Introduction
Kindlin-2 is a member of the focal adhesion protein
family recruited to integrin-containing adhesion sites (1–3).
Kindlin-2 is localized to cell-extracellular matrix (ECM) adhesion
sites, and has been shown to interact directly with the cytoplasmic
tail of β1 and β3-integrin. Kindlin-2 overexpression has been shown
to induce a modest activation of the αIIbβ3-integrin (4). Integrin-mediated cell adhesion
triggers intracellular signaling pathways that control migration,
proliferation, survival, and differentiation of cells including
cancer cells (5). Although
kindlin-2 has been confirmed as an essential element of
bidirectional integrin signaling (6), little is known about the expression of
kindlin-2 in association with human cancer. Recent studies have
shown that kindlin-2 was differentially expressed in prostate,
uterine leiomyosarcoma and malignant mesothelioma (7–9). In
our previous study, we found that kindlin-2 was upregulated at both
the RNA and protein levels in gastric cancer tissues. Moreover,
kindlin-2 expression had a significant positive correlation with
tumor stromal invasion, lymph node metastasis and TNM stage.
Patients with high kindlin-2 expression had significantly poorer
overall survival and progression free survival. Thus, kindlin-2
might play an important role in the invasion and metastasis of
gastric cancer.
Chronic inflammation has been linked to the
development of cancer (10).
Individuals with ulcerative colitis, which is a chronic
inflammatory disease of the colon, have a 10-fold increase in
likelihood of developing colorectal carcinoma. Similarly,
inflammatory conditions of the liver, such as chronic hepatitis and
cirrhosis, are well established risk factors for the development of
hepatocellular carcinoma (11).
Tumor-associated macrophages (TAMs) have been highlighted to play
an important role in malignant progression of neoplasm (12,13).
For many tumors, high numbers of TAMs correlate with lymph node
involvement or the formation of distant metastases (14,15).
Clinical studies have shown a correlation between the high number
of TAMs in the areas of tumors and increased microvessel density
and poor prognosis (16–18). Our previous study showed that the
TAMs play a role in regulating invasion and metastasis in gastric
cancer cells. However, the molecular mechanism underlying the link
between the inflammation mediated by macrophages and gastric cancer
remains unclear.
Although both kindlin-2 and macrophages have been
shown to be correlated with the invasion and metastasis of gastric
cancer in our previous studies, we do not know whether the
kindlin-2 gene is involved in the inflammatory process of promoting
invasion of gastric cancer cells.
IL8, IL10, IL11 have been found to be expressed in
gastric cancer (19–21), but the expressions of IL17b, IL22
and IL24 in gastric cancer remain undefined. Moreover, so far there
have been no reports on the relationship between inflammatory
factors and kindlin-2 expression. In our study, we found that the
kindlin-2 gene was not only significantly elevated when the gastric
cancer cells were co-cultured with TAMs, but this elevation could
also be induced by hypoxia. Furthermore, the interleukin
expressions, with the exception of IL11, were significantly changed
following kindlin-2 downregulation.
Materials and methods
Isolation of monocytes and
macrophages
Mononuclear cells were isolated from the blood of
healthy subjects with density gradient centrifugation (Ficol-Paque;
Amersham, Uppsala, Sweden). There were three layers of liquid after
density gradient centrifugation, mononuclear cells were in the
second cloudy layer, and it was transferred to a clean tube and
centrifuged. The cells were washed with PBS+10% ACD (acid citrate
dextrose solution) solution two times. The cells were counted and
1.4×106 cells were placed on Matrigel (BD Biosciences,
San Jose, CA, USA) covered coverslip (Nalge Nunc International
Corp., Naperville, IL, USA). The isolated cells were grown in
serum-free medium designed for macrophages (Macrophage serum free
medium; Gibco, Paislay, UK) with granulocyte-macrophage
colony-stimulating factor (GM-CSF, 10 ng/ml; ImmunoTools,
Oldenburg, Germany), antibiotics and 5% CO2 at 37°C.
Monocytes adhered to the Matrigel overnight and differentiated to
macrophages due to GM-CSF; other non-adherent mononuclear cells
were removed from the medium the following day. Monocytes were
fully differentiated into macrophages after six days and then used
for experiments. When co-cultured with cancer cells, macrophages
developed into TAMs with special surface marker,
CD14+(22). After
co-culturing with gastric cancer cells, the portion of
CD14+ positive macrophages reached >80% measured with
flow cytometry.
Gastric cancer cell culture
The AGS cell line (CRL-1739) derived from fragments
of a tumor resected from gastric cancer patients, was purchased
from ATCC (American Type Culture Collection). The cells were
cultured in Ham's F12K medium with 2 mM L-glutamine adjusted to
contain 1.5 g/l sodium bicarbonate, 10% fetal bovine serum (FBS)
and antibiotics (100 μg/ml penicillin and 100 U/ml
streptomycin).
The Hs 746T (HTB-135) cell line was purchased from
ATCC. It was derived from a metastatic tumor of the left leg of a
gastric cancer patient. Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) with 4 mM L-glutamine adjusted to contain 1.5
g/l bicarbonate, 4.5 g/l glucose, 10% FBS and antibiotics (100
μg/ml penicillin and 100 U/ml streptomycin).
The NCI-N87 (CRL-5822) cell line was derived from
the metastatic liver tumor of a gastric cancer patient. It was
purchased from ATCC. Cells were cultured in RPMI-1640 medium,
supplemented with 10% FBS and antibiotics (100 μg/ml penicillin and
100 U/ml streptomycin).
All gastric cancer cell lines were maintained at
37°C in a humidified atmosphere with 5% CO2.
Cell sorting by magnetic separation
Cell sorting was performed after gastric cancer
cells were cultured with macrophages for 24 h. Cell sorting was
processed by MACS separator (MACS Miltenyi Biotec, Germany), which
is based on magnetic separation. After Matrigel contained cells
were dissolved and centrifuged, 80 μl of Buffer (degassed PBS with
0.5% BSA and 2 mM EDTA) and 20 μl CD14 MicroBeads (MACS Miltenyi
Biotec) were added to the deposit and incubated for 20 min at 4°C.
Then the cells were washed by Buffer and centrifuged. Cells
resuspended with Buffer were transferred to the prepared LS column
(MACS Miltenyi Biotec). Total effluents which contained gastric
cancer cells and CD14 negative macrophages were collected and
centrifuged. The cell pellets were resuspended in buffer and CD11b
MicroBeads, and the above mentioned separating protocol was
repeated.
Invasion and migration assay (3D dynamic
migration imaging system)
Cells were grown on Matrigel covered coverslip wells
with serum-free medium designed for macrophages. Gastric cancer
cells were grown either alone or with differentiated macrophages on
Matrigel and either in normal (5% CO2 in air) or hypoxic
conditions (CO2 5%, O2 2%, N2
94%). Gastric cancer cells (6×104) were seeded in each
well of the coverslip. Gastric cancer cells were stained with
fluorescent dye (CellTracker green CMFDA; Invitrogen, Eugene, OR,
USA) before imaging. During the invasion phase the cancer cells
invaded in Matrigel were imaged by 3D dynamic migration imaging
system (Olympus Ax70 Research System Microscope, Japan; 12Bit
Cooled Imaging Sensicam camera; PCD Imaging, Kelheim, Germany). The
average migration speed was calculated from the cells which could
be tracked at least for 6 h in one z-plane (ImagePro Plus; Media
Cybernetics, Inc., Bethesda, MD, USA).
Real-time RT-PCR
Total-RNA was isolated from the sorted cells by
membrane binding (RNeasy Mini kit, 74104; Qiagen, Hilden, Germany).
RNA was reverse transcribed to single stranded cDNA by the reverse
transcriptase method (High Capacity cDNA Reverse Transcription kit,
4368814; Applied Biosystems, Bardburg, NJ, USA). The target gene
expression was measured by the real-time RT-PCR method (TaqMan Gene
Expression Assay; Applied Biosystems). GAPDH was used as endogenic
control (TaqMan Endogenous Controls; Applied Biosystems). The PCR
reactions were run in ABI PRISM 7000 sequence detection system.
2−ΔΔCT referred to the fold of the mRNA expression of
one sample compared to the calibration sample. 2−ΔCt was
defined as the fold of mRNA expression of the target gene compared
to GAPDH expression in the same sample.
Western blotting
Gastric cancer cells were lysed in
radioimmunoprecipitation assay (RIPA) buffer (50 mmol/l Tris-Cl,
150 mmol/l NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1%
sodium dodecyl sulfate, pH 7.5) containing 1 mmol/l
phenylmethylsulfonyl fluoride for 30 min on ice. The samples were
centrifuged and the protein concentrations for each sample were
determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories,
Hercules, CA, USA). The protein samples (20 g) were separated by
10% sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis, and then electrotransferred to a nitrocellulose
membrane. After blocking with 5% nonfat dry milk in
Tween-Tris-buffered saline solution [0.1% Tween-20 in 100 mmol/l
Tris-Cl (pH 7.5), 0.9% NaCl], the membranes were incubated with
antibody to kindlin-2 (ab74030; Abcam, USA) at a dilution of 1:600
at 4°C overnight. After washing, the blots were incubated with
horseradish peroxidase conjugated secondary antibodies at a
dilution of 1:10,000 for kindlin-2 for 1 h. The blot was washed and
developed using chemiluminescence (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). For control, we also blotted the membranes
with primary antibodies against β-actin (sc-47778; Santa Cruz
Biotechnology, Inc.) and densitometric data of kindlin-2 were
normalized to those of β-actin levels.
siRNA transfection
In a 6-well tissue culture plate, 2×105
Hs-746T cells/well were seeded in 2 ml antibiotic-free DMEM medium
supplemented with FBS. The cells were incubated at 37°C until the
cells were 60–80% confluent. siRNA transfection kit (sc-45064;
Santa Cruz Biotechnology, Inc.) was used to downregulate the
expression. The cells were divided into three groups: blank control
group, negative control group, and kindlin-2 siRNA group. Six
microliters of kindlin-2 siRNA duplex (sc-10676) or control siRNA
duplex (sc-37007) were added into 100 μl siRNA transfection medium
(sc-36868) (all were from Santa Cruz Biotechnology, Inc.) as the
kindlin-2 siRNA group or negative control group, respectively. In
the blank control group, 6 μl transfection medium was used instead
of duplex. For each transfection group, 6 μl siRNA transfection
(sc-29528; Santa Cruz Biotechnology, Inc.) reagent was added into
100 μl siRNA transfection medium. Thereafter, they were mixed
gently and incubated for 45 min at room temperature. The cells were
washed once with 2 ml siRNA transfection medium. Then the mixed
solution was overlaid the washed cells and incubated for 7 h in a
CO2 incubator. Subsequently, 1 ml DMEM medium containing
2-fold the normal serum and antibiotics concentration was added
without removing the transfection mixture. The cells were incubated
for an additional 24 h. The medium was replaced with normal DMEM
medium the next day. The RNA of cells was extracted after 24 h.
Statistical analysis
Statistical package for social science (SPSS)
version 17.0 was used. Data are expressed as the means ± SEM. One
way ANOVA was used to compare the cell movement speed data and mRNA
expression data between different groups. P<0.05 was considered
to indicate statistically significant differences.
Results
Kindlin-2 relative expression in gastric
cancer cell lines
Kindlin-2 mRNA relative expression (kindlin-2/GAPDH)
was higher in metastatic gastric cancer cell lines than in the
non-metastatic gastric cancer cell line. Kindlin-2 mRNA expression
was the highest in the Hs-746T cell line (658.4±4.8), and the
lowest in the AGS cell line (2.3±0.6). Kindlin-2 mRNA relative
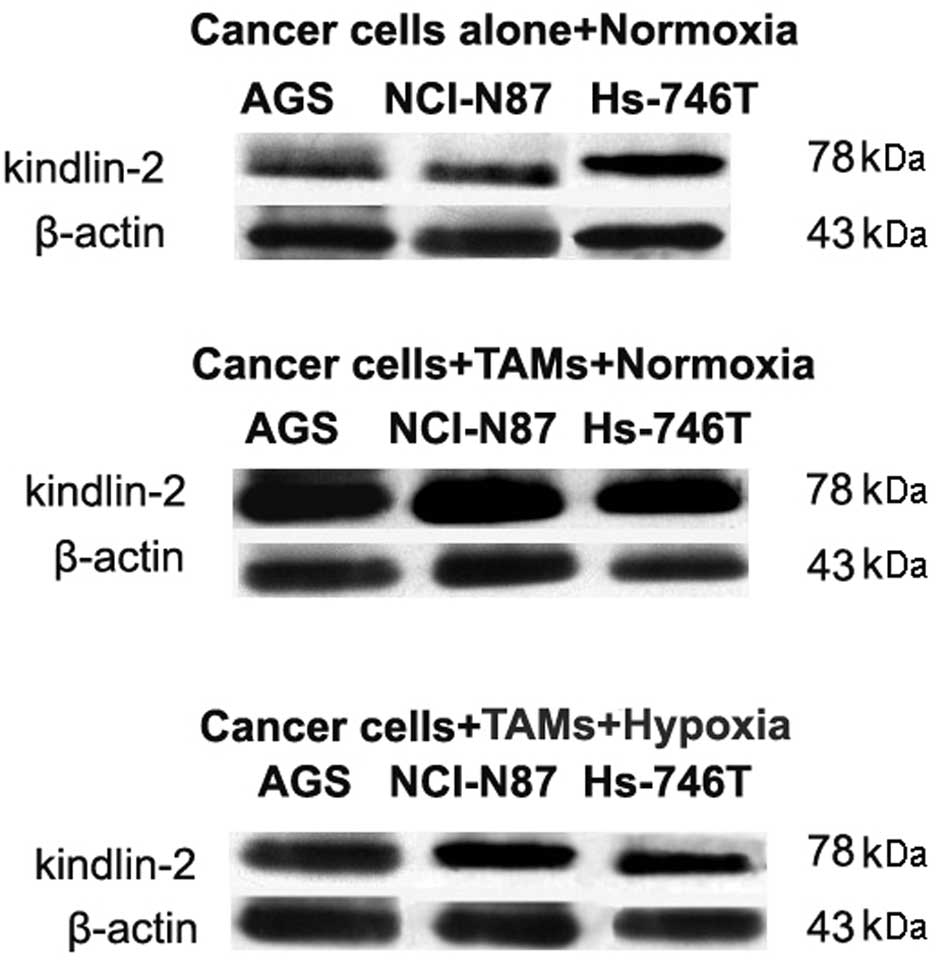
expression in the NCI-N87 cell line was 81.8±4.3 (N=6) (Fig. 1). Kindlin-2 expression in protein
level was verified by western blot analysis (Fig. 2).
Effect of TAMs on kindlin-2 expression in
gastric cancer cell lines under normal or hypoxic conditions
Both kindlin-2 mRNA and protein were upregulated in
all three gastric cancer cell lines, when co-cultured with
macrophages under normal conditions. The elevation of kindlin-2
expression was significantly higher in the non-metastatic cell line
AGS than in the two other cell lines.
Under hypoxic conditions, the induction of kindlin-2
expression by macrophages was significantly downregulated in all
cell lines; AGS (P<0.001), NCI-N87 (P<0.001) and Hs-746T
(P=0.013) (N=6) (Table I). The
effect of macrophage and hypoxia on kindlin-2 expression in protein
level were in accordance with mRNA results (Fig. 2).
 | Table IEffect of macrophages on the kindlin-2
expression in gastric cancer cell lines under normal or hypoxic
conditions. |
Table I
Effect of macrophages on the kindlin-2
expression in gastric cancer cell lines under normal or hypoxic
conditions.
| Kindlin-2 mRNA
relative expression (2−ΔΔCt) |
|---|
|
|
|---|
| Cultured with/without
macrophages under normal conditions | P-value | Cultured with
macrophages under hypoxic/normal conditions (x10−2) | P-value |
|---|
| AGS | 4789.6±149.3 | | 7.1±0.2 | |
| NCI-N87 | 455.1±9.5 | P<0.001a | 5.8±0.0 | 0.159 |
| Hs-746T | 157.7±6.6 | P<0.001a | 0.5±0.2 | 0.036a |
Expression of interleukin in gastric
cancer cell lines with variable kindlin-2 expression
IL8, IL11, IL17b, IL24 expressions were the lowest
in the AGS cell line which also had the lowest kindlin-2
expression, and the highest in the Hs-746T cell line, which had the
highest kindlin-2 expression. There was a significant correlation
between kindlin-2 and interleukin expression, with the exception of
IL10 and IL18 (N=6) (Table II).
IL22 expression was significantly higher the in Hs-746T cell line
than in the other two cell lines. IL18 and IL10 expressions were
significantly lower in the metastatic cell lines compared to AGS
(Table III).
 | Table IICorrelation between kindlin-2 and
interleukin expression. |
Table II
Correlation between kindlin-2 and
interleukin expression.
| Pearson's correlation
co-efficient (r) | P-value |
|---|
| IL8 | 0.988 | <0.001a |
| IL10 | −0.308 | 0.553 |
| IL11 | 0.996 | <0.001a |
| IL17b | 0.919 | 0.010 |
| IL18 | −0.712 | 0.113 |
| IL22 | 0.990 | <0.001a |
| IL24 | 0.993 | <0.001a |
 | Table IIIRelative expression of IL8, IL10,
IL11, IL17b, IL18, IL22 and IL24 mRNA in gastric cancer cell
lines. |
Table III
Relative expression of IL8, IL10,
IL11, IL17b, IL18, IL22 and IL24 mRNA in gastric cancer cell
lines.
|
mRNA
relative expression (target gene/GAPDH, 2−ΔCt) |
|---|
|
|
|---|
| IL8
(x10−4) | P-value | IL10
(x10−5) | P-value | IL11
(x10−4) | P-value | IL17b
(x10−7) | P-value | IL18
(x10−4) | P-value | IL22
(x10−7) | P-value | IL24
(x10−7) | P-value |
|---|
| AGS | 2.1±0.1 | | 8.3±0.6 | | 1.4±0.2 | | 4.7±0.1 | | 23.7±0.9 | | 3.6±0.1 | | 4.8±0.1 | |
| NCI-N87 | 13.9±0.4 | 0.066 | 4.4±0.0 | 0.004a | 3.0±0.1 | 0.245a | 13.6±0.0 | 0.990 | 5.6±0.2 | <0.001a | 4.6±0.1 | 0.908 | 70.9±2.5 | 0.862 |
| Hs-746T | 55.4±5.1 | 0.001a | 3.9±0.2 | 0.002a | 37.9±1.3 | <0.001a | 2336.5±764.4 | 0.033 | 2.5±0.0 | <0.001a | 187.4±10.3 | <0.001a | 8464.4±428.9 | <0.001a |
Invasion rate of Hs-746T cells in
Matrigel after downregulation of kindlin-2
The cell invasion rates in Matrigel decreased
significantly following downregulation of kindlin-2 (3.2±0.1 μm/h)
compared to the blank control group (3.9±0.3 μm/h, P<0.001) and
the negative control group (4.0±0.1 μm/h, P<0.001, N=3).
Expression of interleukin in the Hs-746
cell line following downregulation of kindlin-2
As kindlin-2 relative expression was the highest in
the Hs-746T cell line, we chose to downregulate kindlin-2
expression in this cell line. Real-time RT-PCR was used to verify
the effect of kindlin-2 siRNA. We found that the level of mRNA
expression in the kindlin-2 siRNA group (7.6±0.1) was significantly
lower than in the negative control group (25.3±0.2) or in the blank
control group (24.7±0.1), and there was no significant difference
between the negative control and the blank control group (N=6)
(Fig. 3).
IL8 and IL18 expressions were significantly elevated
following downregulation of kindlin-2 expression in the Hs-746T
cell line compared to the blank control group. IL10, IL11, IL17b,
IL22 and IL24 expressions were significantly decreased after
downregulation of kindlin-2 expression (N=6) (Table IV).
 | Table IVIL8, IL10, IL11, IL17b, IL18, IL22
and IL24 mRNA in the different groups of the Hs-746T gastric cancer
cell line. |
Table IV
IL8, IL10, IL11, IL17b, IL18, IL22
and IL24 mRNA in the different groups of the Hs-746T gastric cancer
cell line.
|
mRNA
relative expression (target gene/GAPDH, 2−ΔCt) |
|---|
|
|
|---|
| IL8
(x10−4) | P-value | IL10
(x10−9) | P-value | IL11
(x10−4) | P-value | IL17b
(x10−5) | P-value | IL18
(x10−5) | P-value | IL22
(x10−7) | P-value | IL24
(x10−5) | P-value |
|---|
| Blank control | 9.7±0.9 | | 58.8±0.4 | | 1.1±0.0 | | 2.3±0.0 | | 7.7±0.3 | | 6.5±0.0 | | 5.9±0.0 | |
| Negative
control | 7.7±0.1 | 0.116 | 47.2±2.6 | 0.350 | 1.5±0.0 | 0.055 | 2.1±0.0 | 0.507 | 7.2±0.1 | 0.344 | 6.3±0.0 | 0.150 | 5.6±0.0 | 0.114 |
| Kindlin-2
siRNA | 49.4±0.5 | 0.001a | 3.1±0.1 | 0.014a | 0.6±0.0 | 0.003 | 1.1±0.0 | 0.017a | 10.7±0.2 | 0.021a | 2.1±0.0 | 0.001a | 3.8±0.0 | 0.001a |
Correlation between interleukin
expression and invasion rate in Matrigel following downregulation
of kindlin-2
There was a significantly negative correlation
between IL8, IL18 expression and gastric cancer cell migration rate
in the Hs-746T cell line following downregualtion of kindlin-2
expression. (r=−0.987, P<0.001; r=−0.983, P<0.001). There was
a significantly positive correlation between IL10, IL11, IL17b,
IL22 and IL24 expression and cell migration rate (r=0.842, P=0.036;
r=0.922, P<0.001; r=0.920, P=0.009; r=0.974, P=0.001).
Discussion
In recent years, studies of kindlin-2 as an
important regulator of integrin activation have focused on the
transformation and progression of tumors. In our two previous
studies, we found that both kindlin-2 expression and chronic
inflammation mediated by macrophages had an effect on the invasion
of gastric cancer. However, little is known about the relationship
between kindlin-2 expression and chronic inflammation. In this
study, we found that kindlin-2 expression was upregulated by
macrophages in all three gastric cancer cell lines. We speculated
that kindlin-2 is involved in the process of tumor-associated
inflammation, and tumor-associated macrophages (TAMs) could
increase the invasion of cancer cells by enhancing the adhesion
between cancer cells and extra-cellular matrix dependent on
kindlin-2 upregulation.
The presence of multiple areas of hypoxia is a
hallmark of cancer. A number of recent studies have shown that
macrophages respond to the level of hypoxia in tumors by
upregulating such transcription factors as hypoxia-inducible
factors 1 and 2, which in turn activate a broad array of mitogenic,
proinvasive, proangiogenic, and prometastatic genes (23). However, so far there have been no
reports about the relationship between kindlin-2 expression,
hypoxia and macrophages. In our study, we found that the response
of kindlin-2 expression to the hypoxia with macrophages depended on
the gastric cancer cell lines and it was downregulated most
severely in the metastatic cell line.
Significant results have demonstrated that
inflammation cytokines, especially interleukin, play an important
role in chronic inflammation and cancer progression (24). Although kindlin-2 expression was
found to be induced by TAMs in our study, we did not know the
relationship between kindlin-2 and interleukins in the cancer
cells. IL8 expression has been found in various human types of
cancer including gastric cancer (25). It has been implicated in a variety
of cellular processes, especially in angiogenesis through
stimulation of endothelial cell migration and suppression of
programmed cell death (26). In our
study, IL8 was significantly elevated following downregulation of
kindlin-2 expression with invasion rate in Matrigel decreased,
suggesting that kindlin-2 might inhibit gastric cancer cell
invasion through elevated IL8 expression. However, IL8 expression
was high in the cell line with high kindlin-2 expression, which
might be due to other signal pathways involved in the regulation of
IL8 expression.
Data concerning the level of IL10 in gastric cancer
patients display discrepancy. A study performed on 68 patients
showed no correlation between serum IL10 level and the clinical
course of disease (27). On the
other hand, Szaflarska et al(28) reported that IL10 was elevated mostly
in advanced disease and the increased levels were associated with
significantly poorer survival of patients. Based on our results, we
speculate that IL10 expression might depend on the type of gastric
cancer and although its expression is low in the metastatic cell
line with high kindlin-2 expression, downregulation of kindlin-2
might decrease the invasion of gastric cells through decreasing
further IL10 expression.
IL11 was initially cloned as a mediator of
plasmacytoma cell proliferation (29) and was later found to exhibit a wide
variety of biological effects in neural cells as well as in the
hematopoietic and the immune systems (30). It has previously been shown that
expression of IL11 and its co-receptor were required for signal
transduction and correlates with invasion and proliferation in
gastric, and colorectal cancer (20,31).
We found that IL11 expression was not only high in the gastric
cancer cell line with high kindlin-2 expression, but was also
significantly decreased after inferring kindlin-2 mRNA expression
and was positively correlated with invasion rate in Matrigel,
suggesting a positive role of kindlin-2 signaling in IL11
expression for regulating gastric cancer cell invasion.
IL18 has been shown to have potent antitumor effects
that are mediated by the induction of apoptosis and inhibition of
angiogenesis (32,33). However, some reports suggest that
tumors could counterattack the immune system by secreting IL18
(34). The serum IL18 concentration
was reported to be higher in metastatic cancer patients than in
early cancer patients (35). We
found that IL18 was not only weakly expressed in the metastatic
cell line with high kindlin-2 expression, but its expression was
also significantly increased after downregulation of kindlin-2.
Therefore, kindlin-2 might contribute to the invasion of cancer
cells through downregulation of IL18 expression, when macrophages
interact with gastric cancer cells.
There are no previous reports on IL17b, IL22 and
IL24 expression in gastric cancer. In this study, we found that
these genes were expressed in three gastric cancer cell lines.
Their expression correlated with kindlin-2 expression levels
suggesting a role in promoting the invasion of gastric cancer
cells. Moreover, downregulation of kindlin-2 expression induced a
decrease in expression of these three interleukins. Therefore,
kindlin-2 might be involved in the interaction between chronic
inflammation and gastric cancer through positive regulation of 17b,
IL22 and IL24 expression.
In conclusion, the novel adhesion gene kindlin-2
might play a role in promoting the invasion and metastasis of
gastric cancer cells by TAMs and through regulating the expression
of interleukins, such as IL8, IL10, IL11, IL17b, IL22 and IL24.
Acknowledgements
This study was supported by grants from the Sigrid
Juselius Foundation and the Helsinki University Central Hospital
Research Funds.
References
|
1
|
Rogalski TM, Mullen GP, Gilbert MM,
Williams BD and Moerman DG: The UNC-112 gene in Caenorhabditis
elegans encodes a novel component of cell-matrix adhesion
structures required for integrin localization in the muscle cell
membrane. J Cell Biol. 150:253–264. 2000.PubMed/NCBI
|
|
2
|
Wegener KL, Partridge AW, Han J, et al:
Structural basis of integrin activation by talin. Cell.
128:171–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ussar S, Wang HV, Linder S, Fässler R and
Moser M: The Kindlins: subcellular localization and expression
during murine development. Exp Cell Res. 312:42–51. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi X, Ma YQ, Tu Y, et al: The
MIG-2/integrin interaction strengthens cell-matrix adhesion and
modulates cell motility. J Biol Chem. 282:20455–20466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim M, Carman CV and Springer TA:
Bidirectional transmembrane signaling by cytoplasmic domain
separation in integrins. Science. 301:1720–1725. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montanez E, Ussar S, Schifferer M, Bösl M,
Zent R, Moser M and Fässler R: Kindlin-2 controls bidirectional
signaling of integrins. Genes Dev. 22:1325–1330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An Z, Dobra K, Lock JG, Strömblad S,
Hjerpe A and Zhang H: Kindlin-2 is expressed in malignant
mesothelioma and is required for tumor cell adhesion and migration.
Int J Cancer. 127:1999–2008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong X, An Z, Wang Y, et al: Kindlin-2
controls sensitivity of prostate cancer cells to cisplatin-induced
cell death. Cancer Lett. 299:54–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kato K, Shiozawa T, Mitsushita J, et al:
Expression of the mitogen-inducible gene-2 (mig-2) is elevated in
human uterine leiomyomas but not in leiomyosarcomas. Hum Pathol.
35:55–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sgambato A and Cittadini A: Inflammation
and cancer: a multifaceted link. Eur Rev Med Pharmacol Sci.
14:263–268. 2010.
|
|
11
|
Hagemann T, Balkwill F and Lawrence T:
Inflammation and cancer: a double-edged sword. Cancer Cell.
12:300–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukhtar RA, Nseyo O, Campbell MJ and
Esserman LJ: Tumor-associated macrophages in breast cancer as
potential biomarkers for new treatments and diagnostics. Expert Rev
Mol Diagn. 11:91–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang BC, Gao J, Wang J, Rao ZG, Wang BC
and Gao JF: Tumor-associated macrophages infiltration is associated
with peritumoral lymphangiogenesis and poor prognosis in lung
adenocarcinoma. Med Oncol. 28:1447–1452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohno S, Ohno Y, Suzuki N, et al:
Correlation of histological localization of tumor-associated
macrophages with clinicopathological features in endometrial
cancer. Anticancer Res. 24:3335–3342. 2004.PubMed/NCBI
|
|
15
|
Hanada T, Nakagawa M, Emoto A, Nomura T,
Nasu N and Nomura Y: Prognostic value of tumor-associated
macrophage count in human bladder cancer. Int J Urol. 7:263–269.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leek RD, Lewis CE, Whitehouse R, Greenall
M, Clarke J and Harris AL: Association of macrophage infiltration
with angiogenesis and prognosis in invasive breast carcinoma.
Cancer Res. 56:4625–4629. 1996.PubMed/NCBI
|
|
17
|
Fujimoto H, Sangai T, Ishii G, et al:
Stromal MCP-1 in mammary tumors induces tumor-associated macrophage
infiltration and contributes to tumor progression. Int J Cancer.
125:1276–1284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koga F, Kageyama Y, Kawakami S, et al:
Prognostic significance of endothelial Per-Arnt-sim domain protein
1/hypoxia-inducible factor-2alpha expression in a subset of tumor
associated macrophages in invasive bladder cancer. J Urol.
171:1080–1084. 2004. View Article : Google Scholar
|
|
19
|
Rajkumar T, Vijayalakshmi N, Gopal G,
Sabitha K, Shirley S, Raja UM and Ramakrishnan SA: Identification
and validation of genes involved in gastric tumorigenesis. Cancer
Cell Int. 10:452010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jackson CB, Judd LM, Menheniott TR, et al:
Augmented gp130-mediated cytokine signalling accompanies human
gastric cancer progression. J Pathol. 213:140–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Won HH, Kim JW, Kim MJ, Kim S, Park JH and
Lee KA: Interleukin 10 polymorphisms differentially influence the
risk of gastric cancer in East Asians and Caucasians. Cytokine.
51:73–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis C and Murdoch C: Macrophage
responses to hypoxia: implications for tumor progression and
anti-cancer therapies. Am J Pathol. 167:627–635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Culig Z: Cytokine disbalance in common
human cancers. Biochim Biophys Acta. 1813:308–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Savage SA, Abnet CC, Mark SD, et al:
Variants of the IL8 and IL8RB genes and risk for gastric cardia
adenocarcinoma and esophageal squamous cell carcinoma. Cancer
Epidemiol Biomarkers Prev. 13:2251–2257. 2004.PubMed/NCBI
|
|
26
|
Waugh D and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thong-Ngam D, Tangkijvanich P, Lerknimitr
R, Mahachai V, Theamboonelrs A and Poovorawan Y: Diagnostic role of
serum interleukin-18 in gastric cancer patients. World J
Gastroenterol. 12:4473–4477. 2006.PubMed/NCBI
|
|
28
|
Szaflarska A, Szczepanik A, Siedlar M,
Czupryna A, Sierzega M, Popiela T and Zembala M: Preoperative
plasma level of IL-10 but not of proinflammatory cytokines is an
independent prognostic factor in patients with gastric cancer.
Anticancer Res. 29:5005–5012. 2009.PubMed/NCBI
|
|
29
|
Paul SR, Bennett F, Calvetti JA, et al:
Molecular cloning of a cDNA encoding interleukin 11, a stromal
cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl
Acad Sci USA. 87:7512–7516. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du X and Williams DA: Interleukin-11:
review of molecular, cell biology, and clinical use. Blood.
89:3897–3908. 1997.PubMed/NCBI
|
|
31
|
Nakayama T, Yoshizaki A, Izumida S, et al:
Expression of interleukin-11 (IL-11) and IL-11 receptor alpha in
human gastric carcinoma and IL-11 upregulates the invasive activity
of human gastric carcinoma cells. Int J Oncol. 30:825–833.
2007.PubMed/NCBI
|
|
32
|
Okano F and Yamada K: Canine
interleukin-18 induces apoptosis and enhances Fas ligand mRNA
expression in a canine carcinoma cell line. Anticancer Res.
20:3411–3415. 2000.PubMed/NCBI
|
|
33
|
Coughlin CM, Salhany KE, Wysocka M, et al:
Interleukin-12 and interleukin-18 synergistically induce murine
tumor regression which involves inhibition of angiogenesis. J Clin
Invest. 101:1441–1452. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cho D, Song H, Kim YM, et al: Endogenous
interleukin-18 modulates immune escape of murine melanoma cells by
regulating the expression of Fas ligand and reactive oxygen
intermediates. Cancer Res. 60:2703–2709. 2000.PubMed/NCBI
|
|
35
|
Lissoni P, Brivio F, Rovelli F, et al:
Serum concentrations of interleukin-18 in early and advanced cancer
patients: enhancedsecretion in metastatic disease. J Biol Regul
Homeost Agents. 14:275–277. 2000.PubMed/NCBI
|