Introduction
Caffeic acid esters are a component of propolis and
exert various biological activities (1), such as antioxidant (2) and anti-inflammatory effects (3). Caffeic acid derivatives, such as
caffeic acid phenethyl ester (CAPE), have anticancer effects
(4). Previous studies have
indicated that CAPE exerts cytotoxicity via induction of apoptosis
through caspase activation and mitochondrial-mediated pathways
(5,6). Apoptosis induced by mitochondrial
damage involves a decrease in the permeability of the mitochondrial
membrane and is directly regulated by the Bcl-2 protein (7). Downregulation of the Bcl-2 protein
might be a useful method to modulate apoptosis and thereby increase
the chemotherapeutic effect of anticancer drugs (8).
We previously demonstrated that octyl caffeate, a
compound semi-derived from caffeic acid, was 6-fold more potent
that CAPE in terms of cytotoxicity against human leukemia U937
cells (9). Further, previous
studies have demonstrated that undecyl caffeate (caffeic acid
undecyl ester, CAUE), a new caffeic acid derivative, was a highly
potent inhibitor of lipopolysaccharide-induced nitric oxide
production in RAW 264.7 mouse macrophage cells (10). However, the cytotoxic effects and
apoptotic mechanisms by which CAUE act remain unclear. Therefore,
the goal of the present study was to investigate the cytotoxicity
of CAUE and its parent compound, CAPE, and to characterize the
mechanisms by which they induce apoptosis in the human B cell
leukemia cell line NALM-6.
Materials and methods
Materials and cell culture
CAUE were prepared as previously described (10). CAPE and all other reagents, unless
stated, were of the highest grade available and were purchased from
either Sigma (St. Louis, MO, USA) or Wako Pure Chemical Industries,
Ltd. (Osaka, Japan). Normal human lymphocytes were provided by
healthy volunteers and prepared by Ficoll-Paque™ PLUS (Amersham,
Arlington Heights, IL, USA), according to manufacturer’s protocol.
Research protocols were approved by the Ethics Committee of Tohoku
Pharmaceutical University. Human B cell leukemia NALM-6 cells were
supplied by the Cell Resource Center for Biomedical Research,
Tohoku University (Sendai, Japan). All cell culture reagents and
small interfering RNAs (siRNAs) were obtained from Invitrogen Corp.
(Carlsbad, CA, USA). Cells were routinely cultured using standard
methods as described in our previous studies (11,12).
MTT assay
Cytotoxicity was assessed by the MTT [3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] assay,
with slight modification of our previously described method
(13). Briefly, cells were seeded
from 1 to 4×104 cells in 96-well plates. Cells were
incubated with CAUE at indicated concentrations for 24 to 72 h,
followed by the addition of 10 μl of MTT (5 mg/ml saline) to each
well. The sample was incubated for 90 min at 37°C, the supernatant
was aspirated, and the cells were lysed and solubilized by the
addition of 100 μl of 0.04 N HCl in isopropanol. The absorbance of
each well was determined at 590 nm using an Inter-med model NJ-2300
Microplate Reader. Control cells were treated with 0.5% dimethyl
sulfoxide (DMSO) as CAUE vehicle. The viability of cells was
calculated by the following formula: absorbance in treated
sample/absorbance in control ×100%.
Assessment of apoptosis
Detection of apoptotic cells was estimated by
nuclear morphological observation and performed by flow cytometry
using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA,
USA). CellQuest Pro software (Becton Dickinson) was then used to
analyze hypodiploid cells (apoptotic sub-G1 peak), with slight
modification of our previously described method (11).
Western blotting
The effects of cellular signal transduction on
protein expression by CAUE-induced apoptosis or confirmation of
Bcl-2 siRNA knockdown assay were estimated by western blotting
(13). Briefly, after incubation of
cells with the indicated concentration of CAUE, the cells were
washed with phosphate buffered saline (PBS) and lysed. The protein
concentration was measured by the BCA™ protein assay kit (Thermo
Fisher Scientific, Inc. Rockford, IL, USA), according to the
instructions provided by the manufacturer. Samples of each protein
(30 μg) were loaded onto a 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gel. After electrophoresis, the protein was
transferred to a polyvinylidene difluoride (PVDF) membrane. The
protein was blocked with blocking solution (25 mM Tris-HCl, pH 7.4,
137 mM NaCl, 2.68 mM KCl and 5% skim milk) for 1 h and incubated
with antibody overnight at 4°C. The membrane was then washed with
blocking solution without skim milk and incubated with horseradish
peroxidase-linked secondary antibody for 1 h. After washing again
with wash buffer, protein levels were analyzed by enhanced
chemiluminescence with an ECL Western Blotting Detection system
(Amersham). All antibodies used were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA).
Measurement of the mitochondrial membrane
potential
After CAUE treatment, NALM-6 cells were incubated
with 0.3 μM rhodamine 123 (R123) for 15 min at 37°C. Cells were
then washed with PBS and collected by centrifugation and the
collected cells were suspended in 500 μl of PBS. Fluorescence
intensities of R123 were analyzed on a FACSCalibur flow cytometer
set at 488 nm excitation (FL1 blue laser).
Bcl-2 siRNA knockdown assay
siRNA-Bcl-2 (siBcl-2) and siRNA-control [as
non-targeting siRNA; negative control (Neg)] were transfected into
NALM-6 cells using the Neon™ Transfection System (Invitrogen Corp.)
according to the instructions provided by the manufacturer.
Briefly, 40–60% confluent cells were harvested and then washed
twice with PBS. Then, 2×105 cells containing 50 pmol of
each siRNA in 10 μl Neon tip were electroporated at 1380V, pulse
length of 10 msec, and 3 pulse times performed. After culture
overnight without antibiotics, samples were used for assessment of
Bcl-2 expression or detection of CAUE-induced apoptosis. The mRNA
level of Bcl-2 (GenBank accession no. NM_000633.2) was quantified
using the real-time polymerase chain reaction (qPCR) with a Light
Cycler (Roche, Basel, Switzerland). Briefly, total RNA was
extracted from each cell line with the Isogen reagent (Nippon Gene,
Tokyo, Japan), and 0.1 μg of total RNA was then reverse transcribed
to single-strand cDNA using the ReverTra Ace® qPCR RT
kit (Toyobo, Osaka, Japan). Aliquots of the cDNA preparations were
subjected to qPCR analysis using SYBR® Premix Ex Taq™
(Takara Bio, Shiga, Japan) to quantify the expression of each
target gene and the internal standard, β-actin (GenBank accession
no. NM_001101.3), using Light Cycler. The Takara Perfect Real- time
Primers (Takara Bio) were used as primer pairs. The results of all
assays were checked against melting curves to confirm the presence
of single PCR products. Knockdown of Bcl-2 protein was validated by
western blotting, as described above.
Statistical analysis
Statistical analysis was performed using a one-way
analysis of variance (ANOVA) followed by the Williams’ type
multiple comparison test or a Bonferroni test among multiple
groups. A p-value of <0.01 was considered significant.
Results
Cytotoxic effect of CAUE
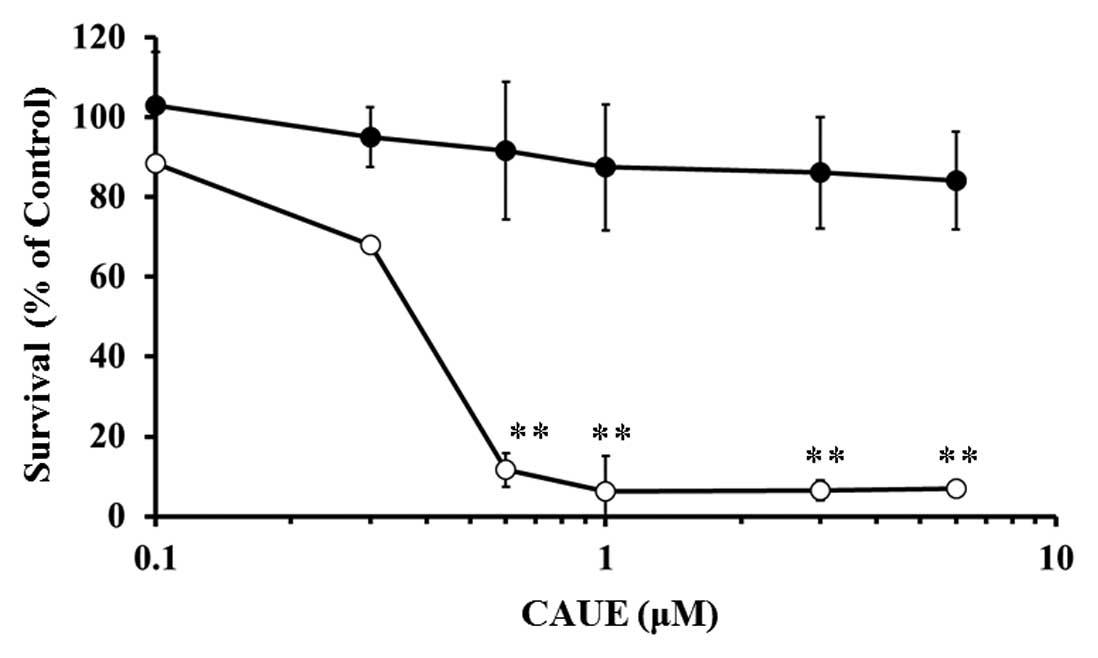
First, we examined the cytotoxic effect of CAUE
incubation for 24 h on normal human lymphocytes or B cell leukemia
NALM-6 cells via the MTT assay (Fig.
1). CAUE treatment in concentrations >0.3 μM resulted in
marked reduction in cell survival of NALM-6 cells but had no
significant effect on normal lymphocytes, even at 6 μM. The 50%
inhibitory concentration (IC50) of CAUE incubation for
24 and 72 h on NALM-6 was 0.33 and 0.16 μM, respectively. The
IC50 of incubation with CAPE for 24 and 72 h in NALM-6
cells was 5.39 and 1.74 μM, respectively. Thus, CAUE was a more
potent cytotoxic agent than CAPE on NALM-6 cells, yet did not exert
a cytotoxic effect on normal cells.
Induction of apoptotic cells by CAUE
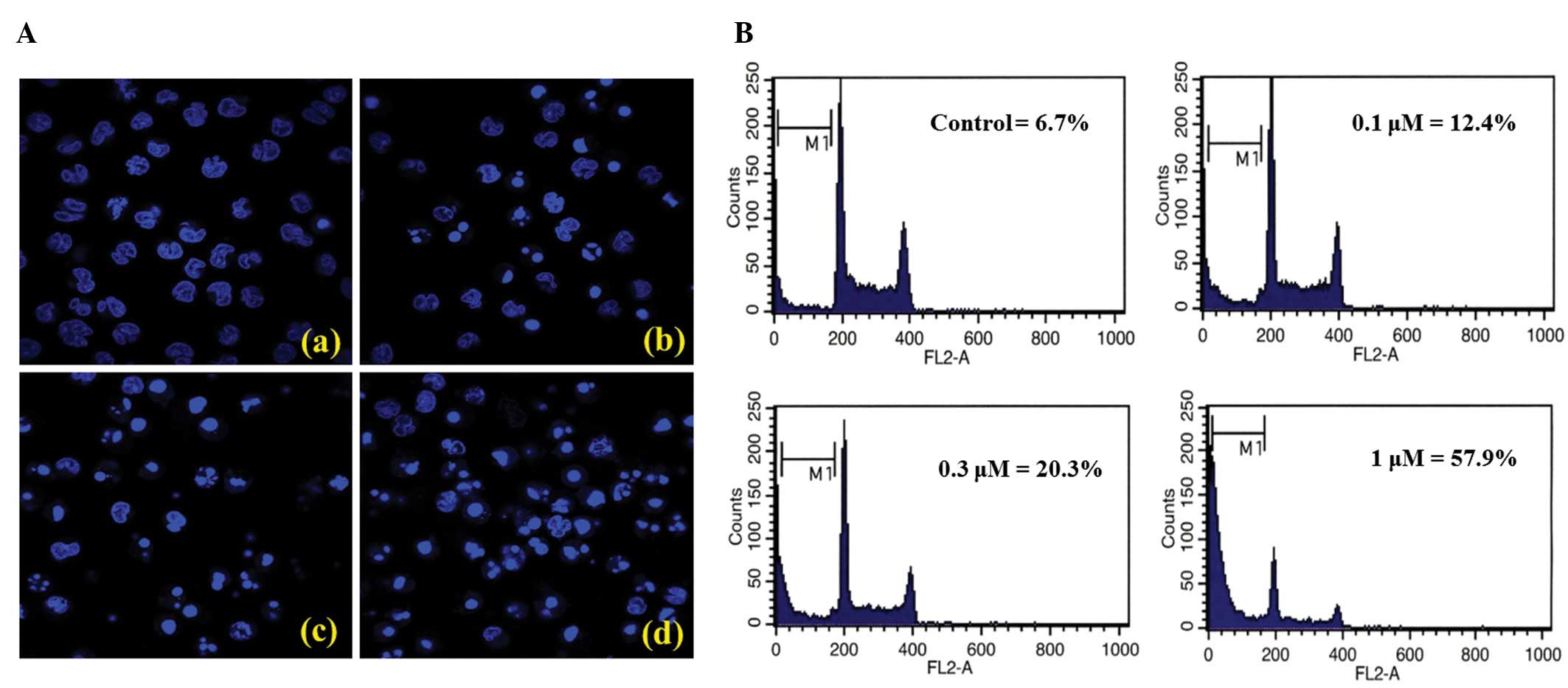
Next, we examined whether CAUE caused apoptosis of
NALM-6 cells by assessing nuclear morphological change and the
presence of hypodiploid cells (sub-G1 peak) using flow cytometry.
Overnight incubation of NALM-6 cells in CAUE produced a
concentration-dependent increase in the typical phenotype of
apoptosis, including nuclear chromatin condensation, apoptotic
bodies (Fig. 2A) as well as an
increase in hypodiploid cells (Fig.
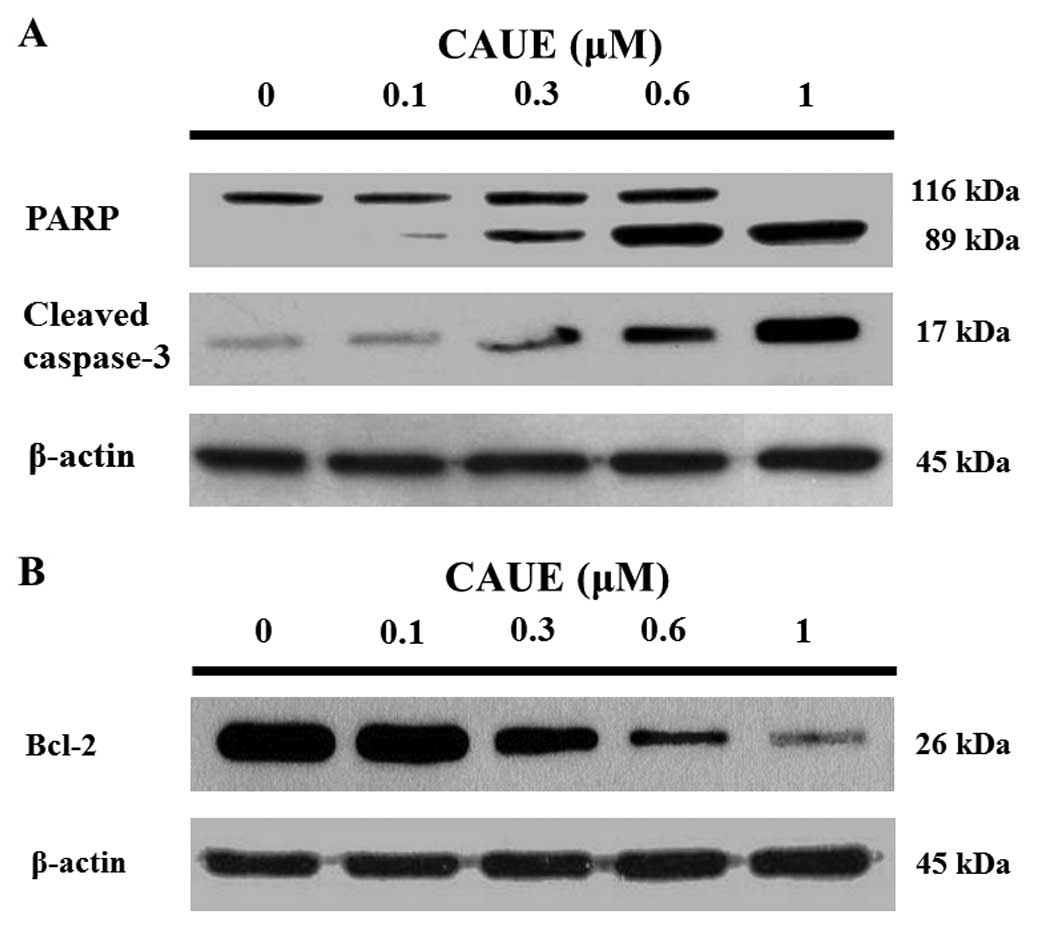
2B). Further, western blotting demonstrated
concentration-dependent cleavage of poly (ADP-ribose) polymerase
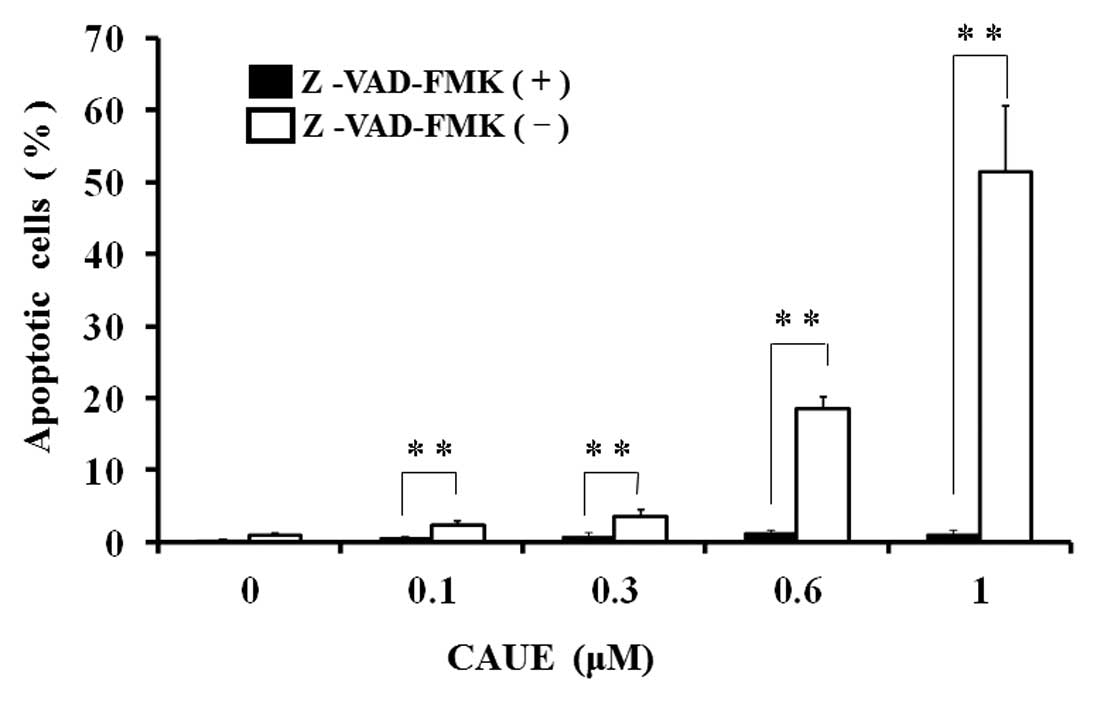
and caspase-3 protein in response to CAUE (Fig. 3A). Z-VAD-FMK, a broad spectrum
caspase inhibitor, completely inhibited CAUE-induced apoptosis
(Fig. 4), which indicated that CAUE
induced apoptosis in a caspase-dependent manner.
Mitochondrial damage by CAUE and effect
of Bcl-2 knockdown
To characterize the involvement of mitochondrial
damage in CAUE-induced apoptosis in NALM-6 cells, we used western
blotting to analyze Bcl-2 protein and a flow cytometry assay to
analyze mitochondrial membrane potential by uptake of R123, a
substrate of mitochondrial membrane permeability. Bcl-2 protein
levels were downregulated in a concentration-dependent manner after
incubation with CAUE for 6 h (Fig.
3B). Likewise, incubation with CAUE for 6 h significantly
reduced mitochondrial membrane potential, and this effect was
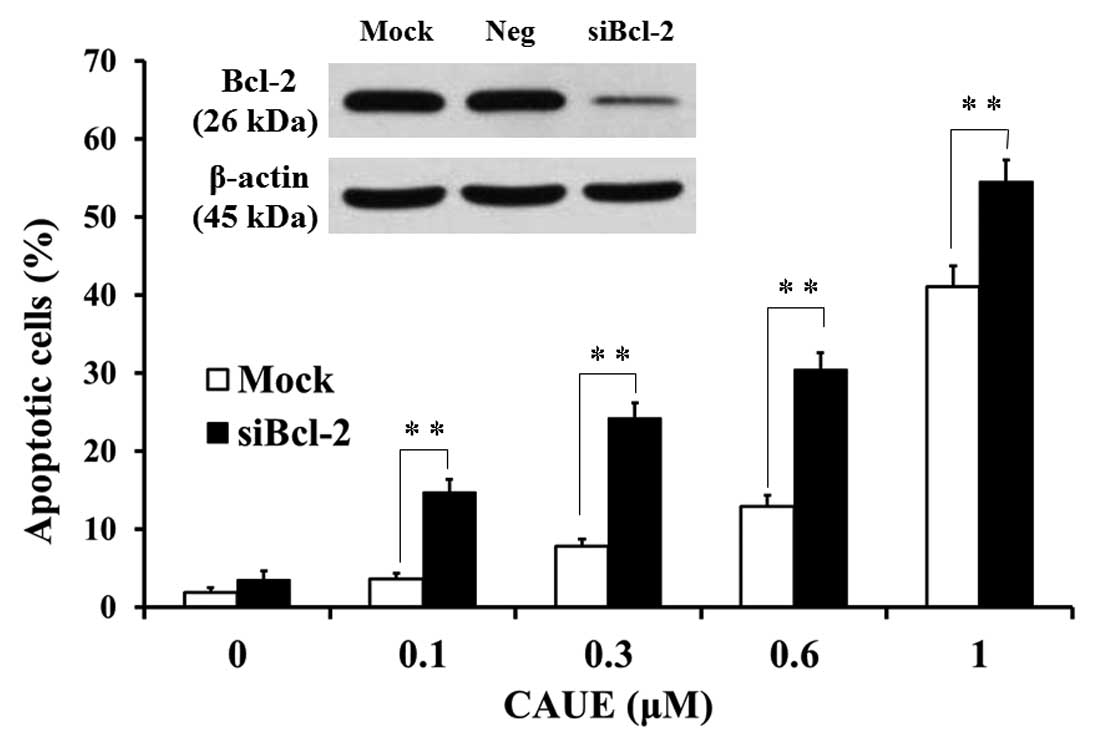
potentiated by incubation with CAUE for 6 h (Fig. 5A and B). In response to siRNA of
Bcl-2 (siBcl-2), Bcl-2 mRNA levels in NALM-6 cells were reduced to
15% of levels seen in cells that underwent mock treatment, while
there was no change in Bcl-2 mRNA levels in the negative control
group (Neg). Further, Bcl-2 protein levels were downregulated in
response to siBcl-2, but did not change in the mock or Neg group
(Fig. 6, inset). CAUE significantly
potentiated the induction of apoptosis in cells treated with
siBcl-2 when compared with the mock group (Fig. 6). These data suggest that induction
of apoptosis by CAUE was mediated by mitochondrial damage and Bcl-2
downregulation.
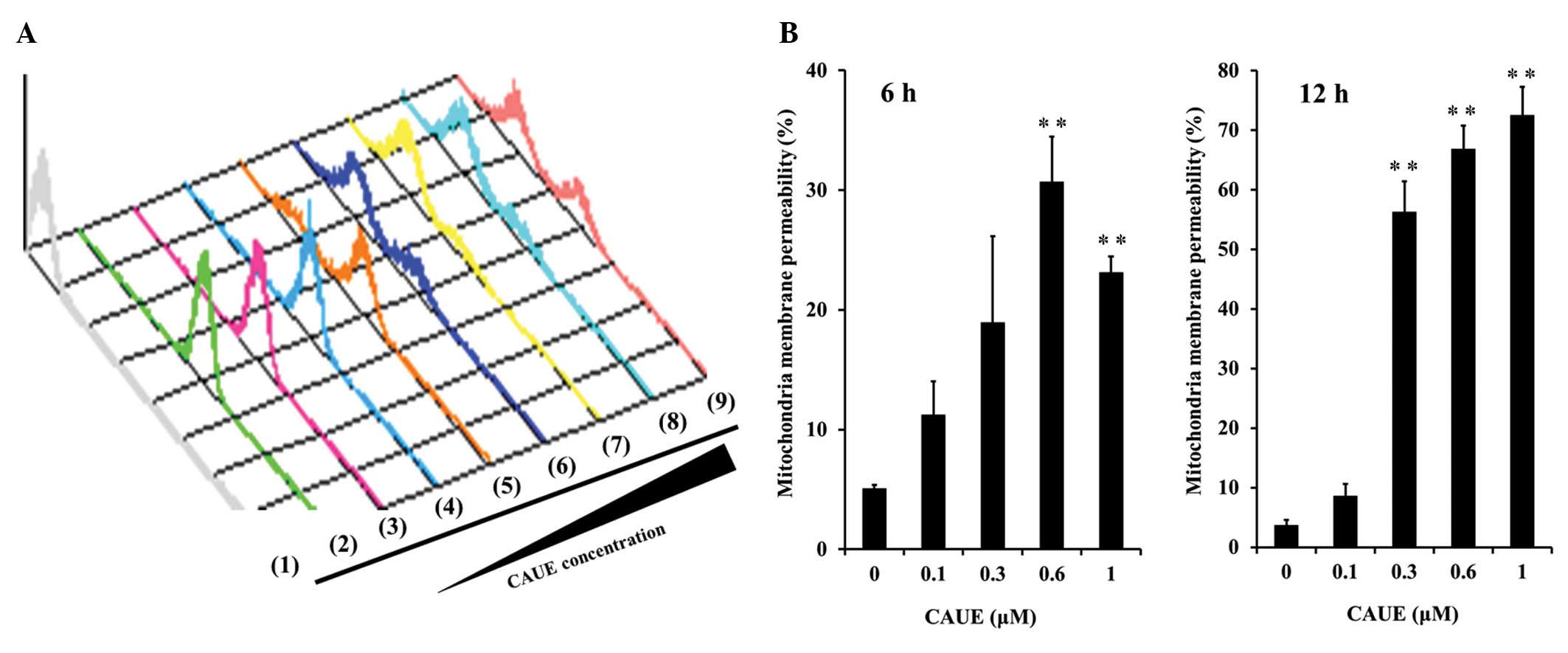 | Figure 5Effects of CAUE on mitochondrial
membrane potential. Mitochondrial membrane potential was assessed
via uptake of R123, a substrate of mitochondrial membrane
permeability, on flow cytometry assay. (A) A typical histogram of
data showing a decrease in uptake of R123 after incubation with
CAUE for 12 h, no incubation with R123 (1; gray), a single
incubation with 0.3 μM R123 (2; green), and CAUE at 0 (3; magenta),
0.1 (4; blue), 0.3 (5; orange), 0.6 (6; dark blue), 1 (7; yellow),
3 (8; light blue), or 6 μM (9; pink). Experiments are
representative of a minimum of three separate experiments. (B)
Quantitative results of mitochondrial membrane permeability
indicating fluorescent intensity of R123 uptake. The results are
means ± SEM of three individual studies. **p<0.01
compared with the control group under the indicated culture
condition. |
Discussion
Chemotherapy is a powerful tool for the treatment of
various cancers, including hematologic malignancies. In particular,
lymphoblastic leukemia is highly aggressive, but frequently curable
with current therapy (14). The
NALM-6 cell line was originally established from the peripheral
blood of a patient with acute lymphoblastic leukemia (15). We employed this cell line in the
present study and assessed the cytotoxic effect of CAUE, a new
caffeic acid derivative, to determine its potential utility as a
treatment for lymphoblastic leukemia. As shown in Fig. 1, CAUE exerted
concentration-dependent cytotoxicity in NALM-6 cells but did not
affect normal lymphocytes. The cytotoxic action of the parental
compound of CAUE, CAPE, has been investigated in tumor cells but
not in normal cells (16,17). The present data suggest that CAUE
and CAPE have similar biologic activity, although CAUE was more
potent than CAPE, as demonstrated by its 10-fold higher cytotoxic
IC50. These results therefore suggest that CAUE is a
powerful chemotherapeutic reagent and has selective action on
leukemia cells.
Apoptosis is an important mechanism by which
anticancer drugs induce an antitumor response (18). The core effectors of apoptosis
encompass proteolytic enzymes of the caspase family, which reside
as latent precursors in most nucleated metazoan cells (19). A majority of studies on apoptosis
are based on the assumption that caspase precursors are activated
by cleavage, a common mechanism for most protease zymogen
activations. Nuclear poly (ADP-ribose) polymerase (PARP) is one of
the main cleavage targets of caspase-3 and appears to be involved
in DNA repair in response to environmental stress (20). Full length PARP (116 kDa) cleave to
a specific 89 kDa form observed during apoptosis (21). As shown in Figs. 2 and 3, treatment with CAUE resulted in
induction of apparent apoptotic features in NALM-6 cells, including
cleaved PARP and activated caspase-3. Further, caspase inhibitor
completely blocked induction of apoptosis by CAUE (Fig. 4). These results suggest that
activation of caspase plays a pivotal role in CAUE-induced
apoptosis in NALM-6.
Apoptosis involves two major pathways, the intrinsic
and extrinsic pathway. The intrinsic pathway involves mitochondria
as initiators of cell death. In this study, the cytotoxic effect of
CAUE was estimated by MTT assay as a reflection of mitochondrial
activity (Fig. 1). It is reasonable
to assume that CAUE-induced apoptosis involves a decrease in
mitochondrial activity. To characterize the apoptotic mechanism by
CAUE, the present study focused on the intrinsic pathway of
apoptosis induction, which involves mitochondrial damage. As shown
in Fig. 5, CAUE induced a
concentration- and time-dependent decrease in mitochondrial
membrane potential. Induction of apoptosis by CAUE has showed
incubation following 6 h (Fig.
2A-b). Incubation with CAUE for 6 h also downregulated Bcl-2
expression (Fig. 3B). These results
suggest that CAUE-induced apoptosis was caused by mitochondrial
damage. As shown in Fig. 6,
CAUE-induced apoptosis was enhanced in the Bcl-2 knockdown
condition induced by siRNA. Several randomized, controlled, phase
III trials have evaluated the utility of antisense Bcl-2 combined
with standard chemotherapy for the treatment of patients with
chronic lymphocytic leukemia, multiple myeloma, malignant melanoma,
or non-small cell lung carcinoma (8). However, attempts to target Bcl-2
therapeutically using antisense technology to inhibit protein
translation have not significantly improved outcomes for cancer
patients, although improved oligonucleotide design may potentially
enhance the efficacy of this approach (22). Various Bcl-2 inhibitors for
anti-apoptotic Bcl-2 proteins are markedly different in terms of
potency and selectivity (23). The
present results suggest that CAUE is a potent inhibitor of Bcl-2
and may be an effective chemotherapy for leukemia.
In conclusion, CAUE has a potent cytotoxic effect in
NALM-6 cells but not on normal lymphocytes, CAUE-induced apoptosis
is mediated by mitochondrial damage and caspase. Thus, CAUE may be
an effective chemotherapy for leukemia, and decreases in Bcl-2
expression via co-treatment with other chemotherapeutical reagent
may enhance the chemotherapeutic action of CAUE on leukemia
patients.
References
|
1
|
Banskota AH, Tezuka Y, Midorikawa K,
Matsushige K and Kadota S: Two novel cytotoxic benzofuran
derivatives from Brazilian propolis. J Nat Prod. 63:1277–1279.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burdock GA: Review of the biological
properties and toxicity of bee propolis (propolis). Food Chem
Toxicol. 36:347–363. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banskota AH, Nagaoka T, Sumioka LY, et al:
Antiproliferative activity of the Netherlands propolis and its
active principles in cancer cell lines. J Ethnopharmacol. 80:67–73.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe MA, Amarante MK, Conti BJ and
Sforcin JM: Cytotoxic constituents of propolis inducing anticancer
effects: a review. J Pharm Pharmacol. 63:1378–1386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC
and Tseng TH: Involvement of tumor suppressor protein p53 and p38
MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma
cells. Biochem Pharmacol. 66:2281–2289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin UH, Song KH, Motomura M, et al:
Caffeic acid phenethyl ester induces mitochondria-mediated
apoptosis in human myeloid leukemia U937 cells. Mol Cell Biochem.
310:43–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta S, Kass GE, Szegezdi E and Joseph B:
The mitochondrial death pathway: a promising therapeutic target in
diseases. J Cell Mol Med. 13:1004–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim R, Emi M, Tanabe K and Toge T:
Therapeutic potential of antisense Bcl-2 as a chemosensitizer for
cancer therapy. Cancer. 101:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ujibe M, Kanno S, Osanai Y, et al:
Octylcaffeate induced apoptosis in human leukemia U937 cells. Biol
Pharm Bull. 28:2338–2341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uwai K, Osanai Y, Imaizumi T, Kanno S,
Takeshita M and Ishikawa M: Inhibitory effect of the alkyl side
chain of caffeic acid analogues on lipopolysaccharide-induced
nitric oxide production in RAW264.7 macrophages. Bioorg Med Chem.
16:7795–7803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanno S, Maeda N, Tomizawa A, Yomogida S,
Katoh T and Ishikawa M: Involvement of p21waf1/cip1
expression in the cytotoxicity of the potent histone deacetylase
inhibitor spiruchostatin B towards susceptible NALM-6 human B cell
leukemia cells. Int J Oncol. 40:1391–1396. 2012.
|
|
12
|
Watanabe K, Kanno S, Tomizawa A, Yomogida
S and Ishikawa M: Acacetin induces apoptosis in human T cell
leukemia Jurkat cells via activation of a caspase cascade. Oncol
Rep. 27:204–209. 2012.PubMed/NCBI
|
|
13
|
Kanno S, Higurashi A, Watanabe Y, Shouji
A, Asou K and Ishikawa M: Susceptibility to cytosine arabinoside
(Ara-C)-induced cytotoxicity in human leukemia cell lines. Toxicol
Lett. 152:149–158. 2004.PubMed/NCBI
|
|
14
|
Cortelazzo S, Ponzoni M, Ferreri AJ and
Hoelzer D: Lymphoblastic lymphoma. Crit Rev Oncol Hematol.
79:330–343. 2011. View Article : Google Scholar
|
|
15
|
Hurwitz R, Hozier J, LeBien T, et al:
Characterization of a leukemic cell line of the pre-B phenotype.
Int J Cancer. 23:174–180. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grunberger D, Banerjee R, Eisinger K, et
al: Preferential cytotoxicity on tumor cells by caffeic acid
phenethyl ester isolated from propolis. Experientia. 44:230–232.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Najafi MF, Vahedy F, Seyyedin M,
Jomehzadeh HR and Bozary K: Effect of the water extracts of
propolis on stimulation and inhibition of different cells.
Cytotechnology. 54:49–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaufmann SH and Vaux DL: Alterations in
the apoptotic machinery and their potential role in anticancer drug
resistance. Oncogene. 22:7414–7430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Satoh MS and Lindahl T: Role of
poly(ADP-ribose) formation in DNA repair. Nature. 356:356–358.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tewari M, Quan LT, O’Rourke K, et al:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate
poly(ADP-ribose) polymerase. Cell. 81:801–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheson BD: Oblimersen for the treatment of
patients with chronic lymphocytic leukemia. Ther Clin Risk Manag.
3:855–870. 2007.PubMed/NCBI
|
|
23
|
Vogler M, Dinsdale D, Dyer MJ and Cohen
GM: Bcl-2 inhibitors: small molecules with a big impact on cancer
therapy. Cell Death Differ. 16:360–367. 2009. View Article : Google Scholar : PubMed/NCBI
|