Introduction
Osteosarcoma is the most common primary malignant
tumor of the bone, occurring most frequently in children and
adolescents (1). Surgery,
radiotherapy and high-dose chemotherapy [with agents such as
doxorubicin, methotrexate, cisplatin, 5-fluorouracil (5-FU),
Taxol® and etoposide] are mainly effective in patients
with localized disease and have improved patient overall survival
over the last several years (2).
However, clinically evident metastatic disease is
present in 10–20% of patients at diagnosis. Despite aggressive
treatment, more than one third of patients develop recurrent
high-grade osteosarcomas, with metastatic disease typically
affecting the lung, liver and bone itself, so that the 5-year
survival rates are still not greater than 60%. The frequent
acquisition of drug-resistant phenotypes and occurrence of
secondary malignancies associated with chemotherapy remain serious
problems (3). Moreover, the toxic
effects of chemotherapy still remain a major drawback in the
treatment of osteosarcoma patients. Thus, there is a pressing need
for the development of new and alternative approaches to the
treatment of osteosarcoma (4).
Combination chemotherapy has received increased
attention in order to identify compounds that may increase the
therapeutic index of clinical anticancer drugs (5). In this regard, dietary supplements,
phytotherapeutic agents and naturally occurring molecules (such as
silibinin, resveratrol, plumbagin, benzyl isothiocyanate,
2-methoxyestradiol, DDTD) with antitumor activity and with low
toxicity to normal tissues have been suggested as possible
candidates for investigation of their synergistic efficacy in
combination with antineoplastic drugs (6–11).
Inorganic phosphate (Pi) is an essential nutrient
for living organisms. It plays a key role in diverse physiological
functions, including osteoblast differentiation and skeletal
mineralization (12). Serum Pi
level is maintained within a narrow range through a complex
interplay between intestinal absorption, exchange with
intracellular and bone storage pools, and renal tubular
reabsorption and depends mainly on the activity of Na/Pi
cotransporters (13). Pi is
abundant in the diet, and intestinal absorption of Pi is efficient
and minimally regulated. The kidney is a major regulator of Pi
homeostasis and can increase or decrease its Pi reabsorptive
capacity to accommodate Pi need. Adequate control of Pi homeostasis
is crucial, as a moderate increase in serum Pi concentration and
polymorphisms in genes involved in Pi metabolism may result in bone
impairment and influence the ageing process and lifespan (14). Relevantly, Pi is emerging as an
important signaling molecule capable of modulating multiple
cellular functions by altering signal transduction pathways, gene
expression and protein abundance in many cell types (15).
Previously, we provided evidence that Pi inhibits
proliferation and aggressiveness of human osteosarcoma U2OS cells
identifying adenylate cyclase, β3 integrin, Rap1, ERK1/2 as
proteins whose expression and function are relevantly affected in
response to Pi (16,17). More recently, we described that in
wild-type p53-containing osteosarcoma U2OS cells, and not in
p53-null Saos and p53 mutant MG63 osteosarcoma cells, Pi is capable
of inducing sensitization to doxorubicin (18). We provided evidence that the
enhancement of doxorubicin-induced cytotoxicity by Pi occurs via
p53-dependent apoptosis and through a mechanism involving ERK1/2
downregulation (18).
Herein, we extended the study of the role of Pi in
the chemosensitivity of osteosarcoma cells to other anticancer
drugs, and we investigated the possible antitumor effects of
treatments based on Pi in combination with either Taxol or 5-FU or
doxorubicin in osteosarcoma U2OS cells. We showed that Pi increases
the antiproliferative response to Taxol similarly to that caused by
doxorubicin. In contrast, Pi did not potentiate the anticancer
effects induced by 5-FU. These effects were paralleled by apoptosis
induction and were cell cycle-dependent.
Materials and methods
Materials
All cell culture materials were from Gibco-Life
Technologies (Gaithersburg, MD, USA). The anticancer drugs
doxorubicin, Taxol (paclitaxel) and 5-FU were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Anti-tubulin antibodies were
obtained from Oncogene-Calbiochem (La Jolla, CA, USA).
Anti-procaspase-3, and anti-poly(ADP ribose) polymerase (PARP)
antibodies were obtained from Upstate Biotechnology Inc. (Lake
Placid, NY, USA). All other antibodies were obtained from Santa
Cruz Biotechnology (San Diego, CA, USA).
Cell culture and treatments
The human osteosarcoma U2OS cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA). U2OS
cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml
streptomycin and 10% fetal bovine serum (FBS) and cultured at 37°C
in a 5% CO2 humidified atmosphere. Unless noted, all
experiments were conducted in the above medium which contained 1 mM
of Pi, and the concentrations listed in the figures are final Pi
medium concentrations. Added Pi was in the form of
NaPO4, pH 7.4, from Sigma-Aldrich (16–19).
Doxorubicin was dissolved in ddH2O and stored at 4°C;
stock solutions of Taxol and 5-FU in 100% dimethylsulphoxide were
maintained at −20°C and thawed immediately before treatment of
human tumor cells. Appropriate drug concentrations were made by
dilution with fresh medium immediately before each experiment to
final concentrations as indicated in the figures (20–22).
Typically, subconfluent cells were split (5×105/10 cm
plate) and grown in 10% serum containing medium. After 24 h, the
medium was removed, the cells were washed with PBS and incubated
with 10% FBS fresh medium (time 0), supplemented or not with Pi and
anticancer drugs, as single agents or in combination, and grown for
the times and at concentrations indicated in the figures. Floating
cells were recovered from culture medium by centrifugation, and
adherent cells were harvested by trypsinization. Both floating and
adherent cells were used in the experiments aimed to study
expression of proteins involved in apoptosis and to perform FACS
analysis (23).
Cell proliferation assay
Viable cells were determined by the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay, as previously described (18,24).
Briefly, cells were seeded in 96-multi-well plates at the density
of 5×103 cells/well. Cells were treated with Pi and
drugs alone or in combination for up to 72 h. Before harvesting,
100 μl of MTT solution (5 mg/ml) was added to each well and
incubated at 37°C for 3 h, then the formazan product was
solubilized by the addition of 100 μl 0.04 N HCl isopropanol. The
optical density of each sample was determined by measuring the
absorbance at 570 vs. 650 nm using an enzyme-linked immunosorbent
assay reader (Molecular Device). Cell proliferation assays were
performed at least three times (in replicates of 6 wells for each
data point in each experiment). Data are presented as means ±
standard deviation for a representative experiment.
Evaluation of subG1 and cell cycle phases
by flow cytometry
After drug treatment, cells were recovered as
previously described in ‘Cell culture and treatments’, fixed by
resuspension in 70% ice-cold methanol/PBS and incubated overnight
at 4°C. After fixing, samples were pelleted at 400 × g for 5 min,
and pellets were washed once with ice-cold PBS and centrifuged for
a further 5 min. Pellets were resuspended in 0.5 ml DNA staining
solution [50 μg/ml of propidium iodide (PI) and 100 μg RNase A in
PBS], and incubated at 37°C for 1 h in the dark. Samples were
transferred to 5-ml Falcon tubes and stored on ice until assayed.
Flow cytometric analysis was performed using a FACSCalibur flow
cytometer (Becton Dickinson, San Jose, CA, USA) interfaced with a
Hewlett-Packard computer (model 310) for data analysis. For the
evaluation of intracellular DNA content, at least 20,000 events for
each point were analyzed, and regions were set up to acquire
quantitative data of cells with fragmented DNA (subG1 or apoptotic
events) compared with the events that fell into the normal G1, S,
G2 regions (23,25).
Flow cytometric analysis of
apoptosis
Annexin V-FITC (fluorescein isothiocyanate) was used
in conjunction with a vital dye, PI, to distinguish apoptotic
(Annexin V-FITC-positive, PI-negative) from necrotic (Annexin
V-FITC-positive, PI-positive) cells (26). Briefly, cells were incubated with
Annexin V-FITC (MedSystems Diagnostics, Vienna, Austria) and PI
(Sigma-Aldrich) in a binding buffer (10 mM HEPES, pH 7.4, 150 mM
NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2) for
10 min at room temperature, washed and resuspended in the same
buffer. Analysis of apoptotic cells was performed by flow cytometry
(FACScan, Becton Dickinson). For each sample, 2×104
events were acquired. Analysis was carried out by triplicate
determination for at least three separate experiments.
Preparation of cell lysates
Cell extracts were prepared as follows. Briefly, 3–5
volumes of RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate,
0.1% SDS) containing 10 μg/ml aprotinin, leupeptin, and 1 mM
phenylmethylsulfonyl fluoride (PMSF) were added to recovered cells.
After incubation on ice for 1 h, samples were centrifuged at 18,000
× g in an Eppendorf microcentrifuge for 15 min at 4°C and the
supernatant (SDS total extract) was recovered. Some aliquots were
taken for protein quantification according to the Bradford method
(27). Others were diluted in 4X
Laemmli buffer, boiled and stored as samples for immunoblot
analysis.
Immunodetection of proteins
Typically, we employed 20–40 μg of total extracts
for immunoblotting. Proteins from cell preparations were separated
by SDS-PAGE and transferred onto nitrocellulose sheets (Schleicher
& Schuell, Dassel, Germany) by a Mini Trans-Blot apparatus
(Bio-Rad, Hercules, CA, USA). Secondary goat anti-rabbit or
anti-mouse antibodies, conjugated with horseradish peroxidase
(Bio-Rad), were used as a detection system (ECL) according to the
manufacturer’s instructions (Amersham Biosciences, UK).
Statistical analysis
Most of experiments were performed at least three
times with replicate samples, except where otherwise indicated.
Data are plotted as means ± SD (standard deviation). The means were
compared using analysis of variance (ANOVA) plus Bonferroni’s
t-test. A P-value of <0.05 was considered to indicate a
statistically significant result. National Institutes of Health
Image J 1.42Q (NIH, Bethesda, MD, USA) software was used for
densitometric analysis.
Results
Pi enhances the cytotoxic effect induced
by doxorubicin and Taxol, but not that induced by 5-FU in U2OS
cells
To further investigate the antitumor action of Pi in
osteosarcoma cells, we analyzed the potential antitumor effects of
a combination of Pi and other commonly used chemotherapeutic
agents. To this purpose, we treated osteosarcoma U2OS cells with
varying concentrations of Taxol and 5-FU, in the presence or
absence of 5 mM Pi, a concentration covering the physiologic range
in humans and in agreement with most of the published studies on
Pi-triggered effects (15–19). Specific treatment conditions were
examined encompassing exposure to no (0 mM, control), 0.5, 1, 5 μM
Taxol, and 5, 10, 50 μM 5-FU in the presence or absence of 5 mM Pi
for 24 h (28–31). We also included in these experiments
parallel cotreatments with Pi and doxorubicin (0.1, 1, 5 μM) as
previously reported (18). After
the treatments, a conventional tetrazolium-based (MTT) assay was
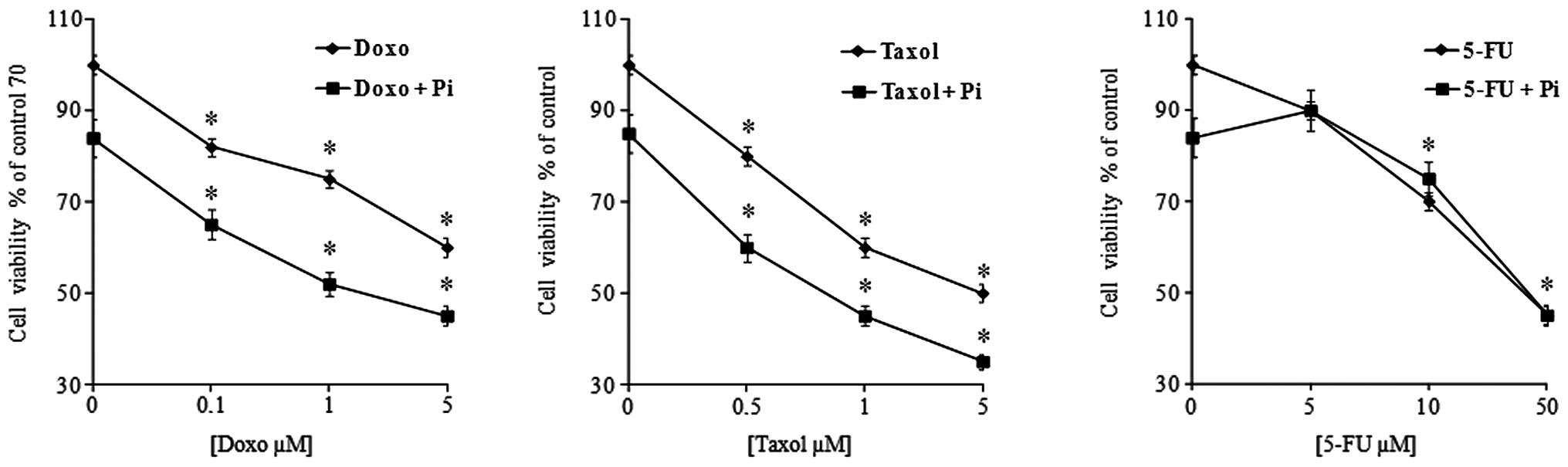
performed (Fig. 1). As expected,
proliferation of U2OS cells was slightly inhibited in a
dose-dependent manner by all three anticancer drugs used (32). Of note, we found that in all
combinations, the presence of Pi strongly enhanced the
antiproliferative effects of doxorubicin and Taxol (at a low dose
of doxorubicin 0.1 μM and Taxol 0.5 μM, the inhibition increased
from 15 to 35% and from 20 to 40% in the presence of Pi,
respectively). The synergistic index (expressed as the ratio
between the growth inhibition induced by the combination of the sum
of the growth inhibitions caused by the single agents) was 1.4 and
1.3 for Doxo + Pi and Taxol + Pi, respectively. On the other hand,
Pi combined with 5-FU did not induce any potentiation of the
cytotoxicity caused by 5-FU alone.
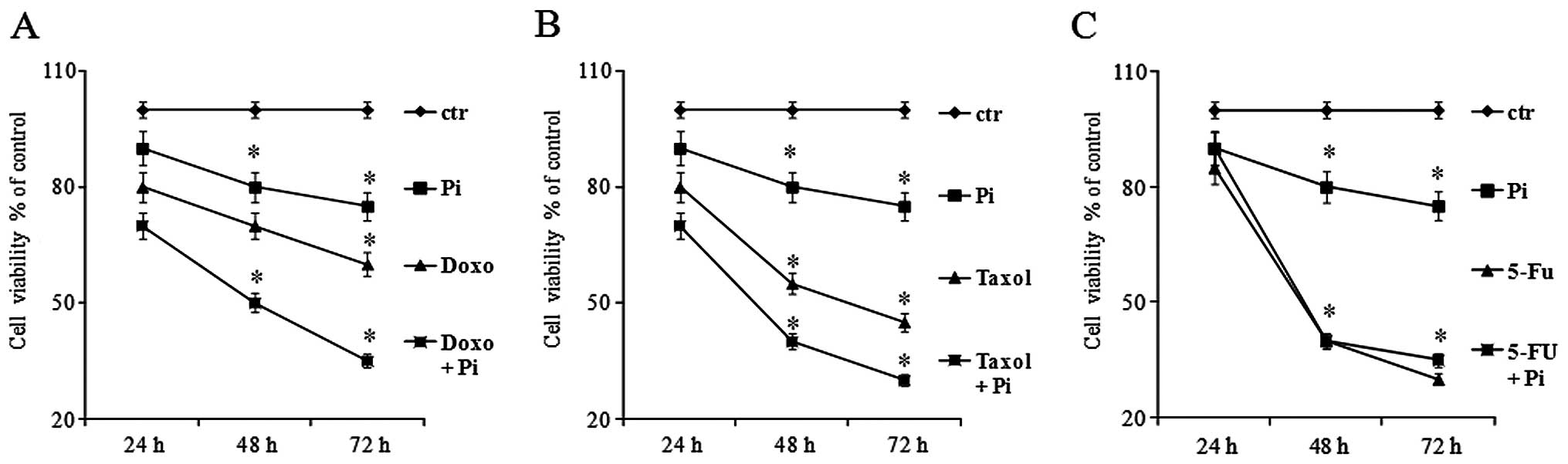
Moreover, we also studied the time-dependency of the
growth inhibition caused by Pi + drug combinations. U2OS cells were
exposed to no (0 mM, control) or low doses of doxorubicin (0.1 μM),
or Taxol (0.5 μM), or 5-FU (5 μM) in the presence or absence of 5
mM Pi for 24, 48 and 72 h (Fig. 2).
As expected, proliferation of U2OS cells was greatly decreased in a
time-dependent manner by all three used agents. Notably, the growth
inhibitory effects induced by Doxo + Pi and Taxol + Pi combinations
were significantly higher than those caused by doxorubicin and
Taxol alone, respectively (for doxorubicin, at 48 h the inhibition
increased from 30 to 50% with an additive/synergistic index of 1.1
and at 72 h from 40 to 65% with an additive/synergistic index of
1.1; for Taxol, at 48 h the inhibition increased from 44 to 61%
with an additive/synergistic index of 1.1; at 72 h from 53 to 72%
with an additive/synergistic index of 1.0). In contrast, no
additive effects were noted with the Pi and 5-FU combination
(Fig. 2).
Pi potentiates the Taxol- and
doxorubicin-induced cytotoxic effect in U2OS cells by inducing
apoptosis
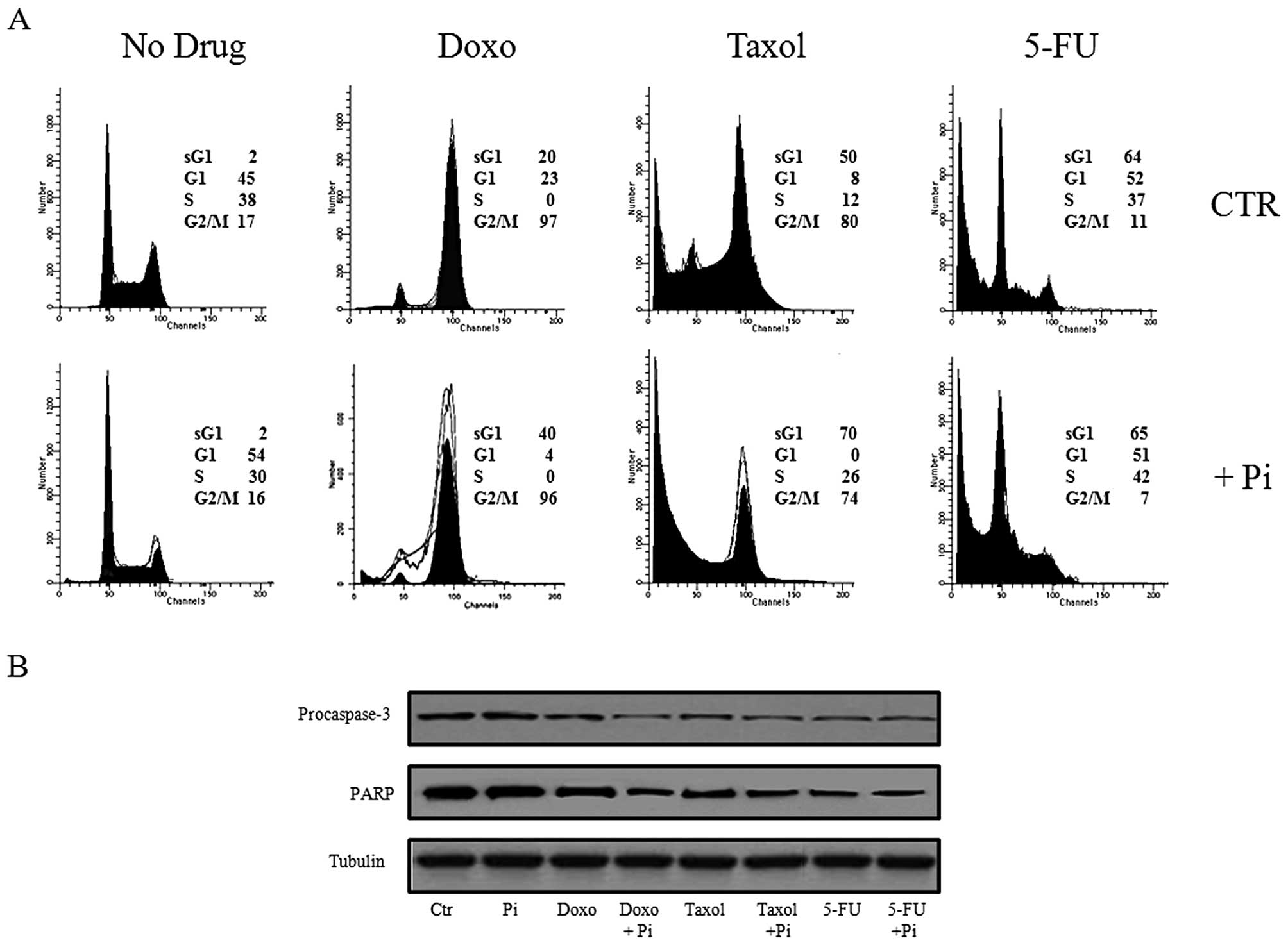
To gain further insight into the enhancement by Pi
of doxorubicin- and Taxol-induced cytotoxicity, we performed
western blotting and flow cytometry-based assays to study the cell
cycle progression and apoptosis (Fig.
3). To this purpose, U2OS cells were exposed to no (0 mM,
control), 0.1 μM doxorubicin, 0.5 μM Taxol, 5 μM 5-FU in the
presence or absence of 5 mM Pi for 48 h and FACS and western blot
analysis were performed (Fig.
3).
As expected, Fig. 3A
shows that Taxol- and doxorubicin-treated U2OS cells were highly
accumulated in the G2/M phase with a concomitant decrease in the
number of cells in the G1 and S phases of the cell cycle, whereas
5-FU caused the cells to accumulate in the G1 and S phases
(28,33). Moreover, a subG1 cell population was
noted in response to doxorubicin (20%) and this was increased in
response to Taxol (50%) and 5-FU (64%). Notably, Fig. 3A shows also that treatment with Doxo
+ Pi and Taxol + Pi combinations induced a marked increase in the
subG1 population when compared to treatment with the single agents
(from 20 to 41% for Doxo + Pi vs. doxorubicin alone; from 50 to 70%
for Taxol + Pi vs. Taxol alone), whereas the treatment with Pi
alone did not cause any relevant change in the subG1 cell fraction.
No increase in the subG1 population occurred following treatment
with the 5-FU + Pi combination when compared to 5-FU treatment
alone.
Accumulation of cells with a hypodiploid DNA content
is consistent with cell death by apoptosis. This is further
confirmed by examining the activation of the terminal caspase-3,
executioner of apoptosis and cleavage of
poly(ADP-ribose)polymerase, PARP, a known target for
apoptosis-associated caspase cleavage (18,29,30).
Fig. 3B shows a
strong decrease in the uncleaved isoform of caspase-3 in Doxo + Pi-
and Taxol + Pi-treated cells suggesting an increase in its activity
correlated with its fragmentation. Doxorubicin, Taxol and 5-FU
alone induced similar effects on caspase-3 activation but to a less
extent. Notably, no decrease in the procaspase-3 protein level was
observed in U2OS cells treated with Pi alone. Finally, we evaluated
the effects of the different treatments on the fragmentation of
PARP, a substrate for caspase-3. The pattern of PARP processing
paralleled that of caspase-3 cleavage (Fig. 3B).
In addition, to further prove the effects of the
different treatments on apoptosis induction, we assessed apoptosis
by FACS analysis after double labeling with Annexin V and PI
(Table I). Overall, the data
indicated that Pi potentiated the Taxol- and doxorubicin-induced
antiproliferative effects (but not the 5-FU-induced ones) by
inducing apoptosis of G2/M-arrested U2OS cells.
 | Table IStudy of apoptosis in the U2OS cell
line. |
Table I
Study of apoptosis in the U2OS cell
line.
| Treatment (48
h) | Necrosis | Late apoptosis | Alive | Early
apoptosis |
|---|
| Control | 0.15 | 0.4 | 99.25 | 0.2 |
| Pi | 0.17 | 0.65 | 98.88 | 0.3 |
| Doxo | 0.37 | 8.83 | 79.18 | 11.62 |
| Doxo + Pi | 0.51 | 25.79 | 61.38 | 11.32 |
| Taxol | 1.2 | 25.1 | 53.38 | 20.32 |
| Taxol + Pi | 1.81 | 36.19 | 33.73 | 28.27 |
| 5-FU | 2.13 | 34.77 | 36.2 | 26.9 |
| 5-FU + Pi | 2.3 | 36.91 | 37.1 | 23.69 |
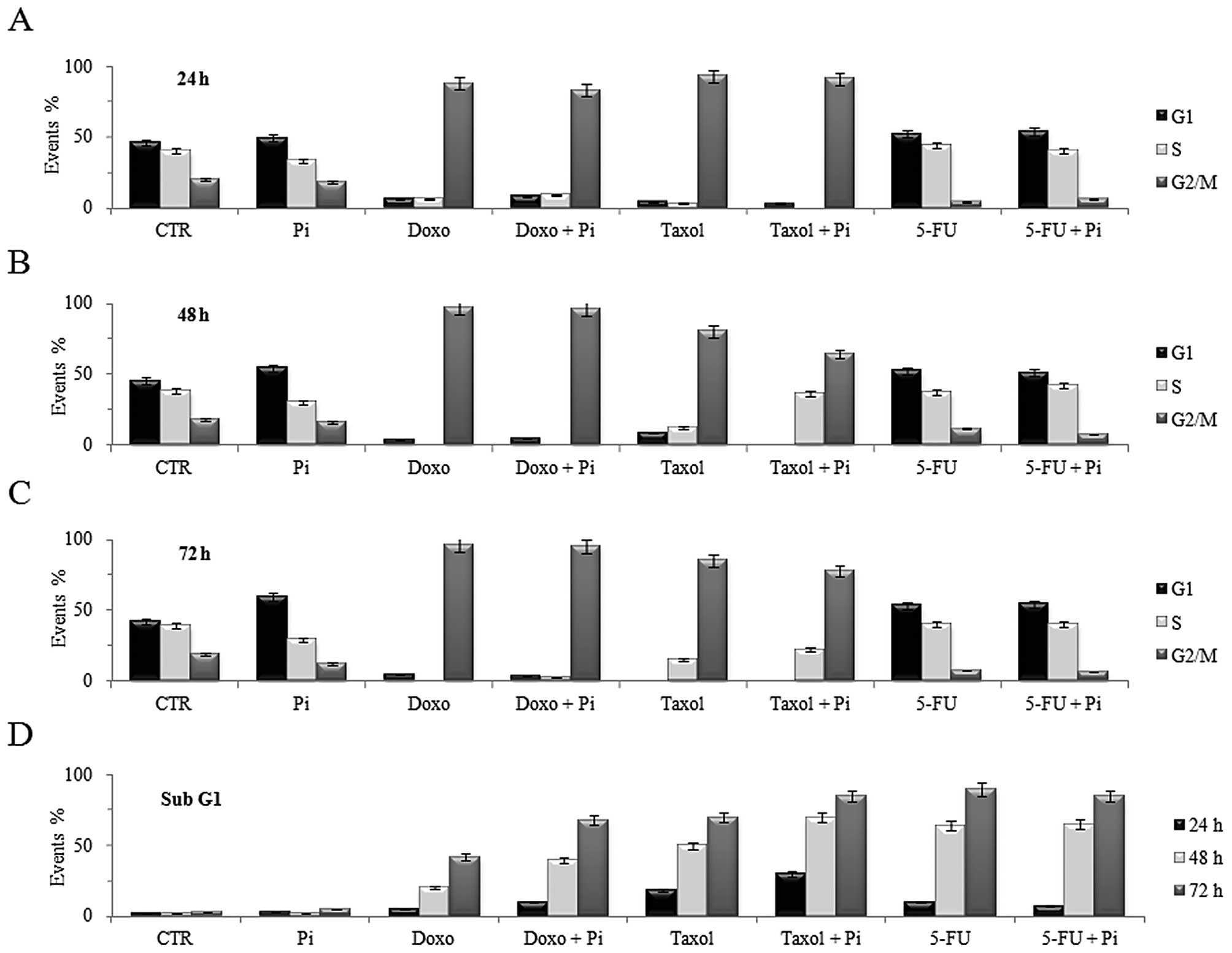
To more effectively evaluate the effects of the
combined treatments with Pi and the above drugs on cell cycle
progression and apoptosis, we also analyzed the subG1 population
and cell cycle distribution of U2OS cells during a time course
(Fig. 4). Doxorubicin-induced G2/M
accumulation was already evident at 24 h (>80% cells in G2) and
increased at 48 h and was maintained up to 72 h (>90% cells in
G2) (Fig. 4A-C). Noteworthy, Doxo +
Pi combination induced a more time-dependent increase in the subG1
population (10, 41 and 68% at 24, 48, 72 h, respectively, compared
to doxorubicin alone), whereas the treatment with Pi alone did not
cause any relevant change in the subG1 cell fraction (Fig. 4D). Moreover, Taxol-induced G2/M
accumulation was already evident at 24 h (>90% cells in G2/M)
and was maintained up to 72 h (Fig.
4A-C). Notably, Taxol + Pi combination induced a stronger
time-dependent increase in subG1 cell population (30, 70 and 85% at
24, 48, 72 h, respectively, compared to Taxol alone) (Fig. 4D). In contrast, a large
time-dependent increase in the subG1 population in response to 5-FU
was evident, but no additive effect by Pi was noted (Fig. 4D).
Discussion
Due to its high metastatic potential and the
frequent acquisition of chemotherapeutic resistance, the clinical
outcome for osteosarcoma remains discouraging despite aggressive
treatments. Thus, novel therapeutic approaches are urgently needed
(3–5). Combination chemotherapy is receiving
increased attention in order to identify compounds that may
increase the therapeutic index of clinical anticancer drugs. In
this regard, naturally occurring molecules with antitumor activity
and with low toxicity to normal tissues have been suggested as
possible candidates for investigation of their synergistic efficacy
in combination with antineoplastic drugs.
Noteworthy, Pi is emerging as an important signaling
molecule capable of modulating multiple cellular functions by
altering signal transduction pathways, gene expression and protein
abundance in many cell types (15).
Previously, we provided evidence that Pi inhibits proliferation and
aggressiveness of human osteosarcoma U2OS cells identifying
adenylate cyclase, β3 integrin, Rap1, ERK1/2 as proteins whose
expression and function are relevantly affected in response to Pi
(16,17).
In agreement with our previous findings, it has been
reported that L/B/K ALP, alkalin phosphatase, whose main action is
to locally increase the Pi levels in the extracellular environment,
inhibits the aggressiveness and the metastatic ability of U2OS
cells, by modulating the expression of genes involved in cell
proliferation and adhesion (34).
More recently, we described that in wild-type p53-containing
osteosarcoma U2OS cells, and not in p53-null Saos and p53-mutant
MG63 osteosarcoma cells, Pi is capable of inducing sensitization to
doxorubicin in a p53-dependent manner (18). To the best of our knowledge, there
have been no studies on the antitumor effect of inorganic phosphate
on osteosarcoma cells, and our above studies are the first
evidence.
In order to gain further insight into the antitumor
action of Pi in osteosarcoma cells, we analyzed the possible
antitumor effects of combined treatments with Pi and other commonly
used chemotherapeutic agents compared to doxorubicin in
osteosarcoma cells. The present study showed that osteosarcoma U2OS
cells are significantly more sensitive to doxorubicin and Taxol
when co-treated with Pi compared to the single use of these agents.
In contrast, combined treatment of Pi with 5-FU did not have an
additive antiproliferative effect compared to 5-FU alone.
The fact that inorganic phosphate, a simple
‘naturally occurring molecule’, combined with relevant
chemotherapeutic agents can achieve this additive cytotoxic effect
illustrates its potential for clinical applications. In addition,
the fact that Pi enhances the antiproliferative effects of
doxorubicin and Taxol in U2OS cells, but not those of 5-FU suggests
that Pi does not act in a widespread way, but can have discrete
effects on cell proliferation depending very likely on cell cycle
phase(s) in which they occur. Doxorubicin, Taxol and 5-FU are
widely used anticancer drugs belonging to different classes of
chemotherapeutics (35).
Doxorubicin, classified as an anthracycline
antiobiotic, is a DNA-damaging agent that generates DNA
double-strand breaks (DNA DSBs) by inhibiting topoisomerase II
(36,37). Doxorubicin is a clinically relevant
antitumor drug widely included in most chemotherapic treatment
protocols for treating human osteosarcoma. It is well known that
treatment of cells (including osteosarcoma cells) with doxorubicin
leads to cell cycle arrest at the G2/M phase and eventually to
apoptosis (33,38,39).
Taxol (paclitaxel) was isolated from the bark of the Pacific yew
Taxus brevifolia and is one of the most powerful antitumor
drugs (40). Taxol acts as an
anticancer agent by targeting microtubules, promoting their
assembly and stabilization. Treatment of cells (including
osteosarcoma cells) with Taxol disrupts the formation of normal
spindles at metaphase, leading to an arrest of cells in the G2/M
phase of the cell cycle and eventually to apoptotic cell death
(28,29).
The antimetabolite and thymidylate synthase
inhibitor 5-FU is a fluoropyrimidine-based compound, acting mainly
in the S phase of the cell cycle to inhibit DNA synthesis (41). Through the enzymatic activity of
uridine phosphorylase, orotate phosphoribosyltransferase and
thymidine kinase, 5-FU is converted intracellularly to several
active metabolites including fluoro(deoxy)uridine monophosphates
[F(d)UMP], all of which interfere with RNA and DNA homeostasis to
induce arrest of cells in the G1/S phase of the cell cycle and
eventually apoptotic cell death (26,31).
Notably, we found that Pi augmented the cytotoxic
effect and showed a synergistic induction of apoptosis in
osteosarcoma U2OS cells when combined with either doxorubicin or
Taxol ‘G2/M blocking’ agents, whereas no additive antiproliferative
effects were noted in combined treatments with Pi and ‘G1/S
blocking’ 5-FU agent. The molecular mechanisms underlying the
Pi-mediated chemosensitivity of osteosarcoma cells to anticancer
drugs are just beginning to be understood and we do know that
further studies and more exhaustive experiments are warranted.
Previously, we provided evidence that Pi inhibits
cell cycle progression of U2OS cells, without the occurrence of
apoptosis, with a G1 cell accumulation and S phase decrease
(16). Moreover, we also described
that the enhancement of doxorubicin-induced cytotoxicity by Pi
occurs via p53-dependent apoptosis and through a mechanism
involving ERK1/2 downregulation (18). A possible role of p53 and/or ERK1/2
in the enhancement of Taxol-induced cytotoxicity by Pi in U2OS is
also under investigation by us, and experiments aimed to
investigate the relationship between the combined treatments with
Pi and chemotherapeutic drugs and their sequence are also planned.
Irrespective of the mechanism(s), we report that Pi acts as a
potent enhancer of doxorubicin- and Taxol-induced cytotoxicity in
osteosarcoma cells.
Combination chemotherapy has received increased
attention in order to identify compounds that may increase the
therapeutic index of clinical anticancer drugs. Pi has been
suggested as an attractive candidate to be investigated. In our
study, Pi was found to have a positive pharmacological interaction
even along with low doses of doxorubicin (0.1 μM) and Taxol (0.5
μM) that are expected to be more tolerable and associated with
minimal undesired side effects in patients, thus increasing the
potential clinical relevance of our data. New drug delivery systems
have been developed that incorporate anticancer drugs into calcium
phosphate cement (CPC) to maintain high concentrations of
anticancer drugs at the local bone site (42). Of note, inorganic phosphate release
and its bone retention from CPC is predicted to occur, thus
affecting Pi concentrations locally.
Collectively, our data support the evidence of Pi as
a signaling molecule and indicate that Pi may act as a potent
enhancer of anticancer drug-induced cytotoxicity in osteosarcoma
cells, suggesting that targeting Pi levels may contribute to the
development of novel modalities for therapeutic intervention in
osteosarcoma.
Acknowledgements
This study was supported by the Italian Minister for
Research (contract grant sponsor, PRIN 2009).
References
|
1
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai X, Ma W, He X and Jha RK: Review of
therapeutic strategies for osteosarcoma, chondrosarcoma, and
Ewing’s sarcoma. Med Sci Monit. 17:177–190. 2011.
|
|
3
|
Kim SY and Helman LJ: Strategies to
explore new approaches in the investigation and treatment of
osteosarcoma. Cancer Treat Res. 152:517–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hattinger CM, Pasello M, Ferrari S, Picci
P and Serra M: Emerging drugs for high-grade osteosarcoma. Expert
Opin Emerg Drugs. 15:615–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gutierrez ME, Kummar S and Giaccone G:
Next generation oncology drug development: opportunities and
challenges. Nat Rev Clin Oncol. 6:259–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raina K and Agarwal R: Combinatorial
strategies for cancer eradication by silibinin and cytotoxic
agents: efficacy and mechanisms. Acta Pharmacol Sin. 28:1466–1475.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szekeres T, Saiko P, Fritzer-Szekeres M,
Djavan B and Jäger W: Chemopreventive effects of resveratrol and
resveratrol derivatives. Ann NY Acad Sci. 1215:89–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian L, Yin D, Ren Y, Gong C, Chen A and
Guo FJ: Plumbagin induces apoptosis via the p53 pathway and
generation of reactive oxygen species in human osteosarcoma cells.
Mol Med Rep. 5:126–132. 2012.PubMed/NCBI
|
|
9
|
Wu CL, Huang AC, Yang JS, et al: Benzyl
isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated
generation of reactive oxygen species causes cell cycle arrest and
induces apoptosis via activation of caspase-3, mitochondria
dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2
OS cells. J Orthop Res. 29:1199–1209. 2011.
|
|
10
|
Maran A, Shogren KL, Benedikt M, Sarkar G,
Turner RT and Yaszemski MJ: 2-Methoxyestradiol-induced cell death
in osteosarcoma cells is preceded by cell cycle arrest. J Cell
Biochem. 104:1937–1945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JT, Fong YC, Li TM, et al: DDTD, an
isoflavone derivative, induces cell apoptosis through the reactive
oxygen species/apoptosis signal-regulating kinase 1 pathway in
human osteosarcoma cells. Eur J Pharmacol. 597:19–26. 2008.
View Article : Google Scholar
|
|
12
|
Yoshiko Y, Candeliere GA, Maeda N and
Aubin JE: Osteoblast autonomous Pi regulation via Pit1 plays a role
in bone mineralization. Mol Cell Biol. 27:4465–4474. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeda E, Taketani Y, Sawada N, Sato T and
Yamamoto H: The regulation and function of phosphate in the human
body. Biofactors. 21:345–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prié D, Beck L, Urena P and Friedlander G:
Recent findings in phosphate homeostasis. Curr Opin Nephrol
Hypertens. 14:318–324. 2005.
|
|
15
|
Khoshniat S, Bourgine A, Julien M, Weiss
P, Guicheux J and Beck L: The emergence of phosphate as a specific
signaling molecule in bone and other cell types in mammals. Cell
Mol Life Sci. 68:205–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naviglio S, Spina A, Chiosi E, et al:
Inorganic phosphate inhibits growth of human osteosarcoma U2OS
cells via adenylate cyclase/cAMP pathway. J Cell Biochem.
98:1584–1596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naviglio S, Di Gesto D, Borrelli V, et al:
Novel molecular mechanisms by inorganic phosphate in osteosarcoma
U2OS cells. Front Biosci. 3:1249–1258. 2011.PubMed/NCBI
|
|
18
|
Spina A, Sorvillo L, Di Maiolo F, et al:
Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS
cells to doxorubicin via a p53-dependent pathway. J Cell Physiol.
228:198–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Camalier CE, Young MR, Bobe G, Perella CM,
Colburn NH and Beck GR Jr: Elevated phosphate activates N-ras and
promotes cell transformation and skin tumorigenesis. Cancer Prev
Res. 3:359–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou J, Gan M, Mao N, Zhu X, Shi Q and Yang
H: Sensitization of osteosarcoma cell line SaOS-2 to chemotherapy
by downregulating survivin. Arch Med Res. 41:162–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olijslagers SJ, Zhang YH, Backendorf C and
Noteborn MH: Additive cytotoxic effect of apoptin and
chemotherapeutic agents paclitaxel and etoposide on human tumour
cells. Basic Clin Pharmacol Toxicol. 100:127–131. 2007.PubMed/NCBI
|
|
22
|
Mao FJ, Sidorova JM, Lauper JM, Emond MJ
and Monnat RJ: The human WRN and BLM RecQ helicases differentially
regulate cell proliferation and survival after chemotherapeutic DNA
damage. Cancer Res. 70:6548–6555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naviglio S, Di Gesto D, Romano M, et al:
Leptin enhances growth inhibition by cAMP elevating agents through
apoptosis of MDA-MB-231 breast cancer cells. Cancer Biol Ther.
8:1183–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naviglio S, Di Gesto D, Illiano F, et al:
Leptin potentiates antiproliferative action of cAMP elevation via
protein kinase A down-regulation in breast cancer cells. J Cell
Physiol. 225:801–809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Naviglio S, Mattecucci C, Matoskova B, et
al: UBPY: a growth-regulated human ubiquitin isopeptidase. EMBO J.
17:3241–3250. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamberti M, Porto S, Marra M, et al:
5-Fluorouracil induces apoptosis in rat cardiocytes through
intracellular oxidative stress. J Exp Clin Cancer Res. 31:60–68.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bradford MM: A rapid and sensitive method
for the quantification of microgram quantities of protein utilizing
the principle of protein dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu KH, Lue KH, Chou MC and Chung JG:
Paclitaxel induces apoptosis via caspase-3 activation in human
osteogenic sarcoma cells (U-2 OS). J Orthop Res. 23:988–994. 2005.
View Article : Google Scholar
|
|
29
|
Zhu JJ, Li FB, Zhou JM, Liu ZC, Zhu XF and
Liao WM: The tumor suppressor p33ING1b enhances taxol-induced
apoptosis by p53-dependent pathway in human osteosarcoma U2OS
cells. Cancer Biol Ther. 4:39–47. 2005.PubMed/NCBI
|
|
30
|
Song B, Wang Y, Xi Y, et al: Mechanism of
chemoresistance mediated by miR-140 in human osteosarcoma and colon
cancer cells. Oncogene. 28:4065–4074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Im YS, Shin HK, Kim HR, et al: Enhanced
cytotoxicity of 5-FU by bFGF through up-regulation of uridine
phosphorylase 1. Mol Cells. 28:119–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koto K, Murata H, Kimura S, et al:
Zoledronic acid inhibits proliferation of human fibrosarcoma cells
with induction of apoptosis, and shows combined effects with other
anticancer agents. Oncol Rep. 24:233–239. 2010.PubMed/NCBI
|
|
33
|
Varmeh S and Manfredi JJ: Overexpression
of the dual specificity phosphatase, Cdc25C, confers sensitivity on
tumor cells to doxorubicin-induced cell death. Mol Cancer Ther.
7:3789–3799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zucchini C, Bianchini M, Valvassori L, et
al: Identification of candidate genes involved in the reversal of
malignant phenotype of osteosarcoma cells transfected with the
liver/bone/kidney alkaline phosphatase gene. Bone. 34:672–679.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Agner J, Falck J, Lukas J and Bartek J:
Differential impact of diverse anticancer chemotherapeutics on the
Cdc25A-degradation checkpoint pathway. Exp Cell Res. 302:162–169.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gewirtz DA: A critical evaluation of the
mechanisms of action proposed for the antitumor effects of the
anthracycline antibiotics adriamycin and daunorubicin. Biochem
Pharmacol. 57:727–741. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tentner AR, Lee MJ, Ostheimer GJ, Samson
LD, Lauffenburger DA and Yaffe MB: Combined experimental and
computational analysis of DNA damage signaling reveals
context-dependent roles for Erk in apoptosis and G1/S arrest after
genotoxic stress. Mol Syst Biol. 31:1–18. 2012.
|
|
38
|
Yuan XW, Zhu XF, Huang XF, et al:
Interferon-alpha enhances sensitivity of human osteosarcoma U2OS
cells to doxorubicin by p53-dependent apoptosis. Acta Pharmacol
Sin. 28:1835–1841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chiosi E, Spina A, Sorrentino A, et al:
Change in TNF-alpha receptor expression is a relevant event in
doxorubicin-induced H9c2 cardiomyocyte cell death. J Interferon
Cytokine Res. 27:589–597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao P and Astruc D: Docetaxel
nanotechnology in anticancer therapy. Chem Med Chem. 7:952–972.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lopez-Heredia MA, Kamphuis GJ, Thüne PC,
Öner FC, Jansen JA and Walboomers XF: An injectable calcium
phosphate cement for the local delivery of paclitaxel to bone.
Biomaterials. 32:5411–5416. 2011. View Article : Google Scholar : PubMed/NCBI
|