis, the expression of Bax and Bcl-2 in the LS174T
cells treated with D-limonene were determined using western blot
analysis. D-limonene treatment resulted in an upregulation of Bax
and a slight downregulation of Bcl-2 (Fig. 4), which resulted in an increase in
the ratio of Bax to Bcl-2, therefore favored apoptosis. Since the
release of cytochrome c from the mitochondria triggers the
activation of pro-caspase 9, we measured cytosol cytochrome
c. D-limonene significantly increased the level of cytosol
cytochrome c (Fig. 4), which
further suggested that D-limonene-induced apoptosis is partly
mediated through the mitochondrial pathway.
D-limonene inhibits phosphorylation of
Akt in LS174T cells
Akt is reported to promote cell survival by
inhibiting apoptosis and its activation correlates with
phosphorylation at Thr308 and Ser473 residues, so the effects of
D-limonene on the amount and phosphorylation of Akt were evaluated
using western blot analysis. D-limonene did not significantly alter
the protein level of Akt, but it decreased the levels of
phospho-Akt (Ser473) and phospho-Akt (Thr308) (Fig. 5), which suggested that inactivation
of Akt kinase following D-limonene treatment was due to
dephosphorylation of Akt, rather than reduction in total Akt
protein. We detected the levels of phospho-GSK3β (ser9), which are
phosphorylated and inactivated by Akt. Similarly, D-limonene
significantly decreased the level of phospho-GSK3β (ser9) (Fig. 5). These results suggested that
D-limonene treatment could decrease the activity of Akt.
Discussion
D-limonene, a monocyclic monoterpene with a
lemon-like odor, is reported to have chemopreventive and
chemotherapeutic activity in animal models and cultured tumor cells
(14,28,29).
It has been shown that D-limonene is well tolerated and may have
clinical activity in patients with advanced cancer in a phase I
clinical trial (9). Moreover, Rabi
and Bishayee demonstrated that D-limonene enhanced the antitumor
effect of docetaxel against prostate cancer cells without being
toxic to normal prostate epithelial cells, which suggested that
D-limonene could be used as a potent non-toxic agent to improve the
treatment outcome of hormone-refractory prostate cancer with
docetaxel (30). However, the
molecular mechanism by which D-limonene inhibits the viability of
cancer cells has not been established. In this study, we
demonstrated that D-limonene inhibited the viability of the LS174T
human colon cancer cells in a dose-dependent manner. In particular,
we showed that D-limonene induced cell apoptosis, which is partly
mediated via the mitochondrial pathway. In addition, we also
observed that D-limonene inhibited the PI3K/Akt pathway.
Apoptosis (programmed cell death), is known to be
involved in a variety of biological events. Accumulating evidence
suggests that most anticancer agents can trigger apoptosis in tumor
cells in vivo and in vitro, which appears to be
associated with their effectiveness in prevention of tumor growth
(31–33). It is well known that caspases play
the central role in apoptosis. Caspase-8 and caspase-9 are the
initiator caspases: caspase-8 is usually involved in the extrinsic
death receptor apoptosis pathway, whereas caspase-9 has been linked
to the intrinsic mitochondrial death pathway. They cleave and
activate the downstream effector caspases, such as caspase-3, which
cause PARP cleavage and eventually lead to apoptosis. Previous
reports indicated that D-limonene may induce cellular apoptosis in
some types of cancer (14,16,30).
Moreover, in human leukemia cell lines, the caspase-dependent
mitochondrial death pathway mediated the D-limonene-induced
apoptosis (16). In this study, we
showed that caspase activation is also involved in the
D-limonene-induced apoptosis. Cleaved caspase-9, caspase-3, and
PARP were activated by D-limonene in LS174T cells, but caspase-8
appears not to be involved. Therefore, these results suggest that
apoptosis induction by D-limonene might have occurred through a
mitochondria-mediated pathway.
It is well known that mitochondria play a key role
in apoptotic signal transduction in mammalian cells (34). Bcl-2 and Bax are members of the
Bcl-2 family that regulates apoptosis by control mitochondria
integrity. Although they have highly similar amino acid sequences,
their functions are opposed: Bcl-2 acts to inhibit apoptosis,
whereas Bax counteracts this effect by heterodimerization with
Bcl-2. The ratio of Bcl/Bax dictates the sensitivity of cells to a
wide variety of apoptotic stimuli (35). In mitochondrion-dependent apoptosis,
the disruption of the mitochondrion leads to the release of
cytochrome c into the cytosol. The apoptosome containing
cytochrome c, Apaf-1 and pro-caspase-9 is then assembled,
which results in proteolytic processing and activation of
pro-caspase-9. Active caspase-9 in turn activates pro-caspase-3
initiating a caspase signaling cascade to induce apoptosis
(36). In the present study, we
found that D-limonene decreased the protein level of Bcl-2, but
increased the protein level of Bax, which is consistent with
previous reports (16,28). We also noted a significant increase
of cytochrome c in the cytosol of D-limonene-treated cells.
Thus, these results further suggest that D-limonene has the ability
to induce the intrinsic mitochondrial apoptosis signaling pathway
in LS174T colon cancer cells.
The PI3K/Akt pathway is an important intracellular
signaling pathway, which plays a critical role in controlling
survival and apoptosis. In many types of cancer this pathway is
overactive, supporting cell survival and proliferation (18–20).
Several reports have shown that some anticancer agents induce
apoptosis, in part, by blocking this pathway (37,38).
Activated Akt phosphorylates and inactivates several pro-apoptotic
proteins, including BAD (25), and
caspase-9 (26), inhibiting the
intrinsic apoptotic pathway. Recently, geraniol, an acyclic dietary
monoterpene, was reported to induce apoptosis by inhibition of AKT
signaling (27). Our results showed
that D-limonene decreased not only phosphorylated Akt protein
levels but also Akt activity. Moreover, we also found that
caspase-9, a downstream target of Akt, was cleaved to the active
form by the D-limonene treatment. Collectively, these results
suggest that inhibition of the Akt pathway contributed, at least in
part, to the apoptotic cell death caused by the D-limonene
treatment.
In conclusion, the results from the present study
suggest that D-limonene inhibited the Akt activation and activated
the intrinsic mitochondrial apoptosis signaling pathway in LS174T
cells. Although further studies are required to determine whether
or not these pathways are involved in the anticancer effect of
D-limonene on other cancer cell lines, we believe that this study
may provide mechanistic insights into understanding the molecular
basis of the anticancer effect of D-limonene.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Heilongjiang Province (D2007-79), the Natural
Science Foundation for Young Scientists of Heilongjiang Province
(QC2008C23), the Science Research Foundation of the Health
Department of Heilongjiang (2006-175) and the Heilongjiang
Postdoctoral Science-Research Foundation.
References
|
1
|
Sun J: D-Limonene: safety and clinical
applications. Altern Med Rev. 12:259–264. 2007.PubMed/NCBI
|
|
2
|
Hakim IA, Harris RB and Ritenbaugh C:
Citrus peel use is associated with reduced risk of squamous cell
carcinoma of the skin. Nutr Cancer. 37:161–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maltzman TH, Hurt LM, Elson CE, Tanner MA
and Gould MN: The prevention of nitrosomethylurea-induced mammary
tumors by d-limonene and orange oil. Carcinogenesis. 10:781–783.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elson CE, Maltzman TH, Boston JL, Tanner
MA and Gould MN: Anti-carcinogenic activity of d-limonene during
the initiation and promotion/progression stages of DMBA-induced rat
mammary carcinogenesis. Carcinogenesis. 9:331–332. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uedo N, Tatsuta M, Iishi H, et al:
Inhibition by D-limonene of gastric carcinogenesis induced by
N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett.
137:131–136. 1999.PubMed/NCBI
|
|
6
|
Giri RK, Parija T and Das BR: d-limonene
chemoprevention of hepatocarcinogenesis in AKR mice: Inhibition of
c-jun and c-myc. Oncol Rep. 6:1123–1127. 1999.PubMed/NCBI
|
|
7
|
Nakaizumi A, Baba M, Uehara H, Iishi H and
Tatsuta M: d-Limonene inhibits N-nitrosobis(2-oxopropyl)amine
induced hamster pancreatic carcinogenesis. Cancer Lett. 117:99–103.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wattenberg LW, Sparnins VL and Barany G:
Inhibition of N-nitrosodiethylamine carcinogenesis in mice by
naturally occurring organosulfur compounds and monoterpenes. Cancer
Res. 49:2689–2692. 1989.PubMed/NCBI
|
|
9
|
Vigushin DM, Poon GK, Boddy A, et al:
Phase I and pharmacokinetic study of D-limonene in patients with
advanced cancer. Cancer Research Campaign Phase I/II Clinical
Trials Committee. Cancer Chemother Pharmacol. 42:111–117. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steele VE, Kelloff GJ, Wilkinson BP and
Arnold JT: Inhibition of transformation in cultured rat tracheal
epithelial cells by potential chemopreventive agents. Cancer Res.
50:2068–2074. 1990.PubMed/NCBI
|
|
11
|
Maltzman TH, Christou M, Gould MN and
Jefcoate CR: Effects of monoterpenoids on in vivo DMBA-DNA adduct
formation and on phase I hepatic metabolizing enzymes.
Carcinogenesis. 12:2081–2087. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van der Logt EM, Roelofs HM, van Lieshout
EM, Nagengast FM and Peters WH: Effects of dietary anticarcinogens
and nonsteroidal anti-inflammatory drugs on rat gastrointestinal
UDP-glucuronosyltransferases. Anticancer Res. 24:843–849.
2004.PubMed/NCBI
|
|
13
|
Manuele MG, Barreiro Arcos ML, Davicino R,
Ferraro G, Cremaschi G and Anesini C: Limonene exerts
antiproliferative effects and increases nitric oxide levels on a
lymphoma cell line by dual mechanism of the ERK pathway:
relationship with oxidative stress. Cancer Invest. 28:135–145.
2010. View Article : Google Scholar
|
|
14
|
Lu XG, Zhan LB, Feng BA, Qu MY, Yu LH and
Xie JH: Inhibition of growth and metastasis of human gastric cancer
implanted in nude mice by d-limonene. World J Gastroenterol.
10:2140–2144. 2004.PubMed/NCBI
|
|
15
|
Hata T, Sakaguchi I, Mori M, et al:
Induction of apoptosis by Citrus paradisi essential oil in human
leukemic (HL-60) cells. In Vivo. 17:553–559. 2003.PubMed/NCBI
|
|
16
|
Ji J, Zhang L, Wu YY, Zhu XY, Lv SQ and
Sun XZ: Induction of apoptosis by d-limonene is mediated by a
caspase-dependent mitochondrial death pathway in human leukemia
cells. Leuk Lymphoma. 47:2617–2624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcription and translation. Nat Rev
Cancer. 5:921–929. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niedermeier M, Hennessy BT, Knight ZA, et
al: Isoform-selective phosphoinositide 3′-kinase inhibitors inhibit
CXCR4 signaling and overcome stromal cell-mediated drug resistance
in chronic lymphocytic leukemia: a novel therapeutic approach.
Blood. 113:5549–5557. 2009.
|
|
20
|
Cui B, Tao J and Yang Y: Studies on the
expression patterns of class I PI3K catalytic subunits and its
prognostic significance in colorectal cancer. Cell Biochem Biophys.
62:47–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Page C, Lin HJ, Jin Y, et al:
Overexpression of Akt/AKT can modulate chemotherapy-induced
apoptosis. Anticancer Res. 20:407–416. 2000.PubMed/NCBI
|
|
22
|
Opel D, Naumann I, Schneider M, Bertele D,
Debatin KM and Fulda S: Targeting aberrant PI3K/Akt activation by
PI103 restores sensitivity to TRAIL-induced apoptosis in
neuroblastoma. Clin Cancer Res. 17:3233–3247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia-Echeverria C and Sellers WR: Drug
discovery approaches targeting the PI3K/Akt pathway in cancer.
Oncogene. 27:5511–5526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crowder RJ and Freeman RS: Glycogen
synthase kinase-3 beta activity is critical for neuronal death
caused by inhibiting phosphatidylinositol 3-kinase or Akt but not
for death caused by nerve growth factor withdrawal. J Biol Chem.
275:34266–34271. 2000. View Article : Google Scholar
|
|
25
|
Szanto A, Bognar Z, Szigeti A, Szabo A,
Farkas L and Gallyas F Jr: Critical role of bad phosphorylation by
Akt in cytostatic resistance of human bladder cancer cells.
Anticancer Res. 29:159–164. 2009.PubMed/NCBI
|
|
26
|
Cardone MH, Roy N, Stennicke HR, et al:
Regulation of cell death protease caspase-9 by phosphorylation.
Science. 282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SH, Park EJ, Lee CR, et al: Geraniol
induces cooperative interaction of apoptosis and autophagy to
elicit cell death in PC-3 prostate cancer cells. Int J Oncol.
40:1683–1690. 2012.PubMed/NCBI
|
|
28
|
Chaudhary SC, Siddiqui MS, Athar M and
Alam MS: D-Limonene modulates inflammation, oxidative stress and
Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum Exp
Toxicol. 31:798–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaji I, Tatsuta M, Iishi H, Baba M, Inoue
A and Kasugai H: Inhibition by d-limonene of experimental
hepatocarcinogenesis in Sprague-Dawley rats does not involve
p21(ras) plasma membrane association. Int J Cancer. 93:441–444.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rabi T and Bishayee A: d-Limonene
sensitizes docetaxel-induced cytotoxicity in human prostate cancer
cells: Generation of reactive oxygen species and induction of
apoptosis. J Carcinog. 8:92009. View Article : Google Scholar
|
|
31
|
Khan N, Adhami VM and Mukhtar H: Apoptosis
by dietary agents for prevention and treatment of cancer. Biochem
Pharmacol. 76:1333–1339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsieh CC, Hernandez-Ledesma B and de Lumen
BO: Lunasin, a novel seed peptide, sensitizes human breast cancer
MDA-MB-231 cells to aspirin-arrested cell cycle and induced
apoptosis. Chem Biol Interact. 186:127–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baysan A, Yel L, Gollapudi S, Su H and
Gupta S: Arsenic trioxide induces apoptosis via the mitochondrial
pathway by upregulating the expression of Bax and Bim in human B
cells. Int J Oncol. 30:313–318. 2007.PubMed/NCBI
|
|
35
|
Uren RT, Dewson G, Chen L, et al:
Mitochondrial permeabilization relies on BH3 ligands engaging
multiple prosurvival Bcl-2 relatives, not Bak. J Cell Biol.
177:277–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen M, Guerrero AD, Huang L, et al:
Caspase-9-induced mitochondrial disruption through cleavage of
anti-apoptotic BCL-2 family members. J Biol Chem. 282:33888–33895.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krystal GW, Sulanke G and Litz J:
Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks
growth, promotes apoptosis, and enhances sensitivity of small cell
lung cancer cells to chemotherapy. Mol Cancer Ther. 1:913–922.
2002.PubMed/NCBI
|
|
38
|
Liu X, Shi Y, Giranda VL and Luo Y:
Inhibition of the phosphatidylinositol 3-kinase/Akt pathway
sensitizes MDA-MB468 human breast cancer cells to cerulenin-induced
apoptosis. Mol Cancer Ther. 5:494–501. 2006. View Article : Google Scholar : PubMed/NCBI
|