Introduction
Regulation of the cell cycle is important in
cellular proliferation, and therefore the loss of cell cycle
control is involved in carcinogenesis (1). Cyclins and cyclin-dependent kinases
(CDKs), in association with each other, play pivotal roles in
promoting the transition of cells from the G1 phase to the S phase
of the cell cycle by phosphorylating the tumor-suppressor
retinoblastoma (RB) protein (2,3).
Cyclin-CDK complex activation is negatively regulated by CDK
inhibitors (CKIs). The first family of CKIs, referred to as the
CIP/KIP family, consists of p21WAF1/Cip1,
p27Kip1 and p57Kip2. Each member inhibits a
broader spectrum of cyclin/CDK complexes including cyclin E/A-CDK2
and cyclin D-CDK4/6 (4,5). The second family of CKIs is called the
INK4 family, which comprises p15INK4b,
p16INK4a, p18INK4c and p19INK4d.
These molecules are specific inhibitors of cyclin D-CDK4/6
complexes (5,6). Many studies have shown that RB is
directly, or indirectly, inactivated in most human cancers.
Abnormalities leading to malignancies frequently relate to loss or
dysfunction of tumor-suppressor molecules including members of
these two CKI families, which activate the RB pathway (5,7).
Perillyl alcohol (POH) is a naturally occurring
monoterpene found in the essential oils of cherry, lemongrass,
gingergrass, cranberry, perilla, mint, lavender, sage, wild
bergamot, caraway and celery seeds (8,9). It
has been shown that POH exerts antitumor activity against malignant
tumor cells in vitro and in vivo. POH inhibits the
growth of various types of malignant tumor cells in vitro
through blockade of proliferation, angiogenesis and migration, and
induction of differentiation and apoptosis (10–14).
Regarding the antiproliferative activity of POH, this monoterpene
is reported to cause cell cycle arrest at the G1 phase through
downregulation of cyclin D1 and upregulation of
p21WAF1/Cip1 in murine mammary transformed cells or
through upregulation of both p21WAF1/Cip1 and
p27Kip1 in human pancreatic adenocarcinoma cells
(15,16). Moreover, POH significantly inhibits
the growth of mammary and liver tumors in rodent models (12,17).
Based on these preclinical data, clinical studies using POH have
commenced in patients with advanced malignancies. However, two
phase II studies in patients with refractory metastatic breast
cancer and metastatic androgen-independent prostate cancer reported
that no objective responses were observed (18,19).
On the other hand, a recent clinical study showed that intranasal
administration of POH increased the overall survival of patients
with recurrent glioblastoma (20).
In this study, we demonstrated that POH caused G1
arrest in malignant tumor cells through p15INK4b and
p21WAF1/Cip1 induction leading to the dephosphorylation
of the RB protein. We suggest that not only p21WAF1/Cip1
but also p15INK4b could be important molecular targets
that mediate the antitumor effects of POH.
Materials and methods
Cell culture and reagents
Human immortalized keratinocyte HaCaT cells were a
kind gift from Dr N.E. Fusenig, German Cancer Research Center,
Heidelberg, Germany. Human colon cancer cell lines HT-29 and SW620
were obtained as cell lines of NCI-60 from the NCI Developmental
Therapeutics Program (NCI DTP). These cells were maintained in DMEM
supplemented with 10% fetal bovine serum, 4 mM L-glutamine, 100
U/ml penicillin and 100 μg/ml streptomycin. Cell cultures were
incubated at 37°C in a humidified atmosphere with 5%
CO2. POH was purchased from Wako (320-52902; Osaka,
Japan), dissolved in dimethyl sulfoxide (DMSO) and diluted to the
final concentrations in each volume of culture medium used.
Growth inhibition assay
Cells were plated at 5×104 cells in
12-well plates. One day after inoculation of cells, various
concentrations of POH were added to the culture medium. From the
first to the second day after plating, the numbers of viable cells
were counted using a trypan blue dye exclusion test.
Cell cycle analysis
For flow cytometry, 5×104 cells were
plated in 12-well plates. One day later, unsynchronized cells were
exposed to 1.0 mM POH for 24 h. The cells were then treated with
Triton X-100 and RNase A, and their nuclei were stained with
propidium iodide before DNA content was measured using a BD FACS
Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). At
least 10,000 cells were counted and the ModFit LD V2.0 software
package (BD Biosciences) was used to analyze the data.
Protein isolation and western blot
analysis
Cells were lysed in SDS buffer [50 mM Tris-HCl (pH
7.5), 1% SDS]. The protein extract was then boiled for 5 min and
loaded onto a 12% (for p15INK4b and
p21WAF1/Cip1 detection), a 10% (for α-tubulin detection)
or a 5% (for RB detection) polyacrylamide gel, subjected to
electrophoresis and transferred to a nitrocellulose membrane. The
following primary antibodies were used: anti-p15INK4b
(sc-612; Santa Cruz Biotechnology, Inc., Santa Cruz, CA)
anti-p21WAF1/Cip1 (sc-397; Santa Cruz Biotechnology,
Inc.), anti-p27Kip1 (sc-528; Santa Cruz Biotechnology,
Inc.), anti-pRB (554136; BD Pharmingen) and anti-α-tubulin (CP06;
Calbiochem, San Diego, CA). The signal was then developed with
Chemi-Lumi One (Nacalai Tesque, Kyoto, Japan) or Immobilon Western
(EMD Millipore, MA).
RNA isolation and real-time reverse
transcription (RT)-PCR
Real-time RT-PCR analysis was performed as
previously described (21). The
GeneAmp5700 (Applied Biosystems, CA) was used to quantify the
expression level of p15INK4b and p21WAF1/Cip1
mRNAs and normalized to β2MG mRNA. Real-time RT-PCR primer probes
for p15INK4b (Hs00394703), p21WAF1/Cip1
(Hs00355782) and β2MG (Hs99999907) were purchased from Applied
Biosystems.
Transfection and luciferase assay
The p15INK4b-luciferase fusion plasmid
was described previously (22).
HaCaT cells were seeded at 1.6×105 cells/well in 6-well
plates. One day later, cells were transfected with the plasmid or
pGVB2 (a vacant control; 2.5 μg) using Lipofectamine LTX and Plus
reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s
instructions. After 24 h, transfected cells were treated with POH
at various concentrations for 12 h and then harvested. Luciferase
assays were then performed using luciferase assay reagents
(Promega, Madison, WI) and a luminometer.
Small interfering RNA (siRNA)
The p15INK4b (CDKN2B HSS141533) and the
negative control (Negative Universal Control High #3) siRNAs were
purchased from Invitrogen. The p21WAF1/Cip1 siRNA (s415)
was purchased from Ambion (Carlsbad, CA). One day before
transfection, HaCaT cells were seeded at 9×104
cells/well in 6-well plates without antibiotics. The
p15INK4b, p21WAF1/Cip1 or a negative control
siRNA (20 nM) was transfected into cells using Lipofectamine
RNAiMax (Invitrogen) according to the manufacturer’s instructions.
Twenty-four hours after the transfection, cells were treated with
1.0 mM POH for 24 h and then harvested.
Statistical analysis
Statistical evaluation of the data was performed
using the Student’s t-test for simple comparison between treatments
and controls. p<0.05 was considered to indicate a statistically
significant difference.
Results
Cell growth inhibition and G1 arrest by
POH in HaCaT cells
We first investigated the antiproliferative effects
of POH in human immortalized keratinocyte HaCaT cells. The growth
of HaCaT cells was measured in the presence or absence of various
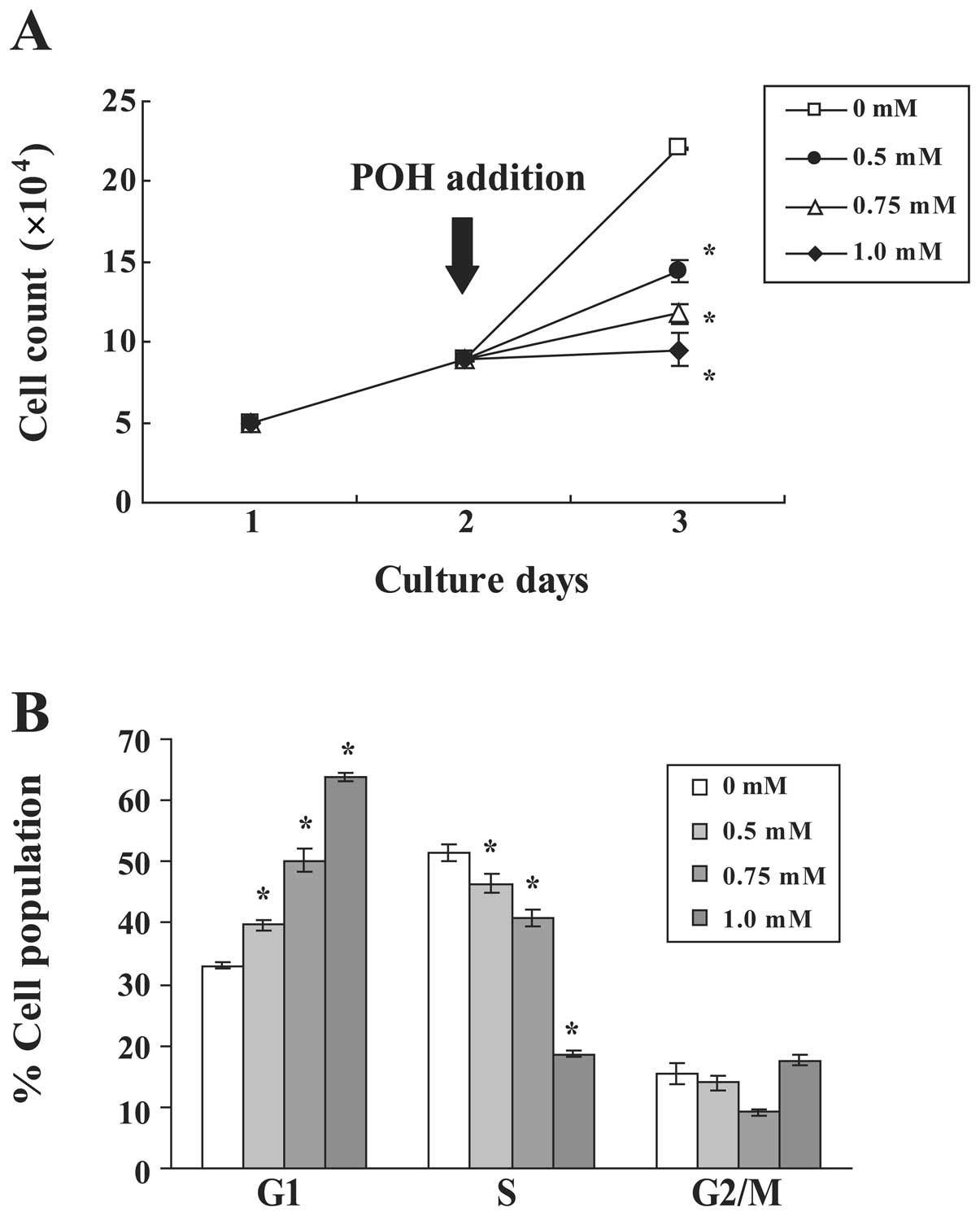
concentrations of POH (Fig. 1A).
POH inhibited the growth of HaCaT cells in a dose-dependent manner.
Notably, 1.0 mM POH had a cytostatic effect. To examine the effects
of POH on cell cycle progression, the DNA content of cell nuclei
was measured by flow cytometry. POH increased the percentage of
cells in the G1 phase and decreased the percentage of cells in the
S phase in a dose-dependent manner (Fig. 1B). These data demonstrate that POH
arrests the HaCaT cell cycle at the G1 phase.
p15INK4b and
p21WAF1/Cip1 induction and hypophosphorylation of the RB
protein by POH in HaCaT cells
We aimed to elucidate whether cell cycle-associated
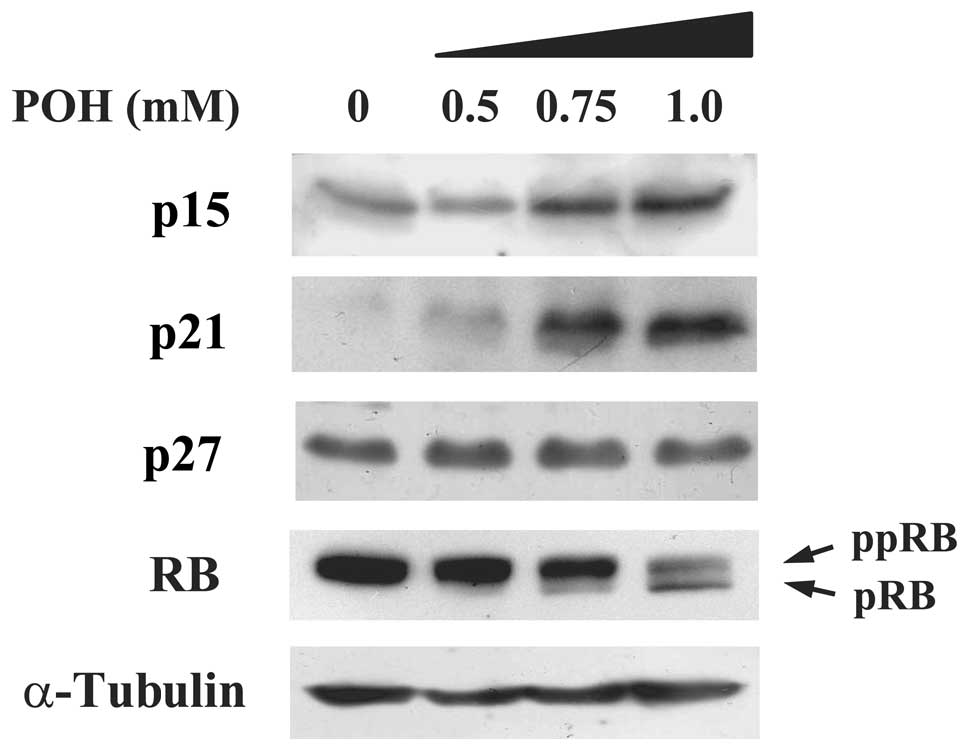
molecules are influenced by treatment with POH in HaCaT cells. We
discovered that POH increased p15INK4b protein
expression in a dose-dependent manner (Fig. 2). Additionally, POH increased
p21WAF1/Cip1, which is consistent with previous studies
(15,16). Of note, POH had no effect on the
protein expression levels of p27Kip1. Both
p15INK4b and p21WAF1/Cip1 are members of the
CKI families and subsequently dephosphorylate the RB protein
leading to G1 cell cycle arrest. We, therefore, examined whether
POH alters the phosphorylation status of RB. A hyperphosphorylated
form of the RB protein was converted into a hypophosphorylated form
by POH treatment in a dose-dependent manner (Fig. 2). Taken together, these results
indicate that POH elevates p15INK4b and
p21WAF1/Cip1 protein levels, and subsequently converts a
hyperphosphorylated form of the RB protein into a
hypophosphorylated form in HaCaT cells.
Mechanisms of p15INK4b and
p21WAF1/Cip1 induction by POH in HaCaT cells
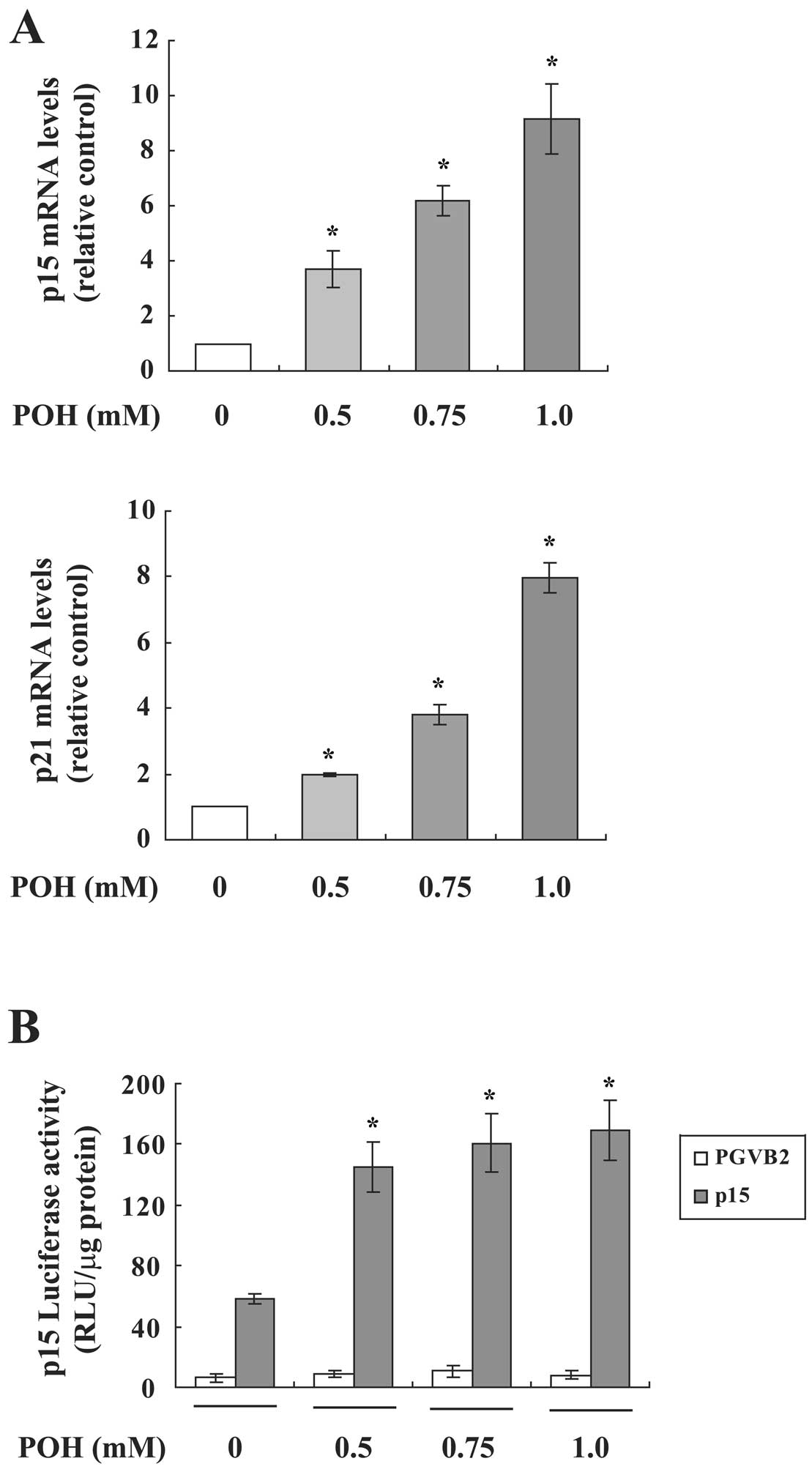
We next investigated whether POH affects
p15INK4b and p21WAF1/Cip1 mRNA expression in
HaCaT cells using real-time RT-PCR. Both mRNAs were significantly
increased by POH in a dose-dependent manner (Fig. 3A). Since these mRNAs are induced by
POH, we analyzed the effect of POH on the promoter activity using
p15INK4b or p21WAF1/Cip1 promoter-luciferase
fusion plasmids in a transient assay. POH upregulated the promoter
activity of p15INK4b (Fig.
3B), however, POH did not elevate that of
p21WAF1/Cip1 (data not shown). These results suggest
that p15INK4b and p21WAF1/Cip1 are
differentially regulated by POH.
p15INK4b and
p21WAF1/Cip1 are important targets of POH-induced G1
arrest
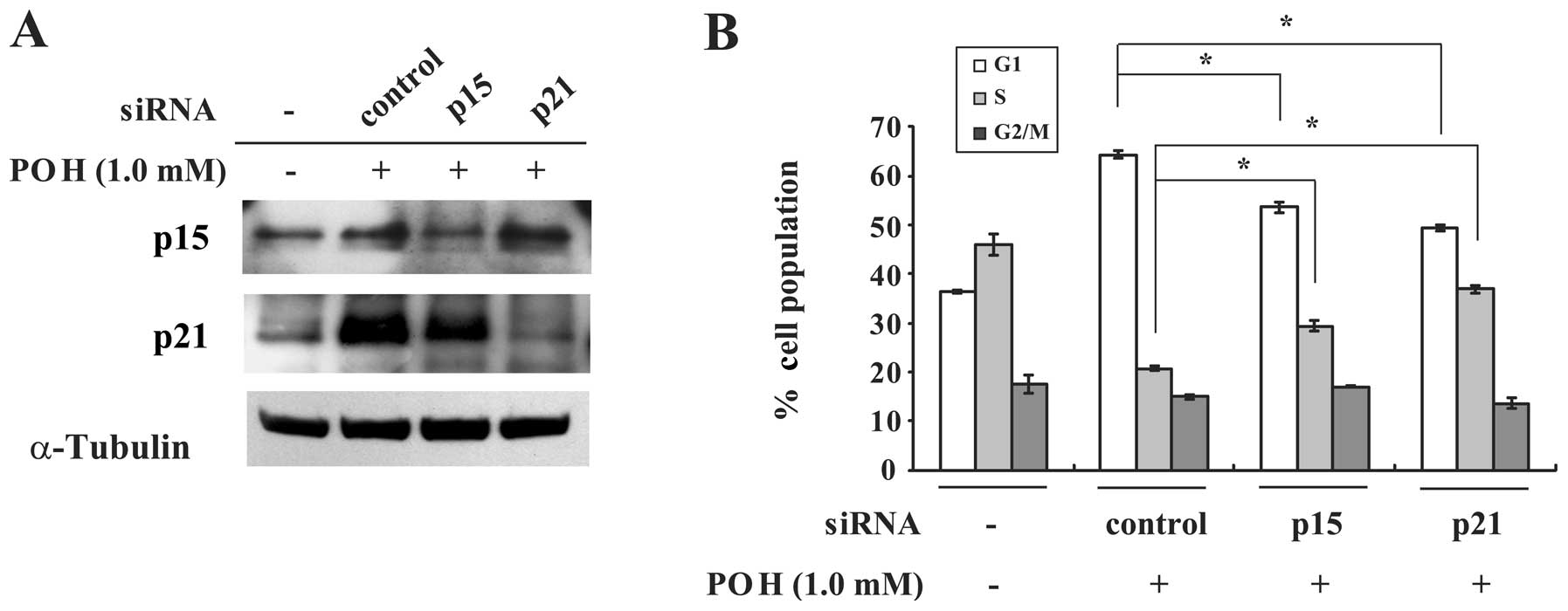
The present results raise the possibility that
upregulation of p15INK4b and p21WAF1/Cip1
proteins by POH contributes to its induction of G1 arrest. If these
molecules are key targets of POH-induced G1 arrest,
p15INK4b or p21WAF1/Cip1-depleted cells
should be insensitive to the effect of POH. Transfection of HaCaT
cells with p15INK4b or p21WAF1/Cip1 siRNA
impaired the induction of these proteins by POH (Fig. 4A). Additionally, these siRNAs
significantly restored POH-altered percentages of the G1 and S cell
populations when compared with the control siRNA (Fig. 4B). These results imply that both
p15INK4b and p21WAF1/Cip1 play pivotal roles
in POH-induced G1 arrest.
POH causes G1 arrest through induction of
p15INK4b and p21WAF1/Cip1 in other cancer
cell lines
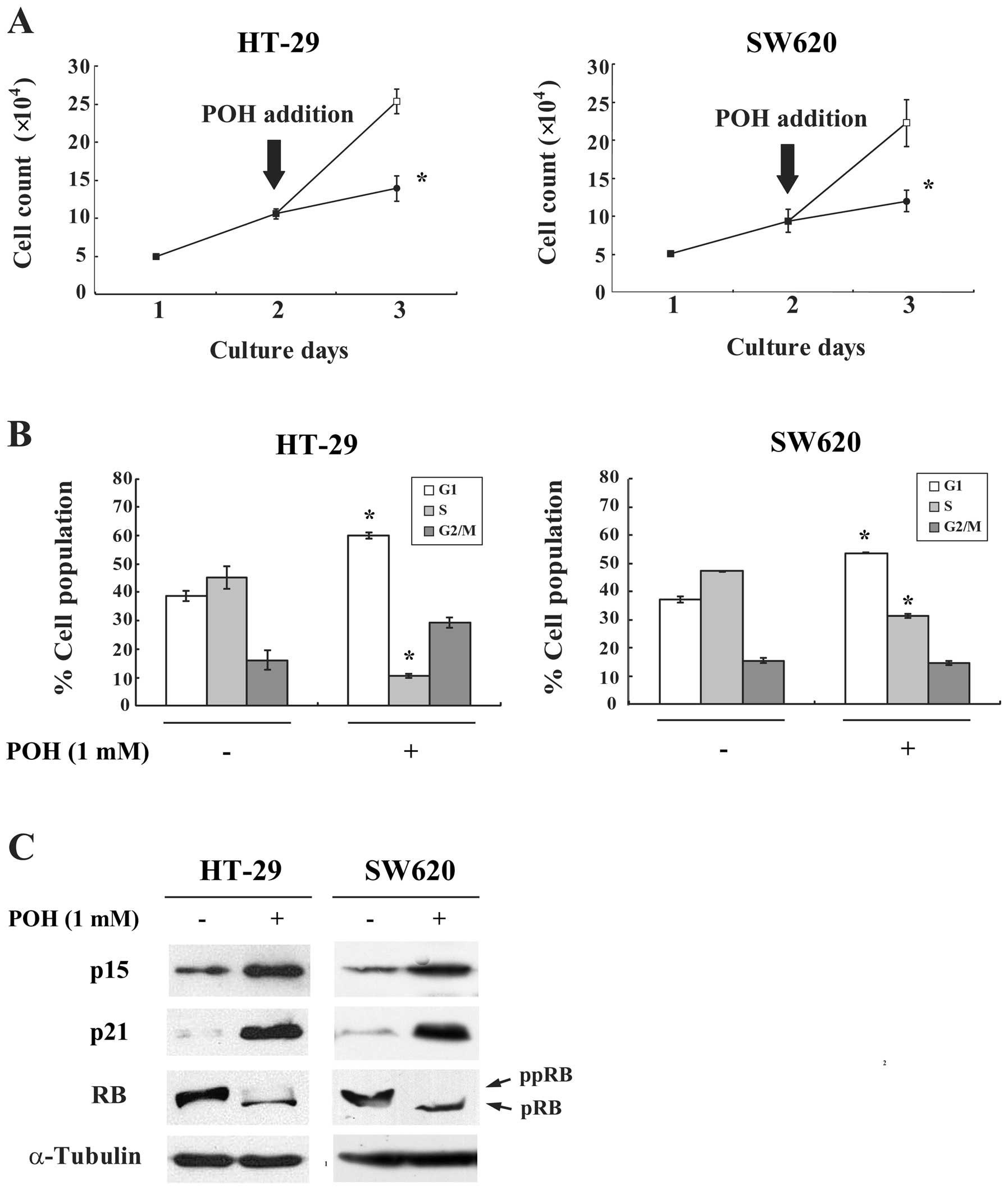
To investigate whether the effects of POH on G1
arrest could be observed more generally, other cancer cell lines,
HT-29 and SW620, were similarly assayed. POH inhibited the
proliferation and caused G1 arrest in these cells (Fig. 5A and B). Moreover, POH increased
p15INK4b and p21WAF1/Cip1 protein expression
and hypophosphorylated the RB protein in both cell lines (Fig. 5C). Taken together, these results
suggest that POH has antitumor activity against various malignant
tumor cells through induction of p15INK4b and
p21WAF1/Cip1 and subsequent G1 arrest.
Discussion
Numerous studies have shown that dysfunction of the
RB pathway is the most frequent event in human malignant tumors
(6,7). Therefore, we focused our studies on
agents that reactivate RB function through induction of the two CKI
families. As a result, we previously demonstrated that
p15INK4b is upregulated by a histone deacetylase
inhibitor trichostatin A, a naturally occurring compound
indole-3-carbinol, an epidermal growth factor receptor inhibitor
gefitinib (ZD1839) and a novel MEK inhibitor JTP-70902 (22–25).
Additionally, we found that p21WAF1/Cip1 is increased by
trichostatin A, a dietary flavonoid apigenin and a plant alkaloid
cryptolepine (26–28).
p16INK4a and p15INK4b are
encoded within the INK4a/ARF/INK4b locus on chromosome 9p21.
Deletion of this locus is the most frequent cytogenetic abnormality
of the RB pathway in human hematopoietic malignancies (6). On the other hand, in many malignant
solid tumors, p16INK4a is inactivated through not only
gene deletions, but also point mutations or transcriptional
silencing by methylation of the promoter. In contrast to
p16INK4a, however, alteration of the p15INK4b
gene is a rare event in solid tumors (6,29).
Moreover, among the INK4 family, p15INK4b has a function
similar to that of p16INK4a. These studies suggest that
p15INK4b may act as a replacement for
p16INK4a when p16INK4a is inactivated.
Krimpenfort et al indicated that p15INK4b can
fulfill a critical backup function for p16INK4a in human
tumors with p16INK4a deficiency (30). In the present study, we showed that
depletion of p15INK4b protein using siRNA suppressed the
G1-arresting activity of POH. These findings suggest that induction
of p15INK4b by POH could be, at least partially,
involved in its antiproliferative activity. Taken together, the
ability of POH to induce p15INK4b might be useful for
inhibiting the growth of solid tumors where the
p16INK4a-RB pathway is inactivated.
p21WAF1/Cip1 is known to be a major
effector of the tumor suppressor p53. Therefore,
p21WAF1/Cip1 is regarded as a tumor-suppressor gene
(31). On the other hand, it has
been shown that p21WAF1/Cip1 plays oncogenic roles in
certain cellular circumstances through its ability to suppress
apoptosis and promote the assembly of cyclin D with CDK4 and CDK6
(32–34). Thus, these data indicate that
p21WAF1/Cip1 induction confers a growth advantage in
tumor development in certain type of cancers, while it has the
opposite effect in others. We revealed that POH upregulated
p21WAF1/Cip1 as well as p15INK4b proteins and
subsequently caused G1 arrest in three malignant tumor cell lines.
Additionally, we showed that depletion of p21WAF1/Cip1
protein using siRNA rendered HaCaT cells insensitive to POH-induced
G1 arrest. These data suggest that the induction of
p21WAF1/Cip1 by POH could be at least partially involved
in its antiproliferative activity.
POH is readily metabolized to perillic acid (PA) and
dihydroperillic acid (DHPA) in animals, whereas in humans PA is the
major circulating metabolite (17,35).
Haag et al(17) reported
that in a rat mammary cancer model administration of a 2.5% POH
diet for 3 weeks caused complete regression in 22 out of 27 (81%)
primary tumors, while the plasma levels of POH metabolites were
approximately 800 μM in rats given a 2% POH diet for 10 weeks.
Based on the data from preclinical models, POH has been tested in
phase I and II clinical trials in patients with refractory solid
malignancies. The mean peak PA plasma levels ranged between 415 and
630 μM and minimal toxicities were observed in patients when doses
of POH at 1600 or 2100 mg/m2 were administered orally
(36–38). Recently, an encouraging clinical
study carried out by da Fonseca et al(20), showed that intranasal administration
of 440 mg POH increased the overall survival of patients with
recurrent GBM when compared with untreated controls.
We revealed that POH upregulated p15INK4b
as well as p21WAF1/Cip1 protein and subsequently caused
G1 arrest in three malignant tumor cell lines. Additionally, we
showed that depletion of the p15INK4b or
p21WAF1/Cip1 protein rendered HaCaT cells resistant to
POH-induced G1 arrest. These results indicate that induction of
both p15INK4b and p21WAF1/Cip1 is at least
partially associated with sensitivity to the antiproliferative
effect of POH. POH driven activation of RB function through
induction of CKIs may contribute to new strategies which have been
termed ‘gene-regulating chemotherapy’ for the treatment of
malignancies. (39,40). In short, POH is promising as a
molecular-targeted anticancer drug against a variety of malignant
tumors.
Acknowledgements
We thank Dr Y. Matsuzaki for his helpful discussion
and useful advice. We were supported by a Grant-in-Aid from the
Japanese Ministry of Education, Culture, Sports, Science and
Technology.
Abbreviations:
|
POH
|
perillyl alcohol
|
|
CDK
|
cyclin-dependent kinase
|
|
CKI
|
CDK inhibitor
|
|
RB
|
retinoblastoma
|
|
siRNA
|
small interfering RNA
|
|
RT-PCR
|
reverse transcription-PCR
|
References
|
1
|
Massague J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dowdy SF, Hinds PW, Louie K, Reed SI,
Arnold A and Weinberg RA: Physical interaction of the
retinoblastoma protein with human D cyclins. Cell. 73:499–511.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roussel MF: The INK4 family of cell cycle
inhibitors in cancer. Oncogene. 18:5311–5317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burkhart DL and Sage J: Cellular
mechanisms of tumour suppression by the retinoblastoma gene. Nat
Rev Cancer. 8:671–682. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelloff GJ, Crowell JA, Hawk ET, Steele
VE, Lubet RA, Boone CW, Covey JM, Doody LA, Omenn GS, Greenwald P,
Hong WK, Parkinson DR, Bagheri D, Baxter GT, Blunden M, Doeltz MK,
Eisenhauer KM, Johnson K, Knapp GG, Longfellow DG, Malone WF,
Nayfield SG, Seifried HE, Swall LM and Sigman CC: New agents for
cancer chemoprevention. J Cellular Biochem (Suppl). 26:1–28. 1996.
View Article : Google Scholar
|
|
9
|
Crowell PL: Prevention and therapy of
cancer by dietary monoterpenes. J Nutr. 129:S775–S778.
1999.PubMed/NCBI
|
|
10
|
Stark MJ, Burke YD, McKinzie JH, Ayoubi AS
and Crowell PL: Chemotherapy of pancreatic cancer with the
monoterpene perillyl alcohol. Cancer Lett. 96:15–21. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi W and Gould MN: Induction of
differentiation in neuro-2A cells by the monoterpene perillyl
alcohol. Cancer Lett. 95:1–6. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mills JJ, Chari RS, Boyer IJ, Gould MN and
Jirtle RL: Induction of apoptosis in liver tumors by the
monoterpene perillyl alcohol. Cancer Res. 55:979–983.
1995.PubMed/NCBI
|
|
13
|
Wagner JE, Huff JL, Rust WL, Kingsley K
and Plopper GE: Perillyl alcohol inhibits breast cell migration
without affecting cell adhesion. J Biomed Biotechnol. 2:136–140.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loutrari H, Hatziapostolou M, Skouridou V,
Papadimitriou E, Roussos C, Kolisis FN and Papapetropoulos A:
Perillyl alcohol is an angiogenesis inhibitor. J Pharmacol Exp
Ther. 311:568–575. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi W and Gould MN: Induction of
cytostasis in mammary carcinoma cells treated with the anticancer
agent perillyl alcohol. Carcinogenesis. 23:131–142. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiseman DA, Werner SR and Crowell PL: Cell
cycle arrest by the isoprenoids perillyl alcohol, geraniol, and
farnesol is mediated by p21Cip1 and p27Kip1
in human pancreatic adenocarcinoma cells. J Pharmacol Exp Ther.
320:1163–1170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haag JD and Gould MN: Mammary carcinoma
regression induced by perillyl alcohol, a hydroxylated analog of
limonene. Cancer Chemother Pharmacol. 34:477–483. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu G, Oettel K, Bailey H, Ummersen LV,
Tutsch K, Staab MJ, Horvath D, Alberti D, Arzoomanian R, Rezazadeh
H, McGovern J, Robinson E, DeMets D and Wilding G: Phase II trial
of perillyl alcohol (NSC 641066) administered daily in patients
with metastatic androgen-independent prostate cancer. Invest New
Drugs. 21:367–372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bailey HH, Attia S, Love RR, Fass T,
Chappell R, Tutsch K, Harris L, Jumonville A, Hansen R, Shapiro GR
and Stewart JA: Phase II trial of daily oral perillyl alcohol (NSC
641066) in treatment-refractory metastatic breast cancer. Cancer
Chemother Pharmacol. 62:149–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
da Fonseca CO, Simão M, Lins IR, Caetano
RO, Futuro D and Quirico-Santos T: Efficacy of monoterpene perillyl
alcohol upon the survival rate of patients with recurrent
glioblastoma. J Cancer Res Clin Oncol. 137:287–293. 2011.PubMed/NCBI
|
|
21
|
Koyama M, Izutani Y, Goda AE, Matsui TA,
Horinaka M, Tomosugi M, Fujiwara J, Nakamura Y, Wakada M, Yogosawa
S, Sowa Y and Sakai T: Histone deacetylase inhibitors and
15-deoxy-Delta12,14-prostaglandin J2 synergistically
induce apoptosis. Clin Cancer Res. 16:2320–2332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hitomi T, Matsuzaki Y, Yokota T, Takaoka Y
and Sakai T: p15INK4b in HDAC inhibitor-induced growth
arrest. FEBS Lett. 554:347–350. 2003.
|
|
23
|
Matsuzaki Y, Koyama M, Hitomi T, Kawanaka
M and Sakai T: Indole-3-carbinol activates the cyclin-dependent
kinase inhibitor p15INK4b gene. FEBS Lett. 576:137–140.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koyama M, Matsuzaki Y, Yogosawa S, Hitomi
T, Kawanaka M and Sakai T: ZD1839 induces p15INK4b and
causes G1 arrest by inhibiting the mitogen-activated protein
kinase/extracellular signal-regulated kinase pathway. Mol Cancer
Ther. 6:1579–1587. 2007.PubMed/NCBI
|
|
25
|
Yamaguchi T, Yoshida T, Kurachi R,
Kakegawa J, Hori Y, Nanayama T, Hayakawa K, Abe H, Takagi K,
Matsuzaki Y, Koyama M, Yogosawa S, Sowa Y, Yamori T, Tajima N and
Sakai T: Identification of JTP-70902, a
p15INK4b-inductive compound, as a novel MEK1/2
inhibitor. Cancer Sci. 98:1809–1816. 2007.PubMed/NCBI
|
|
26
|
Sowa Y, Orita T, Minamikawa S, Nakano K,
Mizuno T, Nomura H and Sakai T: Histone deacetylase inhibitor
activates the WAF1/Cip1 gene promoter through the Sp1 sites.
Biochem Biophys Res Commun. 241:142–150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takagaki N, Sowa Y, Oki T, Nakanishi R,
Yogosawa S and Sakai T: Apigenin induces cell cycle arrest and
p21/WAF1 expression in a p53-independent pathway. Int J Oncol.
26:185–189. 2005.PubMed/NCBI
|
|
28
|
Matsui TA, Sowa Y, Murata H, Takagi K,
Nakanishi R, Aoki S, Yoshikawa M, Kobayashi M, Sakabe T, Kubo T and
Sakai T: The plant alkaloid cryptolepine induces
p21WAF1/Cip1 and cell cycle arrest in a human
osteosarcoma cell line. Int J Oncol. 31:915–922. 2007.PubMed/NCBI
|
|
29
|
Esteller M, Corn PG, Baylin SB and Herman
JG: A gene hypermethylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
30
|
Krimpenfort P, Ijpenberg A, Song JY, van
der Valk M, Nawijn M, Zevenhoven J and Berns A: p15INK4b
is a critical tumour suppressor in the absence of
p16Ink4a. Nature. 448:943–947. 2007.
|
|
31
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
LaBaer J, Garrett MD, Stevenson LF,
Slingerland JM, Sandhu C, Chou HS, Fattaey A and Harlow E: New
functional activities for the p21 family of CDK inhibitors. Genes
Dev. 11:847–862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Canman CE, Gilmer TM, Coutts SB and Kastan
MB: Growth factor modulation of p53-mediated growth arrest versus
apoptosis. Genes Dev. 9:600–611. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abukhdeir AM and Park BH: p21 and p27:
roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phillips LR, Malspeis L and Supko JG:
Pharmacokinetics of active drug metabolites after oral
administration of perillyl alcohol, an investigational
antineoplastic agent, to the dog. Drug Metab Dispos. 23:676–680.
1995.PubMed/NCBI
|
|
36
|
Ripple GH, Gould MN, Stewart JA, Tutsch
KD, Arzoomanian RZ, Alberti D, Feierabend C, Pomplun M, Wilding G
and Bailey HH: Phase I clinical trial of perillyl alcohol
administered daily. Clin Cancer Res. 4:1159–1164. 1998.PubMed/NCBI
|
|
37
|
Ripple GH, Gould MN, Arzoomanian RZ,
Alberti D, Feierabend C, Simon K, Binger K, Tutsch KD, Pomplun M,
Wahamaki A, Marnocha R, Wilding G and Bailey HH: Phase I clinical
and pharmacokinetic study of perillyl alcohol administered four
times a day. Clin Cancer Res. 6:390–396. 2000.PubMed/NCBI
|
|
38
|
Hudes GR, Szarka CE, Adams A, Ranganathan
S, McCauley RA, Weiner LM, Langer CJ, Litwin S, Yeslow G, Halberr
T, Qian M and Gallo JM: Phase I pharmacokinetic trial of perillyl
alcohol (NSC 641066) in patients with refractory solid
malignancies. Clin Cancer Res. 6:3071–3080. 2000.PubMed/NCBI
|
|
39
|
Sakai T: Molecular cancer epidemiology -
the present status and future possibilities. Jpn J Hygiene.
50:1036–1046. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sowa Y and Sakai T: Butyrate as a model
for ‘Gene-regulating chemoprevention and chemotherapy’. Biofactors.
2:283–287. 2000.
|