Introduction
Hepatocellular carcinoma (HCC) is a major cause of
mortality, with a high prevalence in Asia and Africa and an
increase in the incidence rates in Western countries (1). Patients with chronic hepatitis B or C
virus infection as well as patients with liver cirrhosis of
different etiologies are at high risk for development of liver
cancer. Monitoring of these patients requires liver ultrasonography
(US) every 6 months, for early detection of HCC (2,3). The
sensitivity of US varies depending on the size of the lesion and
the skills of the operator. For small lesions <1 cm in diameter,
the sensitivity is 50%; it increases to 70% for lesions
approximately 1 cm and reaches 90% when >5 cm, with specificity
between 48 and 94% (4). Other
radiographic tests with greater sensitivity and specificity are not
suitable for monitoring. Contrast-enhanced CT or
gadolinium-enhanced MRI are usually performed to evaluate
suspicious lesions detected by US (5–8).
The most widely used serum marker for HCC is
α-fetoprotein (AFP). It demonstrates low specificity (76%), which
increases when elevated cut-off levels are used, but exhibits
concomitant loss of sensitivity (maximum 60%) (9–11).
Therefore, 40% of patients diagnosed radiographically with HCC have
low serum AFP levels. Additionally, AFP can be found to be elevated
in patients who are at high risk for HCC and have no radiological
evidence of a liver tumor, due to temporal activation of the AFP
gene (12). Due to this poor
performance, previous recommendations (2) have been altered and serum AFP testing
is not currently included in the instructions for HCC surveillance
(3).
Furthermore, for the screening and evaluation of the
disease, novel HCC markers have been clinically implemented.
AFP-L3, an AFP glycoform with strong binding capacity for lens
culinaris agglutinin (LCA), has been used in Japan as a specific
marker for early HCC diagnosis for several years (13). Another marker which has also been
shown to be present in 50–60% of patients with HCC is des-γ
carboxyprothrombin (DCP or PIVKA-II), a prothrombin form lacking
carboxylation (14–16). The performance characteristics of
these new HCC markers might be differentiated by ethnicity,
etiology, stage of liver disease and total AFP levels (12,17,18).
In Greece, as well as in other areas of the Mediterranean basin,
where the etiology of HCC is primarily chronic viral hepatitis
(1,19), the implementation of these tests has
not been studied extensively.
In our study, we evaluated the combined testing of
AFP-L3 and DCP as HCC markers in patients with serum AFP levels
that were not in accordance with radiological findings. We
investigated one group of patients with definite HCC characterized
by either low or moderately elevated total AFP and a second group
of patients with mild to moderate AFP elevation which showed no
evidence of HCC when evaluated by extensive imaging.
Materials and methods
Patients
One hundred and fifty consecutive patients were
hospitalized or seen in the Liver Unit of Hippokration Hospital,
Athens, with the diagnosis of HCC, during the period 2009–2010.
From this cohort, 60 patients (40%) had serum total AFP <200
ng/ml. At the same time, 50 patients with persistently elevated AFP
>10 ng/ml, without radiographic or other evidence of HCC for at
least one year of follow-up, were identified. Informed consent was
obtained from all patients. The study was approved by the
Institution’s Ethics Committee.
AFP-L3% and DCP
Total serum AFP was determined by routine
immunoassays (mainly Architect, Abbott) at the time of visit or
hospitalization. Serum aliquots were collected and stored at −70°C
until further analysis. AFP-L3% and DCP were measured by an
automated Liquid Phase Binding Assay System (LiBASys, Wako). A
liquid-phase binding reaction between antigen and antibody was used
with bound and free forms separated by column chromatography
(20,21). Total AFP and the L3 fraction were
measured simultaneously. An AFP-L3% assay, using on-chip
electrokinetic reaction and separation by affinity electrophoresis
(μTAS, Wako i30) was performed when AFP-L3 was undetectable by
LiBASys (22). AFP-L3% >10% and
DCP >7.5 ng/ml were considered positive.
Statistical analysis
Data entry and statistical analysis were performed
with the statistical package IBM SPSS (version 20, SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate
statistically significant differences and all reported p-values
were based on two-tailed tests. Corrected χ2 or
two-tailed Fisher’s exact test and t-test or the Mann-Whitney test
were used for qualitative and quantitative data, when
appropriate.
Results
Patients
The underlying liver disease for the 60 patients
studied with HCC was indicated as chronic viral hepatitis in 43/60
patients (71.5%), as alcohol-related liver disease in 2 patients
(3%), and as cryptogenic liver disease, including steatohepatitis,
in 15 patients (25%). Their mean AFP was 22 ng/ml (median 8.5
ng/ml, range 1.7–173 ng/ml) and in the vast majority (88.5%) <50
ng/ml. The group of patients without radiographic evidence of HCC
consisted of 22 patients with chronic viral infection (40%), 1 (2%)
with alcohol-related liver disease and 27 with cryptogenic disease
(54%). Their mean AFP was 22 ng/ml (median 15 ng/ml, range
10.15–140 ng/ml). Patient characteristics are shown in Table I.
 | Table ICharacteristics of studied patients
with misleading total AFP. |
Table I
Characteristics of studied patients
with misleading total AFP.
| Patients with
HCC | Patients without
HCC |
|---|
|
|
|
|---|
| Male | Female | Total | Male | Female | Total |
|---|
| Number (N) | 48 | 12 | 60 | 15 | 35 | 50 |
| Median age in years
(range) | 66.5 (48–85) | 70 (38–79) | 67 (38–85) | 48 (34–73) | 67 (40–82) | 63 (34–82) |
| Multifocal HCC
(%) | 13 (27) | 5 (42) | 18 (30) | 0 (0) | 0 (0) | 0 (0) |
| HBV (%) | 28 (58) | 1 (8) | 29 (48) | 3 (20) | 4 (11) | 7 (14) |
| HCV (%) | 7 (15) | 7 (58) | 14 (23) | 3 (20) | 12 (34) | 15 (30) |
| Alcohol (%) | 2 (4) | 0 (0) | 2 (3) | 1 (7) | 0 (0) | 1 (2) |
| Other (%) | 11 (23) | 4 (33) | 15 (25) | 8 (53) | 19 (54) | 27 (54) |
| Median AFP ng/ml
(range) | 8 (1.7–173) | 12.5 (2.0–128) | 8.5 (1.7–173) | 13.6 (10.2–30) | 15.5 (10.4–140) | 15 (10.2–140) |
AFP-L3% and DCP in patients with HCC
AFP-L3% by LiBASys was positive in 12/60 (20%)
patients with HCC with mean levels of 48.7% (median 49.8%, range
13.2–89.6%). Total AFP in patients positive for AFP-L3% was
12.4–137.2 ng/ml (mean 38.6, median 22.1 ng/ml) by LIBASys compared
to common methods that determined total AFP to be 9–173 ng/ml (mean
52.4, median 41.9 ng/ml). Furthermore, total AFP values by LiBASys
were lower in 45/60, equal in 13/60, and minimally higher in 2/60
cases than previously determined, with a mean difference of −60%.
In one of the 34 patients with AFP <10 ng/ml, as indicated by
routine methodology, AFP levels were found to be >10 ng/ml by
LiBASys (14.4 ng/ml). In this patient, both AFP-L3% and DCP were
positive. AFP-L3% was not detectable in any of the remaining 33
patients, whereas DCP was positive in 13 patients. DCP was positive
in a total of 26/60 (43%) patients with median levels of 115 ng/ml.
Eighteen of the 26 DCP positive patients were negative for AFP-L3%.
A total of 30 patients (50%) tested positive for either DCP or
AFP-L3 by LiBASys. Supplementary AFP-L3% testing on μTAS revealed
10 more positive patients. The sensitivity of AFP-L3% increased
significantly, reaching 37% (assuming all samples positive by
LiBASys would be positive by μTAS), although 14 of the 26 DCP
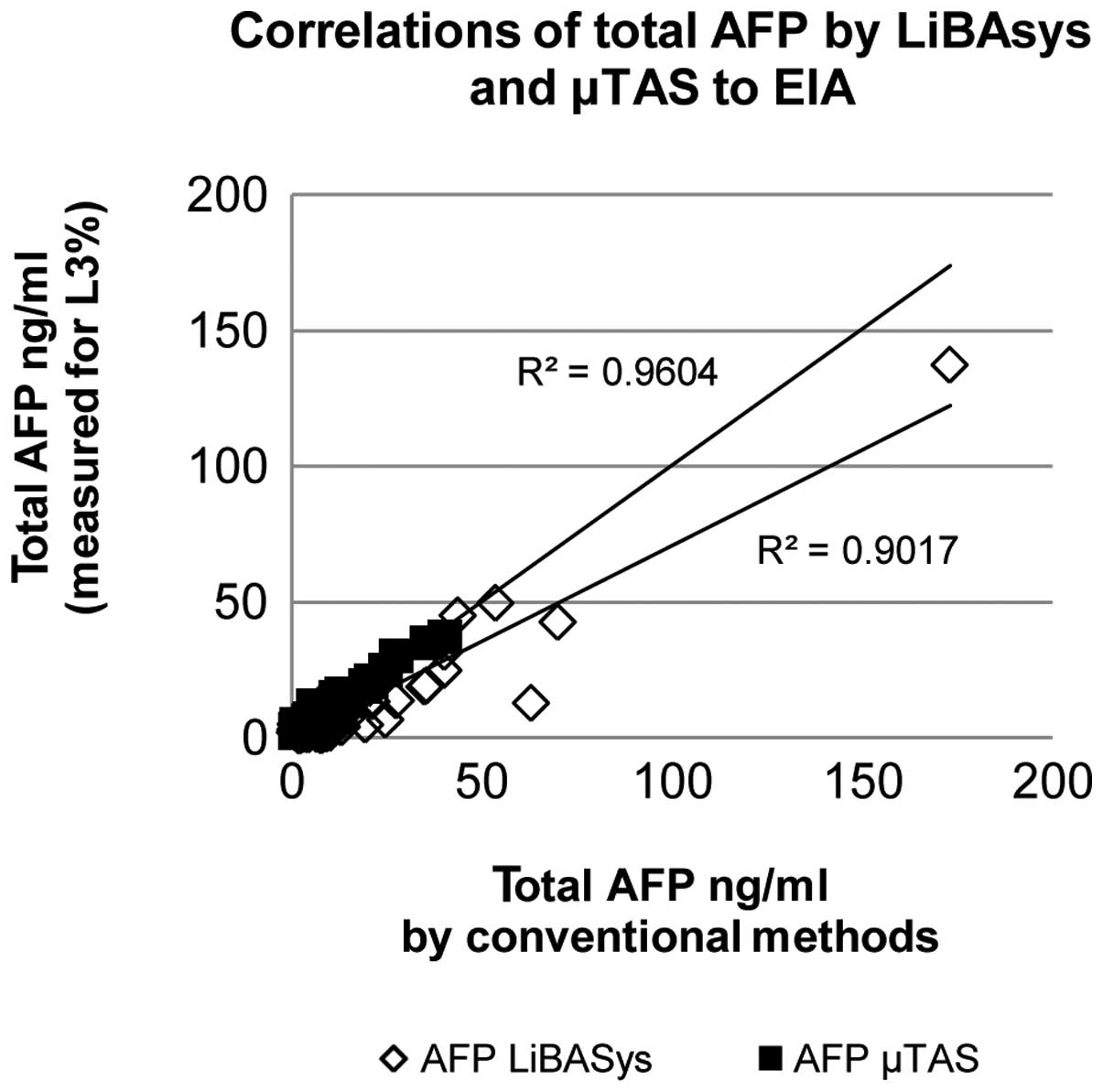
positive patients remained AFP-L3 negative (Table II). Total AFP by μTAS correlated
better with values obtained by routine immunoassays (mean
difference 0.7 ng/ml) (Fig. 1) and
differed from LiBASys (mean difference 4 ng/ml). Overall, 36/60
(60%) patients with HCC were positive for either AFP-L3% or DCP
(sensitivity 60%). In patients with AFP <10 ng/ml, the
sensitivity of combined testing was 56% (19/34). The total AFP
sensitivity (as HCC marker at levels >200 ng/ml) of combined
testing in the whole HCC cohort was 84%. No correlation between
AFP-L3% or DCP and number of lesions, stage of underlying fibrosis
or etiology of HCC was found.
 | Table IIDCP and AFP-L3% in patients with HCC
and non-diagnostic total AFP. |
Table II
DCP and AFP-L3% in patients with HCC
and non-diagnostic total AFP.
| AFP-L3% | |
|---|
|
| |
|---|
| DCP | + (%) | − (%) | Total (%) |
|---|
| + | 12 (20) | 14 (23) | 26 (43) |
| - | 10 (17) | 24 (40) | 34 (57) |
| Total | 22 (37) | 38 (63) | 60 |
AFP-L3% and DCP in patients with high
total AFP and no radiographic evidence of HCC
Among the 50 patients with no evidence of HCC and
persistent AFP >10 ng/ml (mean 22 ng/ml), total AFP by LiBASys
was elevated (mean 26.8 ng/ml, range 11–120 ng/ml) in 14 (28%) and
in normal range (mean 4.1 ng/ml) in 36 (72%) patients. The mean AFP
difference between enzyme immunoassay (EIA) and LiBASys was 9 ng/ml
(median 6.5 ng/ml, range 1.5–36 ng/ml). DCP was negative in all
patients and AFP-L3% in 49/50 patients. The patient mean follow-up
was 3 years (range 1–4 years). One patient with chronic hepatitis C
and AFP 140 ng/ml who tested negative for both AFP-L3% and DCP, was
recently diagnosed with HCC. This patient was not used in the
calculations of sensitivity. In another patient, with AFP 13 ng/ml,
AFP-L3% was found positive by LiBASys (11.7/16%) in the two
different samples that were collected at a time interval of 20 days
(only the first was used for calculations). This patient has not
developed HCC during follow-up. Supplementary AFP-L3% testing on
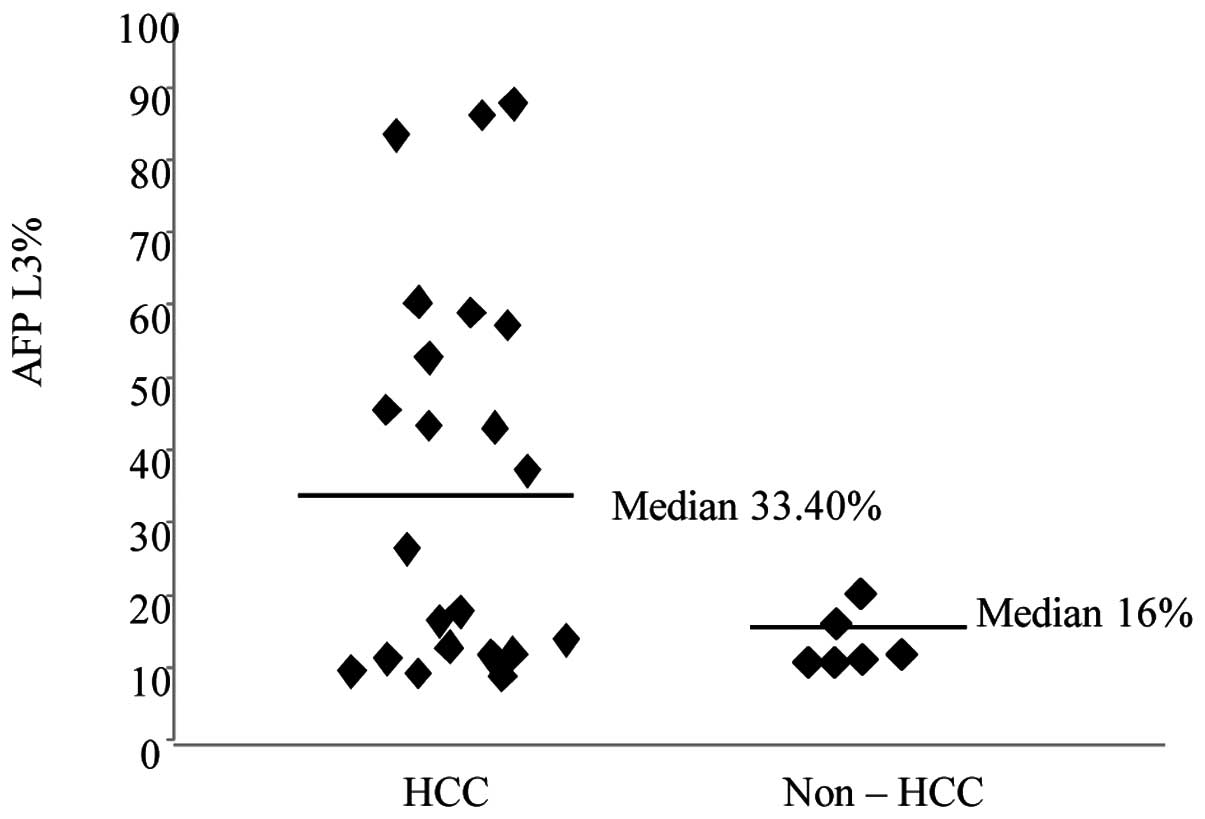
μTAS was positive in 5 more patients. AFP-L3% levels in positive
cases were higher in HCC (mean 38.07%) than in non-HCC patients
(mean 16%) (Fig. 2). The
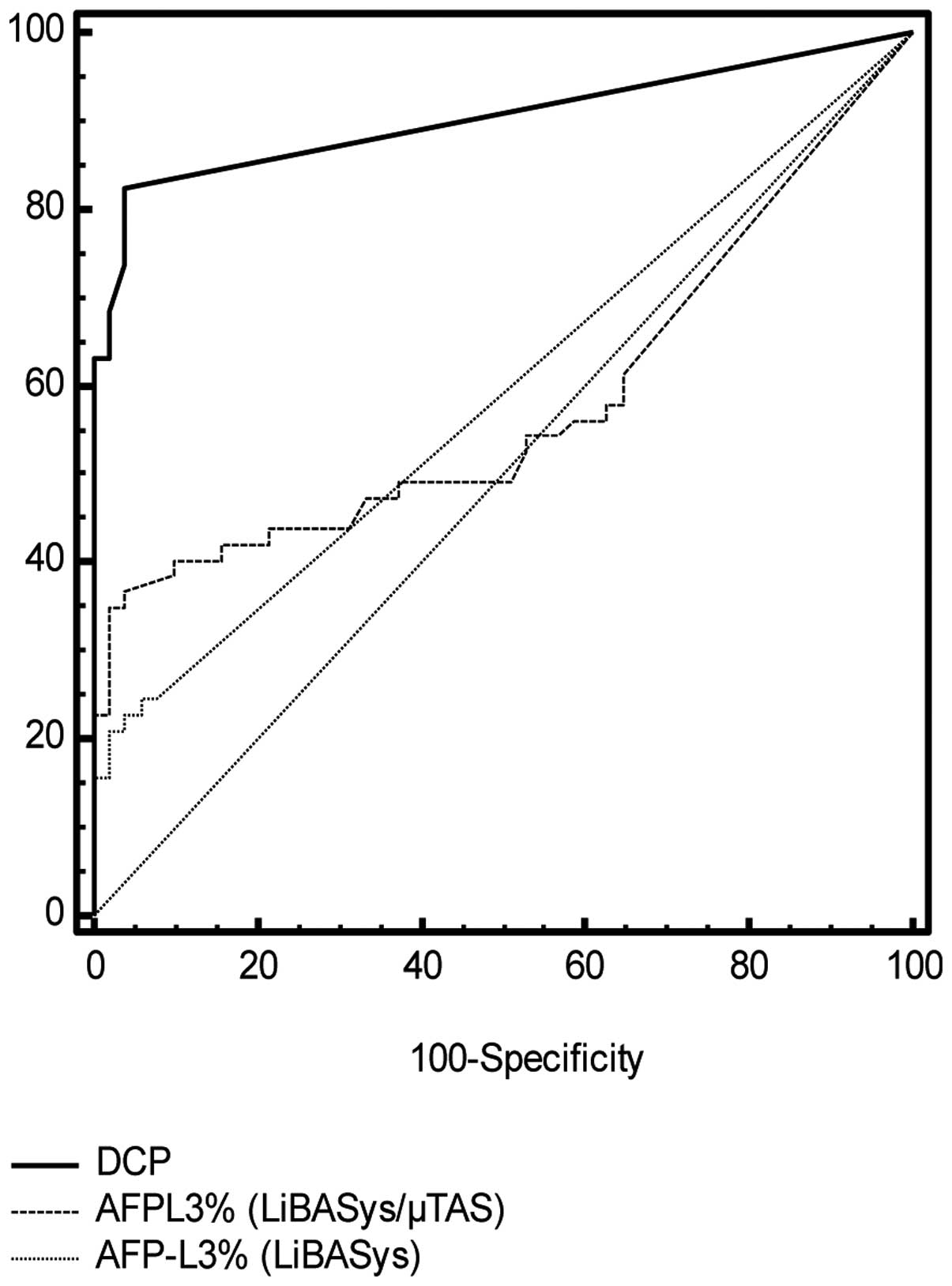
specificity in the tested population was 100% for DCP and 78.6% for
AFP-L3%. Receiver operating characteristic (ROC) curves are shown
in Fig. 3.
Discussion
In the present study, we examined the combined use
of serum HCC markers in two patient populations with chronic liver
disease in which total AFP was inconsistent with radiological
findings. Patients with HCC often demonstrate low levels of serum
AFP compared to patients with benign liver disease which can
present high AFP values, resulting in extensive and repeated
radiological examinations (23).
Several new serum markers have been investigated for HCC, including
proteins, enzymes, cytokines, autoantibodies and molecular markers.
Their combined use appears to increase the accuracy and sensitivity
of serological diagnosis of HCC (24–30).
AFP-L3% and DCP are HCC markers that can be measured
on the same platform, making testing more friendly to clinical
laboratories. AFP-L3 is an isoform of AFP derived only by cancer
cells, and is, thus, one of the most promising new HCC serum
markers. This test is already being used in clinical practices and
routine screening mainly in Asia (31,32).
Previous reports have indicated the effectiveness of the combined
use of AFP-L3% and DCP in HCC surveillance (33,34),
however, their implementation in Western Europe remains limited.
Our results in a cohort of 150 HCC patients showed that combined
measurement of AFP-L3% and DCP by LiBASys, in patients with low
total AFP, increases the sensitivity of serological diagnosis by
20%. In the tested population, DCP demonstrated greater sensitivity
(43 vs. 20%) and specificity (100 vs. 98%) compared to AFP-L3%. On
the other hand, the LiBASys analyzer persistently yielded lower
total serum AFP compared to routine methods.
The implementation of the μTAS on-chip immunoassay
(35) increased the sensitivity of
combined testing by 10%. In previous studies, this sensitive
AFP-L3% assay was applied in patients with total AFP <20 ng/ml
with promising results (36–38).
The cut-off level that should be used for optimal results with this
new assay has yet to be fully clarified. In one study, AFP-L3% was
>10 in 14.8%, >7 in 26.7% and >5 in 41.5% of patients with
HCC (36). Other investigators
demonstrated sensitivity of 44.6 and specificity of 71.2% using a
5% limit, in patients with low AFP (37). The cut-off level of 6–7% was
suggested in a study involving HCC-treated patients with low AFP
(38). High sensitive AFP-L3 was
found useful in predicting recurrence of HCC after treatment with a
cut-off of 5% (39). In our study,
sera from patients with HCC, with low total AFP (mean 9.4 ng/ml)
and undetectable AFP-L3% by LiBASys, were tested with μTAS. This
resulted in improvement of AFP-L3% sensitivity since it was found
positive in 10 additional sera. We used the same cut-off level of
10% as in the LiBASys assay. At lower cut-off values, the
sensitivity would further increase, adding 5 more HCC patients at
levels >7% (sensitivity 45%) and 10 more with a >5% fraction,
(sensitivity 53%), but with a significant concomitant decrease in
specificity at levels of 44–72%. AFP-L3 at levels between 5 and 10%
by μTAS was observed in 27 (54%) patients without any radiographic
evidence of HCC.
In patients with increased AFP of non-neoplastic
origin, as determined by thorough radiographic studies, in a
relatively long period of follow-up, the combined DCP/AFP-L3%
testing displayed high specificity (88%). In 49 of 50 individuals
with mean follow-up of 3 years and persistently increased AFP with
no evidence of HCC, only 1 patient was found positive for AFP-L3%
by LiBASys and none for DCP. With the implementation of a more
sensitive assay for AFP-L3%, a total of 6 patients with no
radiographic evidence of HCC were found to be positive at levels
>10%. These patients are under close follow-up with no
radiographic evidence of HCC as yet.
The values of total AFP with μTAS were higher than
LiBASys and much closer to routine immunoassays. This may
contribute to the increased sensitivity of the assay and could make
possible the complete replacement of solitary AFP measurements to
combination testing with AFP-L3% and DCP on one instrument.
With combined testing, 60% of patients with HCC and
non-diagnostic total AFP had at least one positive tumor marker,
AFP-L3% and/or DCP. This approach seems to offer a more sensitive
laboratory diagnostic procedure than total AFP and may contribute
to earlier detection of HCC and improved survival (40). It has also been suggested that a
relationship exists between these serum tumor markers and the stage
or outcome of the disease (41,42).
However, in our study, AFP-L3% and DCP were not associated with the
cause, number of lesions or the outcome of HCC. This could be due
to the study population, which, in the great majority, consisted of
hospitalized patients with symptoms of advanced liver disease, and
the cross-sectional type of the study.
In conclusion, combined testing of DCP and AFP-L3%
was found to be of major benefit for the diagnosis of HCC. The
higher specificity of these markers, as compared to total AFP, may
aid in supporting the benign nature of elevated AFP as a result of
hepatocyte regeneration in some patients with chronic liver
disease.
Acknowledgements
We thank Professor S.J. Hadziyannis for his
contribution and Wako Inc. and local distributors for the provision
of reagents.
Abbreviations:
|
AFP-L3%
|
α-fetoprotein L3%
|
|
DCP
|
des-γ carboxyprothrombin
|
|
HCC
|
hepatocellular carcinoma
|
|
LCA
|
lens culinaris agglutinin
|
References
|
1
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases.
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005.
|
|
3
|
Bruix J and Sherman M: American
Association for the Study of the Liver. Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colli A, Fraquelli M, Casazza G, Massironi
S, Colucci A, Conte D and Duca P: Accuracy of ultrasonography,
spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing
hepatocellular carcinoma: a systematic review. Am J Gastroenterol.
101:513–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicolau C, Vilana R, Bianchi L and Brú C:
Early-stage hepatocellular carcinoma: the high accuracy of
real-time contrast-enhanced ultrasonography in the assessment of
response to percutaneous treatment. Eur Radiol. 17(Suppl 6):
F80–F88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maruyama H, Yoshikawa M and Yokosuka O:
Current role of ultrasound for the management of hepatocellular
carcinoma. World J Gastroenterol. 14:1710–1719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SK, Kim SH, Lee WJ, Kim H, Seo JW,
Choi D, Lim HK, Lee SJ and Lim JH: Preoperative detection of
hepatocellular carcinoma: ferumoxides-enhanced versus mangafodipir
trisodium-enhanced MR imaging. AJR Am J Roentgenol. 179:741–750.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Snowberger N, Chinnakotla S, Lepe RM,
Peattie J, Goldstein R, Klintmalm GB and Davis GL: Alpha
fetoprotein, ultrasound, computerized tomography and magnetic
resonance imaging for detection of hepatocellular carcinoma in
patients with advanced cirrhosis. Aliment Pharmacol Ther.
26:1187–1194. 2007. View Article : Google Scholar
|
|
9
|
Kudo M: The 2008 Okuda lecture: Management
of hepatocellular carcinoma: from surveillance to molecular
targeted therapy. J Gastroenterol Hepatol. 25:439–452. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trevisani F, D’Intino PE, Morselli-Labate
AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S,
Roda E and Bernardi M: Serum alpha-fetoprotein for diagnosis of
hepatocellular carcinoma in patients with chronic liver disease:
influence of HBsAg and anti-HCV status. J Hepatol. 34:570–575.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gambarin-Gelwan M, Wolf DC, Shapiro R,
Schwartz ME and Min AD: Sensitivity of commonly available screening
tests in detecting hepatocellular carcinoma in cirrhotic patients
undergoing liver transplantation. Am J Gastroenterol. 95:1535–1538.
2000. View Article : Google Scholar
|
|
12
|
Taketa K: Alpha-fetoprotein: reevaluation
in hepatology. Hepatology. 12:1420–1432. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Mallory T and Satomura S: AFP-L3: a
new generation of tumor marker for hepatocellular carcinoma. Clin
Chim Acta. 313:15–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malaguarnera G, Giordano M, Paladina I,
Berretta M, Cappellani A and Malaguarnera M: Serum markers of
hepatocellular carcinoma. Dig Dis Sci. 55:2744–2755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stefaniuk P, Cianciara J and
Wiercinska-Drapalo A: Present and future possibilities for early
diagnosis of hepatocellular carcinoma. World J Gastroenterol.
16:418–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tateishi R, Yoshida H, Matsuyama Y, Mine
N, Kondo Y and Omata M: Diagnostic accuracy of tumor markers for
hepatocellular carcinoma: a systematic review. Hepatol Int.
2:17–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huo TI, Hsia CY, Chu CJ, Huang YH, Lui WY,
Wu JC, Lee PC, Chi CW and Lee SD: The predictive ability of serum
alpha-fetoprotein for hepatocellular carcinoma is linked with the
characteristics of the target population at surveillance. J Surg
Oncol. 95:645–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volk ML, Hernandez JC, Su GL, Lok AS and
Marrero JA: Risk factors for hepatocellular carcinoma may impair
the performance of biomarkers: a comparison of AFP, DCP, and
AFP-L3. Cancer Biomark. 3:79–87. 2007.PubMed/NCBI
|
|
19
|
Raptis I, Koskinas J, Emmanouil T and
Hadziyannis S: Changing relative roles of hepatitis B and C viruses
in the aetiology of hepatocellular carcinoma in Greece.
Epidemiological and clinical observations. J Viral Hepat.
10:450–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamagata Y, Shimizu K, Nakamura K, Henmi
F, Satomura S, Matsuura S and Tanaka M: Simultaneous determination
of percentage of Lens culinaris agglutinin-reactive
alpha-fetoprotein and alpha-fetoprotein concentration using the
LiBASys clinical auto-analyzer. Clin Chim Acta. 327:59–67. 2003.
View Article : Google Scholar
|
|
21
|
Owen WE, Roberts RF and Roberts WL:
Performance characteristics of the LiBASys des-gamma-carboxy
prothrombin assay. Clin Chim Acta. 389:183–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kagebayashi C, Yamaguchi I, Akinaga A,
Kitano H, Yokoyama K, Satomura M, Kurosawa T, Watanabe M, Kawabata
T, Chang W, Li C, Bousse L, Wada HG and Satomura S: Automated
immunoassay system for AFP-L3% using on-chip electrokinetic
reaction and separation by affinity electrophoresis. Anal Biochem.
388:306–311. 2009.
|
|
23
|
Eleftheriou N, Heathcote J, Thomas HC and
Sherlock S: Serum alpha-fetoprotein levels in patients with acute
and chronic liver disease. Relation to hepatocellular regeneration
and development of primary liver cell carcinoma. J Clin Pathol.
30:704–708. 1977. View Article : Google Scholar
|
|
24
|
Hsieh SY, He JR, Yu MC, Lee WC, Chen TC,
Lo SJ, Bera R, Sung CM and Chiu CT: Secreted ERBB3 isoforms are
serum markers for early hepatoma in patients with chronic hepatitis
and cirrhosis. J Proteome Res. 10:4715–4724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jirun P, Zhang G, Kim HK, Ha SA, Zhongtian
J, Shishi Q, Zhuqingqing C, Lei G, Yoo J, Kim S, Park YG, Wang J,
Yang Y, Xu Z, Huang Z, Lee YK, Song EY and Kim JW: Clinical utility
of alpha fetoprotein and HCCR-1, alone or in combination, in
patients with chronic hepatitis, liver cirrhosis and hepatocellular
carcinoma. Dis Markers. 30:307–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hua D, Hu Y, Wu YY, Cheng ZH, Yu J, Du X
and Huang ZH: Quantitative methylation analysis of multiple genes
using methylation-sensitive restriction enzyme-based quantitative
PCR for the detection of hepatocellular carcinoma. Exp Mol Pathol.
9:455–460. 2011. View Article : Google Scholar
|
|
27
|
Qu KZ, Zhang K, Li H, Afdhal NH and
Albitar M: Circulating microRNAs as biomarkers for hepatocellular
carcinoma. J Clin Gastroenterol. 45:355–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu T, Xue R, Dong L, Wu H, Zhang D and
Shen X: Rapid determination of serological cytokine biomarkers for
hepatitis B virus-related hepatocellular carcinoma using antibody
microarrays. Acta Biochim Biophys. 43:45–51. 2011.
|
|
29
|
Yasmin Anum MY, Looi ML, Nor Aini AH,
Merican I, Wahidah A, Mohd Radzi AH, Nor Azizah A and Othman NH:
Combined assessment of TGF-beta-1 and alpha-fetoprotein values
improves specificity in the diagnosis of hepatocellular carcinoma
and other chronic liver diseases in Malaysia. Med J Malaysia.
64:223–227. 2009.PubMed/NCBI
|
|
30
|
Kang X, Sun L, Guo K, Shu H, Yao J, Qin X
and Liu Y: Serum protein biomarkers screening in HCC patients with
liver cirrhosis by ICAT-LC-MS/MS. J Cancer Res Clin Oncol.
136:1151–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Subwongcharoen S, Leelawat K,
Treepongkaruna SA and Narong S: Serum AFP and AFP-L3 in clinically
distinguished hepatocellular carcinoma from patients with liver
masses. J Med Assoc Thai. 94:S46–S51. 2011.PubMed/NCBI
|
|
32
|
Tanwandee T, Setthasin S,
Charatcharoenwitthaya P, Chainuvati S, Leelakusolvong S, Pausawasdi
N, Srikureja W, Pongprasobchai S, Manatsathit S, Kachintorn U, Ekpo
P and Senawong S: Clinical utility of lens culinaris
agglutinin-reactive alpha-fetoprotein in the diagnosis of
hepatocellular carcinoma: evaluation in a Thai referral population.
J Med Assoc Thai. 92:S49–S56. 2009.
|
|
33
|
Sterling RK, Jeffers L, Gordon F, Venook
AP, Reddy KR, Satomura S and Kanke F: Utility of Lens culinaris
agglutinin-reactive fraction of alpha-fetoprotein and
des-gamma-carboxy prothrombin, alone or in combination, as
biomarkers for hepatocellular carcinoma. Clin Gastroenterol
Hepatol. 7:104–113. 2009. View Article : Google Scholar
|
|
34
|
Donati M, Brancato G and Donati A:
Clinical biomarkers in hepatocellular carcinoma (HCC). Front
Biosci. 2:571–577. 2011.
|
|
35
|
Tamura Y, Igarashi M, Kawai H, Suda T,
Satomura S and Aoyagi Y: Clinical advantage of highly sensitive
on-chip immunoassay for fucosylated fraction of alpha-fetoprotein
in patients with hepatocellular carcinoma. Dig Dis Sci.
55:3576–3583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toyoda H, Kumada T, Tada T, Kaneoka Y,
Maeda A, Kanke F and Satomura S: Clinical utility of highly
sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in
hepatocellular carcinoma patients with alpha-fetoprotein <20
ng/ml. Cancer Sci. 102:1025–1031. 2011. View Article : Google Scholar
|
|
37
|
Oda K, Ido A, Tamai T, Matsushita M,
Kumagai K, Mawatari S, Saishoji A, Kure T, Ohno K, Toyokura E,
Imanaka D, Moriuchi A, Uto H, Oketani M, Hashiguchi T and Tsubouchi
H: Highly sensitive lens culinaris agglutinin-reactive
α-fetoprotein is useful for early detection of hepatocellular
carcinoma in patients with chronic liver disease. Oncol Rep.
26:1227–1233. 2011.
|
|
38
|
Morimoto M, Numata K, Nozaki A, Kondo M,
Moriya S, Taguri M, Morita S, Konno M, Sugo A, Miyajima E, Maeda S
and Tanaka K: Novel Lens culinaris agglutinin-reactive fraction of
α-fetoprotein: a biomarker of hepatocellular carcinoma recurrence
in patients with low α-fetoprotein concentrations. Int J Clin
Oncol. 17:373–379. 2012.
|
|
39
|
Kobayashi M, Hosaka T, Ikeda K, Seko Y,
Kawamura Y, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase
Y and Kumada H: Highly sensitive AFP-L3% assay is useful for
predicting recurrence of hepatocellular carcinoma after curative
treatment pre- and postoperatively. Hepatol Res. 41:1036–1045.
2011.
|
|
40
|
Tong MJ, Sun HE, Hsien C and Lu DS:
Surveillance for hepatocellular carcinoma improves survival in
Asian-American patients with hepatitis B: results from a
community-based clinic. Dig Dis Sci. 55:826–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toyoda H, Kumada T, Osaki Y, Oka H and
Kudo M: Role of tumor markers in assessment of tumor progression
and prediction of outcomes in patients with hepatocellular
carcinoma. Hepatol Res. 37(Suppl 2): S166–S171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shiina S, Tateishi R, Arano T, Uchino K,
Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto
T, Yoshida H, Omata M and Koike K: Radiofrequency ablation for
hepatocellular carcinoma: 10-year outcome and prognostic factors.
Am J Gastroenterol. 107:569–577. 2012.PubMed/NCBI
|