Introduction
Gastric cancer is the second most fatal malignancy
worldwide (1). Despite recent
advances in surgical technique, diagnostic methods and chemotherapy
regimens, no effective targeting therapy is available for gastric
cancer, as the molecular mechanisms underlying gastric cancer
development remain unclear (2,3).
microRNAs (miRNAs, miRs) are short, 19–22 nucleotide
long, on-coding RNAs that influence cellular processes at the
post-transcriptional level by targeting the 3′ untranslated region
of mRNA, causing reduced translation of proteins or degradation of
mRNAs (4–6). miRNAs are dysregulated in various
human types of cancer, including gastric adenocarcinoma (GA), and
play crucial roles in tumorigenesis, cell growth, differentiation
and apoptosis (7,8). Thus, miRNAs may function as tumor
suppressor genes or oncogenes (9).
Accumulating evidence suggests that the miRNA-200 family serve as
tumor suppressors in cancer cells. The miR-200 family consists of
two clusters: miR-200b, miR-200a and miR-429 located on chromosome
1; and miR-200c and miR-141 located on chromosome 12 (10,11).
Epithelial-mesenchymal transition (EMT) is a
pathological event associated with tumor progression and is
considered to influence and promote certain steps in the metastatic
cascade. Characterized by loss of cell-cell adhesion and apex-base
polarity, EMT enhances cell motility and metastasis (12,13).
Its prominent hallmarks are loss of expression of epithelial
markers such as E-cadherin and induction of mesenchymal markers
such as N-cadherin and vimentin (14). Ectopic expression of ZEB1, ZEB2,
Snail and Twist result in loss of E-cadherin-mediated cell-cell
adhesion, and induction of cell motility, suggesting that
activation of these transcriptional factors results in induction of
EMT phenotypes (15).
In this study, we confirmed the expression of
miR-200a in GA and normal tissue using fluorescent in situ
hybridization (FISH); we also showed that ZEB1 and ZEB2 are
involved in the EMT process and identified target genes of
miR-200a. Transcriptional factors such as Twist1 and Snail2 play
key roles in EMT. We further confirmed reduced miR-200a levels in
GA through downregulated E-cadherin expression and upregulation of
the major activator of the Wnt signaling pathway, β-catenin. We
determined that miR-200a acted as a tumor-suppressor factor and
inhibited the process of EMT and tumor growth.
Materials and methods
Patients and samples
A GA tissue microarray was obtained from Shaanxi
Chaoying Biotechnology Ltd., (Xi’an, China). Pathologic tumor
grades on the microarray were defined, according to the 2007 WHO
criteria, as follows: 4 cases with grade I, 22 with grade II, 34
with grade III and 4 with grade IV; 10 cases were normal gastric
tissue. Each dot was a 1.5-mm diameter tissue sample from an
individual specimen that had been pathologically confirmed. All
microarrays were stored in the dark at 4°C.
In situ hybridization
Using antisense locked nucleic acid (LNA) modified
oligonucleotide probes, in situ hybridization was performed
with the In-Situ Hybridization kit (Boster, Wuhan, China).
Sequences of the LNA/DNA oligonucleotides contained locked nucleic
acids at eight consecutive centrally located bases (indicated by
the underline) as shown: hsa-miR-200a 5′-ACA TCG TTA CCA GAC GAC AGT GTT
A-3′; hsa-miR-200b 5′-TCA TCA TTA CCA GGC AGT ATT
A-3′; and hsa-miR-200c 5′-TCC ATC ATT ACC CGG CAG TAT
TA-3′. Sections were deparaffinized and deproteinated, and
then prehybridized for 2 h in hybridization liquid in a humidified
chamber (50% formamide, 5X SSC). Sections were incubated with 20 μl
LNA-miR-200a, LNA-miR-200b and LNA-miR-200c hybridization solution
at 42°C for 16 h, after washing with phosphate-buffered saline
(PBS) 3 times. miR-200a, miR-200b and miR-200c were labeled with
Cy3-avidin at 0.5 mg/ml and incubated for 2 h at room temperature
in the dark. Nuclei were counterstained with a DAPI karyotyping kit
(GenMed, Boston, MA, USA). After washing with PBS 3 times, sections
were sealed, detected under a fluorescence microscope with an
OptiGrid system and analyzed by IPP6.1 (Olympus, Tokyo, Japan).
Cell culture and transfection
Human stomach adenocarcinoma cell lines, SGC7901,
were obtained from the Laboratory of Neuro-Oncology, Tianjin
Neurological Institute. Cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA)
supplemented with 12% fetal bovine serum (Invitrogen, Carlsbad, CA,
USA), and incubated at 37°C with 5% CO2. The miRNA
mimics and negative controls were synthesized by GenePharma
(Shanghai, China). Transfections with hsa-miR-200a mimics were
performed in serum-free medium 24 h after plating. Cell
transfection used Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions. Mimic sequences were: miR-200a mimic
sense, 5′-UAA CAC UGU CUG GUA ACG AUG U-3′ and anti-sense, 5′-AUC
GUU ACC AGA CAG UGU UAU U-3′; negative control sense, 5′-UUC UCC
GAA CGU GUC ACG UTT-3′ and anti-sense, 5′-ACG UGA CAC GUU CGG AGA
ATT-3′. The mixture was then added to cells; after 4 h, the medium
was changed to complete medium.
Quantative real-time PCR analysis
Total RNA was harvested using TRIzol (Invitrogen)
according to the manufacturer’s protocol. Concentrations of RNA
were determined using NanoDrop® ND-1000. Total RNA (1
μg) was used to synthesize cDNA by reverse transcription using MMLV
reverse transcriptase (Promega Corp., Madison, WI, USA) following
the manufacturer’s protocol. Real-time PCR analysis was performed
to determine the expression of miR-200a in SGC7901 cells 48 h after
transfection with miR-200a mimic or scrambled negative control.
qRT-PCR primers were purchased from GenePharma. All PCR reactions
were performed using standard PCR conditions: stage 1, 95°C for 3
min (1 cycle); stage 2, 95°C for 12 sec, followed by 62°C for 40
sec; stage 3, from 62 up to 95°C, followed by 0.2°C for 2 sec (1
cycle). Expression of U6 was used as an internal control.
Immunohistochemical analysis
Immunostaining was performed on paraffin-embedded
gastric tissue microarrays and paraffin sections of tumor specimens
using the avidin-biotin complex (ABC)-peroxidase method. The
sections were incubated with primary antibodies against ZEB1, ZEB2
and Twist (1:100 dilution, Abcam), E-cadherin and β-catenin (1:100
dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA),
N-cadherin (1:100 dilution, Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China), and Slug (1:100 dilution,
Cell Signaling Technology) overnight at 4°C. They were then treated
with biotinylated secondary antibody (1:100) for 1 h at room
temperature, followed by incubation with ABC-peroxidase for a
further 1 h. After washing with Tris-buffer, sections were stained
with 3,3′-diaminobenzidine (DAB) for 5 min, rinsed in water and
counterstained with hematoxylin. Negative controls were obtained by
substituting primary antibodies with non-immune serum. Sections
with no labeling or with <5% labeled cells were scored as 0; as
1 with 5–30% of cells labeled; as 2 with 31–70% of cells labeled;
and as 3 with ≥71% of cells labeled. Staining intensity was scored
similarly, with 0 for negative staining, 1 for weakly positive, 2
for moderately positive and 3 for strongly positive. Scores for
percentage of positive tumor cells and for staining intensity were
added to generate an immunoreactive score for each specimen. The
products of the quantity and intensity scores were calculated so
that a final score of 0–1 indicated negative expression (−), 2–3
indicated weak expression (+), 4–5 indicated moderate expression
(++) and 6 indicated strong expression (+++). Each sample was
examined separately and scored by two pathologists. Cases with
discrepancies in the scores were discussed to reach a
consensus.
Western blot analysis
Following transfection, cells were washed with
ice-cold PBS three times. The cells were then solubilized in 1%
Nonidet P-40 lysis buffer (20 mM Tris, pH 8.0, 137 mM NaCl, 1%
Nonidet P-40, 10% glycerol, 1 mM CaCl2, 1 mM
MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium
fluoride, 1 mM sodium orthovanadate and a protease inhibitor
mixture), then centrifuged at 20,000 × g for 15 min at 4°C. Protein
concentrations were measured by Nanodrop spectrophotometer (Gene,
USA). Proteins were harvested and 40 μg from each sample were
subjected to SDS-PAGE separation, and then transferred to
polyvinylidene difluoride (PVDF) membranes (Millipore, Bellerica,
MA, USA). The membranes were incubated with primary antibody
against ZEB1 and ZEB2 (1:1,000 dilution, Santa Cruz Biotechnology),
followed by incubation with HRP-conjugated secondary antibody
(1:1,000 dilution, Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd.). Specific proteins were detected using the SuperSignal
Protein Detection kit (Pierce, Rockford, IL, USA). After washing
with stripping buffer, the PVDF membrane was reprobed with
anti-GAPDH antibody (1:1,000 dilutions, Santa Cruz
Biotechnology).
Immunofluorescence staining
Forty-eight hours after transfection, cells were
seeded onto sterile cover slips and washed with cold PBS twice,
fixed with 4% paraformaldehyde in PBS, permeabilized with 0.1%
Triton X-100 for 10 min, and blocked in 1% BSA for 30 min at room
temperature to block non-specific binding. Cells were incubated in
appropriate primary antibodies (ZEB1, ZEB2, Snail2, Twist1,
E-cadherin, N-cadherin and β-catenin) overnight at 4°C. Samples
were washed, incubated with species-specific secondary
rhodamine-labeled antibodies in PBS (1:100 dilution) for 60 min.
Nuclei were stained with DAPI at room temperature for 10 min.
Immunofluorescence was examined using a confocal microscope (Leica
Microsystems, Heidelberg, Germany).
Subcutaneous tumor assay
BALB/c-A 6-week-old nude mice, bred at the
laboratory animal facility, were purchased from the animal center
of the Cancer Institute of Chinese Academy of Medical Science. All
experimental procedures were carried out according to the
regulations and Internal Biosafety and Bioethics Guidelines of the
Tianjin Medical University and the Tianjin Municipal Science and
Technology Commission. The SGC7901 subcutaneous tumor xenografts
were established as previously described (16). When the subcutaneous tumors reached
50 mm3 in size, 18 mice were randomly selected for the
control, scrambled miR-treated (scramble), and miR-200a-treated
groups (n=6 for each group). A mixture of 10 μl oligonucleotides
containing scrambled miR-200a mimics and 10 μl Lipofectamine 2000
was injected into the xenograft tumors in a multi-site injection
manner. The mice in the control group received 10 μl of PBS only.
Treatment was conducted at 2-day intervals, twice. Tumor volume was
measured with a caliper every 2 days, using the formula: volume =
1/2 (length × width2). At the end of a 21-day
observation period, the mice were sacrificed and tumor tissues were
removed for formalin fixation and preparation of paraffin-embedded
sections for immunohistochemical analysis.
Statistical analysis
Data were analyzed with the SPSS 10.0. The one-way
analysis of variance (ANOVA), the χ2 test and the
Pearson’s correlation were used to analyze significance between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
miRNA-200s expression and their
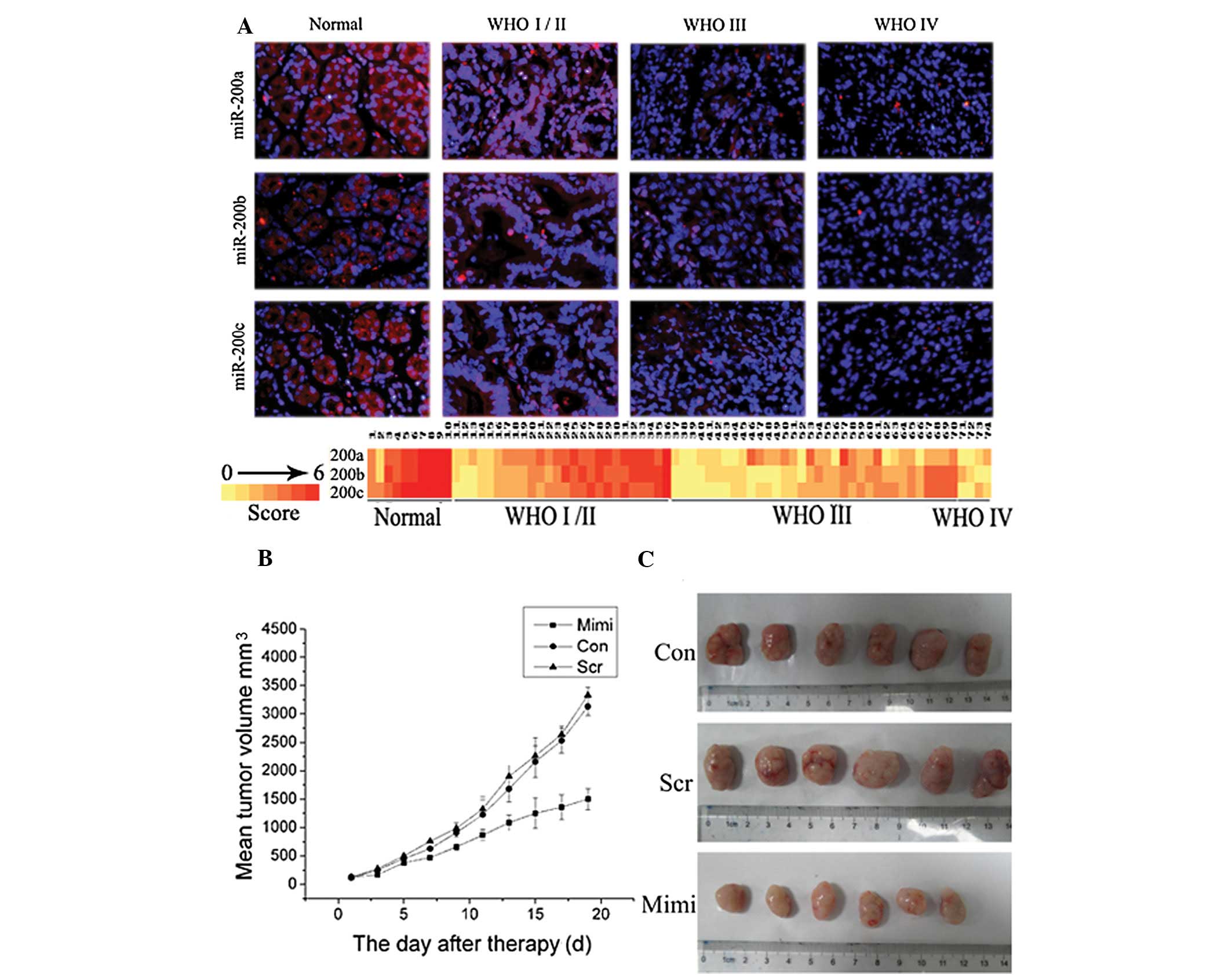
association with pathologic grade in gastric carcinoma
Analysis using FISH showed miR-200a, miR-200b and
miR-200c were expressed in gastric carcinoma; their positive rates
were 70.27% (52/74), 66.21% (49/74) and 68.91% (51/74),
respectively. Levels of miR-200a, miR-200b and miR-200c decreased
significantly in high grade GA (WHO grades III–IV) compared to low
grade GA (WHO grades I–II). Indeed, detectable levels of miR-200a,
miR-200b and miR-200c were found in 84.62% (22/26), 80.77% (21/26)
and 80.77% (21/26) low-grade gastric carcinomas, respectively, but
were detectable in 55.26% (21/38), 50.00% (19/38), 52.63% (20/38)
high-grade GAs, respectively (P<0.05; Fig. 1A). miR-200a, miR-200b and miR-200c
expression negatively correlated with WHO GA grades (Fig. 1A).
miR-200a inhibits xenograft tumor growth
in vivo
Our previous study revealed that overexpression of
miR-200a significantly inhibited SGC7901 cell growth, invasion and
induced G0/G1 phase arrest in
vitro(17). To further
investigate the effect of miR-200a on tumor growth in vivo,
SGC7901 cells were injected into three groups of nude mice to
construct SGC7901 gastric carcinoma xenografts. Main tumor volume
prior to injection with miR-200 mimic-transfected SGC7901 cells was
120±33.11 mm3. During the first 3 days of the
observation period, tumor sizes did not differ significantly among
the three groups (P<0.05). Following treatment, tumors in the
miR-200a-treated group grew slower than those in the control and
scramble group. On Day 7, tumors of the miR-200a-treated group
started to become significantly smaller than in the control groups
(P<0.05). At the end of the study, differences in tumor mass
between the miR-200a-treated group and the control group were
marked (P<0.01); however, tumor volume between control and
scramble mice did not differ significantly (Fig. 1C). Three weeks after injection, the
group with miR-200a mimics formed substantially smaller tumors
(1499.9±361.0 mm3) than did the control (3128.5±309.0
mm3) and the scramble groups (3325.8±278.1
mm3) (Fig. 1B).
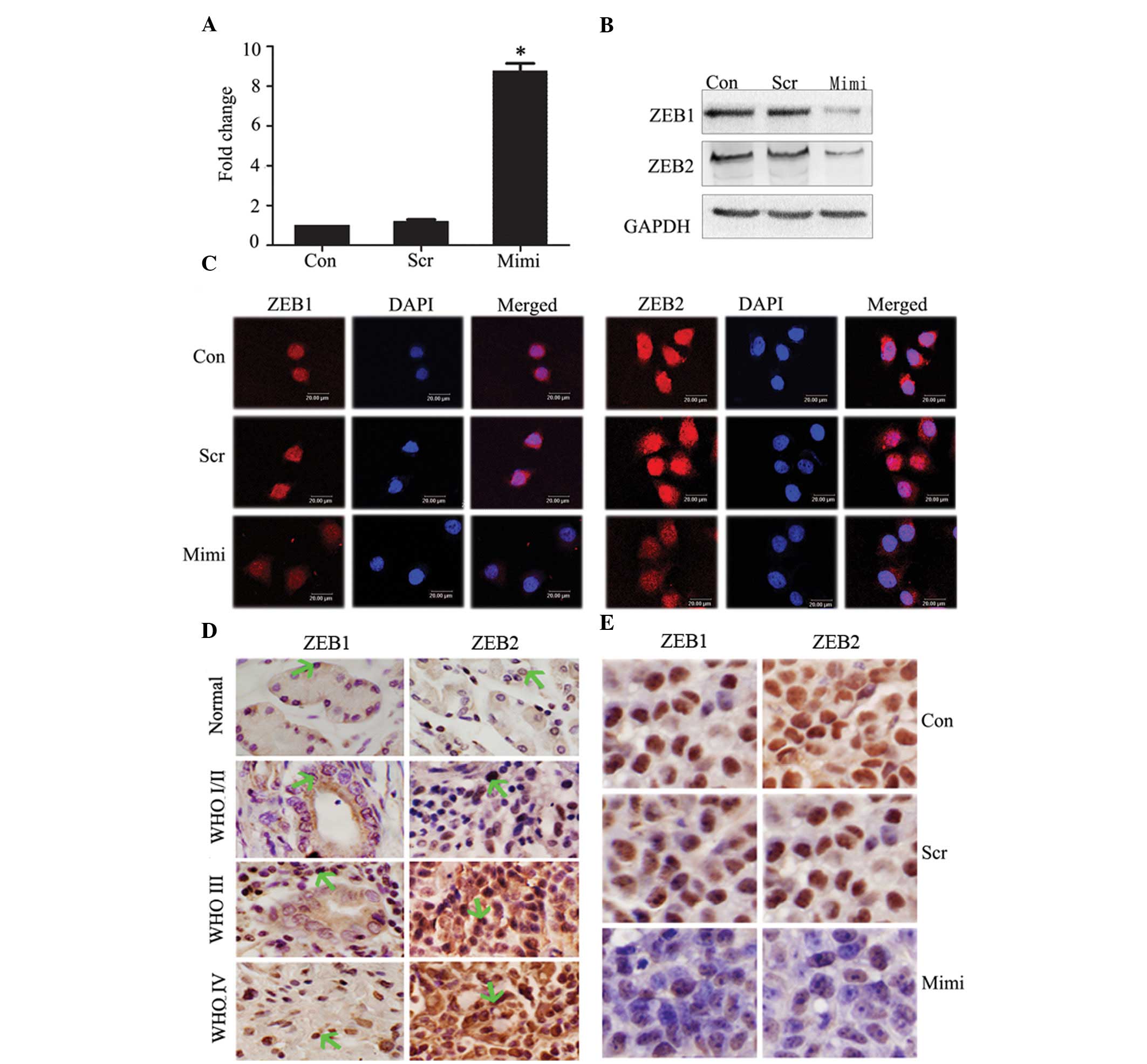
Expression of potential miR-200a target
genes
Forty-eight hours after transfection with miR-200a
mimics in SGC7901 cells, RT real-time PCR showed relative
expression of miR-200a in the miR-200 mimic-transfected group to be
upregulated ~10-fold compared to control groups (P<0.05)
(Fig. 2A). To further determine the
mechanism by which miR-200a regulates EMT and inhibits tumor
growth, we performed a miRNA target search using TargetScan. Some
predicted and validated target genes, such as ZEB1 and ZEB2, which
probably participate in EMT in GA, have been suggested as direct
targets for miR-200a in kidney tubular cells, ovarian cancer and
breast cancer (18–20). We tested the effects of miR-200a
elevation on ZEB1 and ZEB2 protein expression in SGC7901, using
western blot analysis. Overexpression of miR-200a reduced ZEB1 and
ZEB2 protein levels (Fig. 2B).
Immunofluorescence staining of ZEB1 and ZEB2 showed them to be
expressed in the nucleus, but decreased when miR-200a expression
increased (Fig. 2C).
Since finding ZEB1 and ZEB2 to be targets of
miR-200a, we immunohistochemically investigated the relationship
between miR-200a and ZEB1/ZEB2 expression in the same GA tissue
microarray. We have shown that miR-200a negatively correlates with
WHO grades. Expression of ZEB1 and ZEB2 increased significantly in
high-grade GA (WHO grades III–IV) compared to low-grade GA (WHO
grades I–II) (Fig. 2D). Given the
in vivo data of SGC7901 cells following treatment with
miR-200a mimics, immunohistochemical staining analysis of xenograft
tumors taken 21 days after treatment revealed that ZEB1 and ZEB2
levels were downregulated in the miR-200a-treated group compared to
tumors from the scramble and the control groups (Fig. 2E).
According to these data, we found an inverse
correlation between expression of miR-200a and ZEB1/ZEB2 protein in
cells, tissue samples and xenograft tumors. We postulate that
ZEB1/ZEB2 are the targets of miR-200a in GA.
miRNA-200a effects on E-cadherin and the
Wnt/β-catenin pathway inhibits EMT
miR-200a reportedly regulates both the EMT of the
cells and the activity of the Wnt/β-catenin signaling pathway
(17). To further determine the
mechanism by which miR-200a regulates EMT and the activity of the
Wnt/β-catenin signaling pathway, we transfected miR-200a mimics
into SGC7901 cells, and then used immunofluorescence staining to
investigate expression levels and locations of E-cadherin,
N-cadherin and β-catenin proteins. Re-expression of miR-200a
inhibits EMT by upregulating E-cadherin and downregulating
N-cadherin in the cytomembrane; the location of β-catenin shifts
from nucleus to cytoplasm (Fig.
3A). When E-cadherin is reduced or deleted, β-catenin is
released from catenin/cadherin complexes and is translocated to the
nucleus, activating the Wnt/β-catenin signaling pathway. miRNA-200a
acts as a tumor suppressor through its effects on E-cadherin,
thereby suppressing the Wnt/β-catenin pathway. Re-expression of
miR-200a could result in reversal of EMT to MET.
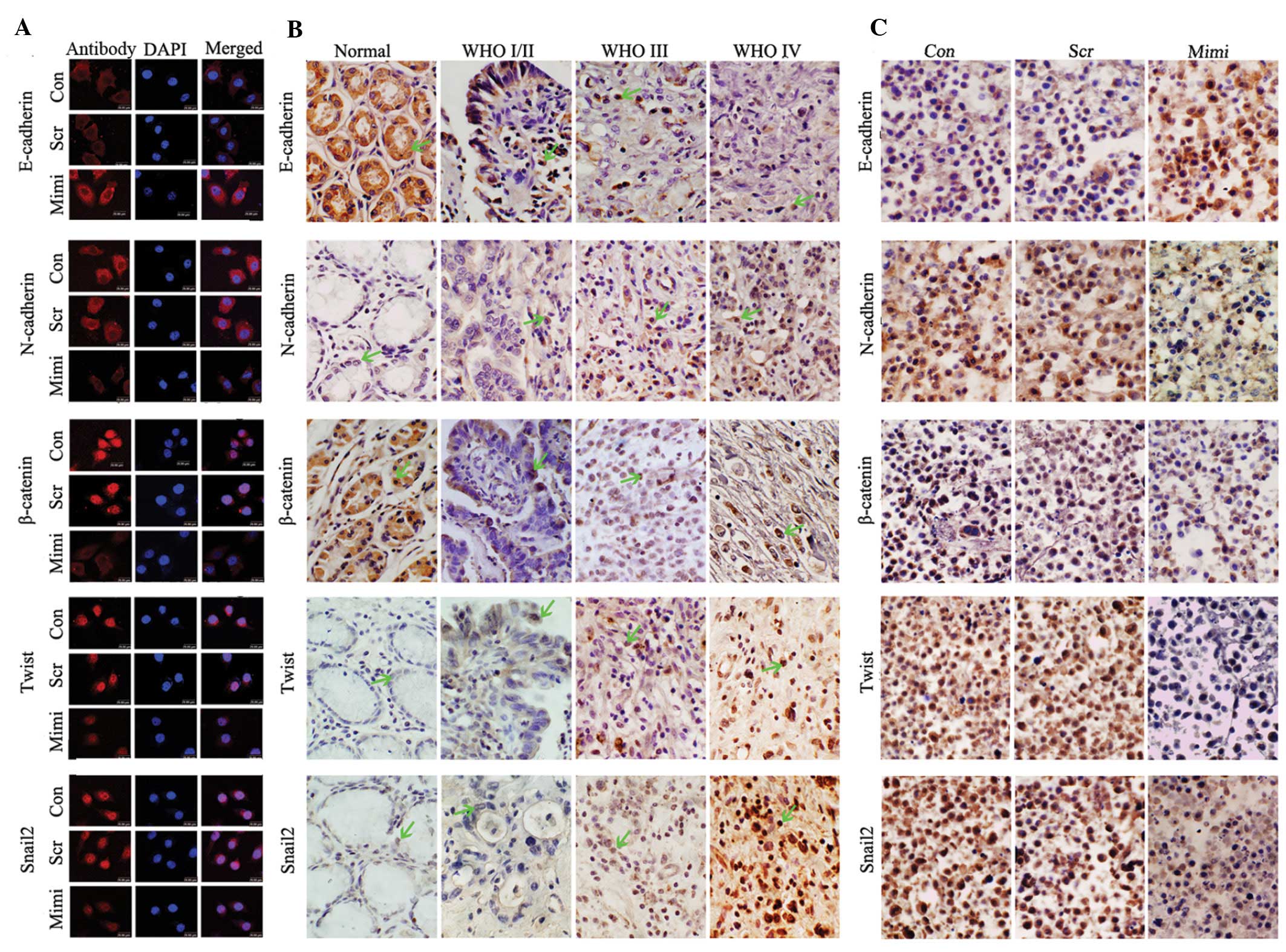 | Figure 3Impact of elevated miR-200a on the
expression of the EMT-associated proteins. (A) Location of
β-catenin shifted from nucleus to cytoplasm in SGC7901 cells when
miR-200a was upregulated, while levels of Snail2 and Twist1
decreased in the nucleus and N-cadherin decreased in cell
membranes, compared to control vector. E-cadherin increased in cell
membranes compared to control vector. (B) Expression of E-cadherin,
N-cadherin, β-catenin, Twist1 and Snail2 was detected in GA and in
normal gastric samples using immunohistochemistry. E-cadherin was
downregulated in GA compared to normal gastric mucosa, and was
negatively correlated with the WHO GA grades. However, levels of
N-cadherin, β-catenin, Twist1 and Snail2 protein were positively
correlated with the WHO GA grades. (C) Immunohistochemical analyses
of xenograft tumors after treatment with miR-200a mimics.
Expression of N-cadherin, β-catenin, Twist1 and Snail2 was
suppressed in xenograft tumors of the miR-200a-mimic treatment
group; however, the level of E-cadherin was increased compared with
control groups. |
These regulators play a crucial role in EMT. The
E-box-binding factor Snail functions as a repressor transcription
factor by directly binding to an E-box in the E-cadherin promoter,
thus directly repressing its transcription. Twist, a basic
helix-loop-helix transcriptional factor, suppresses E-cadherin
transcription, to promote EMT. An analysis of correlations between
miRNA and mRNA expression in publicly available data for NCI60 cell
lines showed Twist1 to be in the top 25 genes negatively correlated
with miR-200 expression (21). To
confirm whether transcription factors change expression when
treated with miR-200a mimics, and thus affect EMT regulation,
immunofluorescence staining of Twist and Snail2 showed that they
decreased in the nucleus, when miR-200a expression is elevated
(Fig. 3A). However, the underlying
mechanism requires further investigation.
We tested expression of E-cadherin, N-cadherin,
β-catenin, Twist and Snail2 immunohistochemically in the gastric
carcinoma tissue microarray, and found that levels of N-cadherin,
β-catenin, Twist1 and Snail2 protein were positively correlated
with the WHO GA grades. However, the expression of E-cadherin was
negatively correlated with the WHO GA grades (Fig. 3B). To verify gene expression levels
induced by miR-200a mimics in vivo, we determined protein
expression of these genes immunohistochemically. N-cadherin,
β-catenin, Twist and Snail2 were prominently downregulated, and
E-cadherin was upregulated in tumor specimens of the miR-200a
mimic-treated group (Fig. 3C).
We confirmed that miR-200a regulates EMT through the
Wnt/β-catenin signaling pathway.
Discussion
microRNAs are dysregulated in cancer and may play
essential roles in tumorigenesis. In this study, we focused on the
miR-200 family, which is reportedly downregulated in prostate
cancer, breast cancer and lung adenocarcinoma (22–24),
but is elevated in ovarian adenocarcinoma and endometrial carcinoma
(25,26). To study the expression of the
miR-200 family during GA progression, we performed in situ
hybridization in a GA tissue microarray. We found that miR-200a,
miR-200b and miR-200c were downregulated in GA compared to normal
gastric mucosa, and were negatively associated with gastric
carcinoma grade.
Our previous study showed that elevated miR-200a in
SGC7901 cells inhibited cell growth and invasion and induced
G0/G1 phase arrest (17). In this study, we found that
upregulated miR-200a suppressed tumor growth in vivo,
confirming our in vitro results. However, the mechanism
involved in this suppression was unclear. Little is known regarding
the effect of miR-200a on ZEB1 and ZEB2 expression in GA. From
western blotting and immunofluorescence staining in SGC7901 cells,
and immunohistochemistry in vivo, we inferred that ZEB1 and
ZEB2 are targets of miR-200a. To verify the significance of the
Wnt/β-catenin signaling pathway and EMT in GA, we performed
immunofluorescence staining in SGC7901, immunohistochemistry in
gastric carcinoma tissue microarray and a tumor xenograft mouse
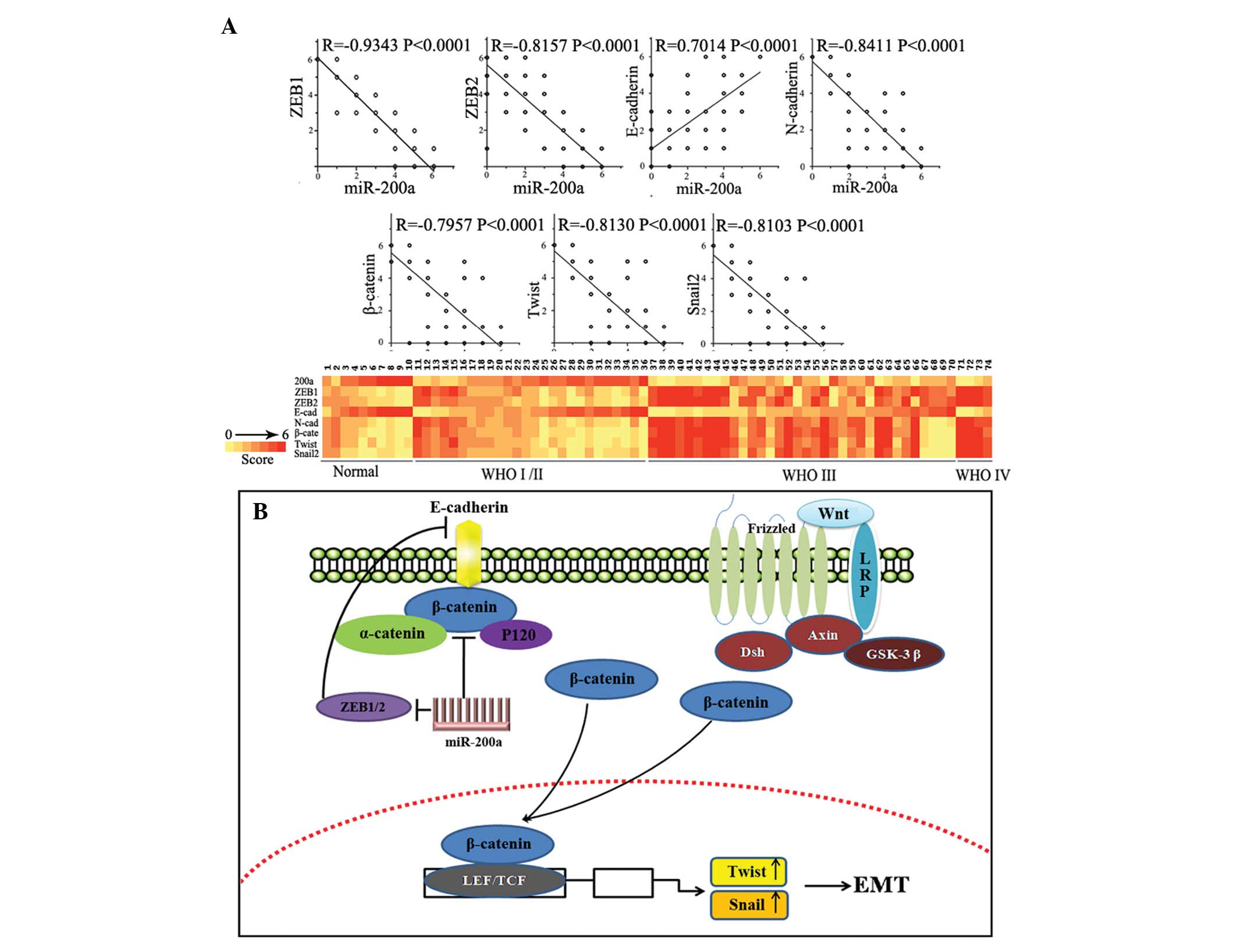
model. The Pearson’s correlation showed significant inverse
correlations existed between miR-200a expression and those of
ZEB1/ZEB2 (rs=−0.9343, P<0.0001 and rs=−0.8153, P<0.0001,
respectively), N-cadherin (rs=−0.8411, P<0.0001), β-catenin
(rs=−0.7957, P<0.0001), Snail2 (rs=−0.7957, P<0.0001) and
Twist (rs=−0.8130, P<0.0001), and a significant positive
correlation existed between miR-200a expression and E-cadherin
expression (rs=0.7014, P<0.0001; Fig. 4A) in the same gastric carcinoma
tissue microarray. This evidence, both in vitro and in
vivo, indicated that miR-200a inhibition caused downregulation
of E-cadherin by targeting ZEB1/ZEB2, meanwhile elevating
expression of β-catenin and relocating mostly membrane-associated
β-catenin to nucleus, thus activating the Wnt/β-catenin signaling
pathway. Aberrant activation of the Wnt/β-catenin signaling pathway
promotes cell proliferation, metastasis and tumorigenesis (30). In conclusion, overexpression of
miR-200a inhibited EMT and delayed tumor growth by increasing
E-cadherin level and reducing expression of N-cadherin and
β-catenin.
Our previous study showed that miR-200a can directly
target β-catenin, and, thus, inhibit the Wnt/β-catenin signaling
pathway. miR-200a inhibits EMT, the initial tumorigenesis step, by
targeting ZEB1/ZEB2, which inhibit E-cadherin by binding to an
E-box element in the E-cadherin gene promoter (27,28).
The cadherin-associated protein β-catenin is critical to the
Wnt/β-catenin signaling pathway (29). In summary, miR-200a regulates the
activity of β-catenin through two types of mechanisms (Fig. 4B).
Previous investigations found that Twist1 required
Snail2 to suppress E-cadherin, which induces EMT and tumor
metastasis in HMLE cells. It is suggested that Twist1 specifically
and directly binded the E-box domain 306 bp from the human Snail2
transcription start site to activate its transcription (31). During Drosophila mesoderm
formation, Twist1 induces Snail1 expression to promote EMT
(32,33), and also induces expression of
mesenchymal markers, such as N-cadherin and fibronectin. These
events appear to be independent of E-cadherin expression during
Drosophila gastrulation, and may function as a potent
transcriptional activator to induce expression of mesenchymal
markers (33,34). The repressor Twist1 is reportedly
induced by its gene promoter being directly activated by canonical
Wnt signaling and the TCF/LEF transcription factors (35). Previous studies suggest that the
strong β-catenin/TCF signaling in sparse SW480 cell cultures induce
the Slug gene, resulting in repression of E-cadherin transcription
(36).
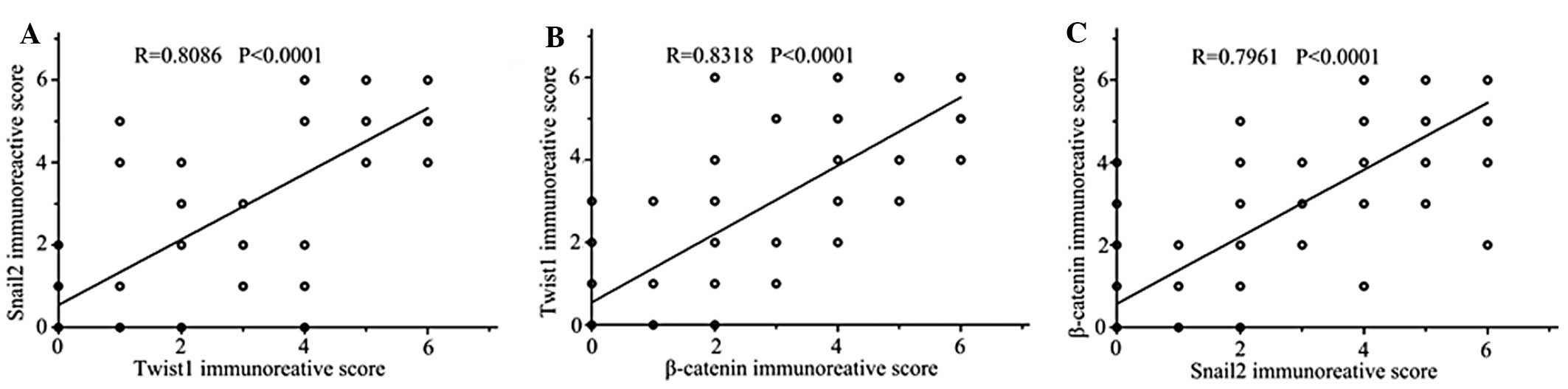
We confirmed for the first time, using SGC7901
cells, that upregulated miR-200 reduced levels of Snail2 and Twist.
To further confirm these results, we performed immunohistochemical
analyses of a tumor xenograft mouse model and GA tissue microarray.
We also found that miR-200 expression was negatively correlated
with Snail2 and Twist1 and analyzed relationships among expression
of Snail2, Twist1 and β-catenin in the same tissue assay (Fig. 5).
In this study, we found fluctuations in miR-200a
expression that were similar to changes in expression of miR-200b
and miR-200c in gastric carcinoma and normal gastric mucosa. A
recent report suggested that miR-200b regulates EMT and promotes
cell proliferation, invasion, and migration by directly targeting
ZEB2 in gastric carcinoma (37).
ZEB1 and ZEB2 are established direct targets of miR-200c (20). miR-200c could regulate migration and
invasion activity through both ZEB1/E-cadherin-dependent and
-independent pathways (38).
However, further investigations are required to clarify whether
miR-200b and miR-200c affect the biological activity of tumor cells
or mediate EMT processes.
Acknowledgements
This study was supported by the Chinese National
Natural Scientific Fund 81172356 and 81172406, and by the Natural
Science Foundation of Tianjin (10JCZDJC18500). The authors thank Dr
Daiming Fan for kindly providing SGC7901 gastric cancer cells and
the members of the Tianjin Laboratory of Neuro-Oncology, Tianjin
Neurological Institute for their technical assistance.
References
|
1
|
Zhang X, Nie Y, Du Y, Cao J, Shen B and Li
Y: MicroRNA-181a promotes gastric cancer by negatively regulating
tumor suppressor KLF6. Tumour Biol. 33:1589–1597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang M, Li C, Nie H, et al: Down-regulated
miR-625 suppresses invasion and metastasis of gastric cancer by
targeting ILK. FEBS Lett. 586:2382–2388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Cao Y, Jie Z, et al: miR-495 and
miR-551a inhibit the migration and invasion of human gastric cancer
cells by directly interacting with PRL-3. Cancer Lett. 323:41–47.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hua Y, Duan S, Murmann AE, et al:
miRConnect: identifying effector genes of miRNAs and miRNA families
in cancer cells. PLoS One. 6:e265212011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Negrini M, Nicoloso MS and Calin GA:
MicroRNAs and cancer - new paradigms in molecular oncology. Curr
Opin Cell Biol. 21:470–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Howe EN, Cochrane DR and Richer JK:
Targets of miR-200c mediate suppression of cell motility and
anoikis resistance. Breast Cancer Res. 13:R452011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schliekelman MJ, Gibbons DL, Faca VM, et
al: Targets of the tumor suppressor miR-200 in regulation of the
epithelial-mesenchymal transition in cancer. Cancer Res.
71:7670–7682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slabakova E, Pernicova Z, Slavickova E,
Starsichova A, Kozubik A and Soucek K: TGF-beta1-induced EMT of
non-transformed prostate hyperplasia cells is characterized by
early induction of SNAI2/Slug. Prostate. 71:1332–1343.
2011.PubMed/NCBI
|
|
14
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davalos V, Moutinho C, Villanueva A, et
al: Dynamic epigenetic regulation of the microRNA-200 family
mediates epithelial and mesenchymal transitions in human
tumorigenesis. Oncogene. 31:2062–2074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao F, Zhang Q, Kang C, et al:
Suppression of matrix metalloproteinase-9 expression by RNA
interference inhibits SGC7901 gastric adenocarcinoma cell growth
and invasion in vitro and in vivo. Med Oncol. 27:774–784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su J, Zhang A, Shi Z, et al: MicroRNA-200a
suppresses the Wnt/β-catenin signaling pathway by interacting with
β-catenin. Int J Oncol. 40:1162–1170. 2012.
|
|
18
|
Gregory PA, Bert AG, Paterson EL, et al:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burk U, Schubert J, Wellner U, et al: A
reciprocal repression between ZEB1 and members of the miR-200
family promotes EMT and invasion in cancer cells. EMBO Rep.
9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiklund ED, Bramsen JB, Hulf T, et al:
Coordinated epigenetic repression of the miR-200 family and miR-205
in invasive bladder cancer. Int J Cancer. 128:1327–1334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barron N, Keenan J, Gammell P, et al:
Biochemical relapses following radical prostatectomy and miR-200a
levels in prostate cancer. Prostate. 7:1193–1199. 2011.PubMed/NCBI
|
|
23
|
Guttilla IK, Adams BD and White BA:
ERalpha, microRNAs, and the epithelial-mesenchymal transition in
breast cancer. Trends Endocrinol Metab. 23:73–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roybal JD, Zang Y, Ahn YH, et al: miR-200
inhibits lung adenocarcinoma cell invasion and metastasis by
targeting Flt1/VEGFR1. Mol Cancer Res. 9:25–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Snowdon J, Zhang X, Childs T, Tron VA and
Feilotter H: The microRNA-200 family is upregulated in endometrial
carcinoma. PLoS One. 6:e228282011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mateescu B, Batista L, Cardon M, et al:
miR-141 and miR-200a act on ovarian tumorigenesis by controlling
oxidative stress response. Nat Med. 17:1627–1635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saydam O, Shen Y, Wurdinger T, et al:
Downregulated microRNA-200a in meningiomas promotes tumor growth by
reducing E-cadherin and activating the Wnt/beta-catenin signaling
pathway. Mol Cell Biol. 29:5923–5940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia H, Ng SS, Jiang S, et al:
miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially
inhibits nasopharyngeal carcinoma cell growth, migration and
invasion. Biochem Biophys Res Commun. 391:535–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han L, Yang Y, Yue X, et al: Inactivation
of PI3K/AKT signaling inhibits glioma cell growth through
modulation of beta-catenin-mediated transcription. Brain Res.
1366:9–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ip YT, Park RE, Kosman D, Yazdanbakhsh K
and Levine M: Dorsal-twist interactions establish snail expression
in the presumptive mesoderm of the Drosophila embryo. Genes
Dev. 6:1518–1530. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leptin M: Twist and snail as positive and
negative regulators during Drosophila mesoderm development.
Genes Dev. 5:1568–1576. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reinhold MI, Kapadia RM, Liao Z and Naski
MC: The Wnt-inducible transcription factor Twist1 inhibits
chondrogenesis. J Biol Chem. 281:1381–1388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, Blechman J, Savagner P and Ben-Ze’Ev A: Autoregulation of
E-cadherin expression by cadherin-cadherin interactions: the roles
of beta-catenin signaling, Slug, and MAPK. J Cell Biol.
163:847–857. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurashige J, Kamohara H, Watanabe M, et
al: MicroRNA-200b regulates cell proliferation, invasion, and
migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg
Oncol. 19:656–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Radisky DC: miR-200c at the nexus of
epithelial-mesenchymal transition, resistance to apoptosis, and the
breast cancer stem cell phenotype. Breast Cancer Res. 13:1102011.
View Article : Google Scholar : PubMed/NCBI
|