Introduction
Rhabdomyosarcoma (RMS), the most common type of
pediatric soft tissue sarcoma, is associated with the skeletal
muscle lineage. Pediatric RMS is categorized into two major
subtypes: embryonal RMS (ERMS) and alveolar RMS (ARMS) based on
their histologic appearance (1).
ERMS shows a more favorable prognosis, while ARMS is more
aggressive with a high frequency of metastases at initial diagnosis
and a worse prognosis (2,3).
ERMS has not been found to be associated with any
diagnostic genetic alterations, but loss of heterozygosity at 11p15
is a common finding (4,5). In contrast, ARMS is associated with
recurrent chromosomal translocations. The translocation
t(2;13)(q35;q14) leading to the PAX3-FOXO1 (P3F) gene fusion was
found to be present in 55% of ARMS cases, while the translocation
t(1;13)(q36;q14) leading to the PAX7-FOXO1 gene fusion was present
in 22% of cases, and 23% of ARMS were fusion-negative (2). ARMS
with the 2;13 translocation is characterized as having
overexpression of P3F relative to wild-type PAX3, at both the RNA
and protein levels (6). In both
PAX3 and P3F, an alternative splice occurs at the intron 2/exon 3
junction resulting in inclusion or exclusion of a glutamine (Q)
residue [PAX3(+Q) or (−Q) forms, respectively] (7). As a result, PAX3(+Q)-FOXO1 and
PAX3(−Q)-FOXO1 are coexpressed in P3F-positive ARMS tumors.
Among RMS tumors, PAX3(+/−Q)-FOXO1-positive ARMS is
the most clinically intractable fusion subtype of pediatric RMS
(1,2,8,9).
However, the histologic classification of RMS into ARMS and ERMS
may be difficult in some cases (9)
and there are no specific drugs for treating specific histologic or
fusion subtypes. Therefore, there is substantial impetus to
elucidate target genes of P3F, which can be used to identify
therapeutic targets and markers for use in RMS diagnosis and
management.
Although several recent studies utilized gene
expression profiles to classify RMS and/or identify target genes of
P3F and PAX7-FOXO1 or P3F only in ARMS (10–16),
more research is needed to validate the function of genes that are
biologically relevant in ARMS development.
In the present study, we identified potential target
genes of P3F by analyzing gene expression profiles from two
independent systems: primary tumors (P3F-positive ARMS and
fusion-negative ERMS) and a cell culture system expressing the
inducible PAX3(+/−Q)-FOXO1-estrogen receptor (ER) ligand binding
domain construct in the pBabe (pB) retroviral vector. Among the
potential target genes of P3F, we focused on cell death or
apoptosis-related [Gremlin1, cysteine knot superfamily 1, BMP
antagonist 1 (GREM1), death-associated protein kinase 1 (DAPK1)]
and development-related [myogenic differentiation 1 (MYOD1) and
hairy/enhancer-of-split related with YRPW motif 1 (HEY1)] genes
since apoptosis and development were significantly overrepresented
functional categories in our gene expression profiles.
Materials and methods
Tumor samples
The tumor specimens (15 ERMS and 16 P3F-positive
ARMS) used for microarray were previously described (15). Independent tumor specimens (20 ERMS
and 17 P3F-positive ARMS) examined for quantitative
reverse-transcription PCR (qRT-PCR) in the validation studies were
previously described (15). The
presence of P3F in ARMS tumor specimens was determined and
confirmed by RT-PCR and/or qRT-PCR (17).
Cell culture
RD ERMS cell line and RD-derived cells transduced
with inducible PAX3(+/−Q)-FOXO1 in pB or pK1 (pK) were maintained
in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Grand
Island, NY, USA) with 10% fetal bovine serum (FBS) (Thermo
Scientific HyClone, Logan, UT, USA). The medium was supplemented
with 1% penicillin/streptomycin (P/S) and 1% antibiotic-antimycotic
(AM) (both from Invitrogen).
DNA constructs and construction of
PAX3(−Q)-FOXO1-ER in pB retroviral vector
Retroviral constructs with several relevant DNA
inserts [pK1-PAX3(+Q)-FOXO1-ER (pK-P3F-ER), pBabe-PAX2(+Q)-FOXO1-ER
(pB-P3F-ER) and pCDNA3-PAX3(+Q)-FOXO1-ER (pCDNA-P3F-ER)] were
previously generated by Dr Frederic G. Barr’s laboratory (18–21). A
modified ER ligand-binding domain was provided by Dr G.I. Evan
(22). Unless it is noted as
PAX3(−Q)-FOXO1, the P3F constructs used in our studies refer to the
PAX3(+Q)-FOXO1 isoform. In order to clone an inducible construct of
PAX3(−Q)-FOXO1-ER into the pB retroviral vector, two consecutive
mutations were introduced, using QuikChange site-directed
mutagenesis (Stratagene; Agilent Technologies, Santa Clara, CA,
USA) followed by multiple subcloning steps. Protocols are available
upon request.
Establishment of ERMS cell culture
systems inducibly expressing P3F
Retroviral transduction was performed as described
previously with modifications (20,21).
Cells that were transduced with retroviral DNA constructs were
selected with puromycin (BD Biosciences, San Jose, CA, USA) at 1
μg/ml for RD-derived cells with PAX3(+/−Q)-FOXO1-ER in pB or in
pK1. Cells carrying inducible PAX3(+/−Q)-FOXO1-ER were treated with
the ligand 4-hydroxytamoxifen (Tmf) (Sigma-Aldrich, St. Louis, MO,
USA), which activates and induces transcriptional activity of
PAX3(+/−Q)-FOXO1-ER by translocating PAX3(+/−Q)-FOXO1-ER to the
nucleus.
Luciferase reporter assays
RD cells expressing PAX3(+/−Q)-FOXO1-ER in pB or pB
alone were plated at 5×104 per well in 24-well plates.
The cells were transfected with 0.3 μg firefly luciferase reporter
DNA containing PAX3 DNA binding sites (6 × PRS9) and 0.015 μg
pRL-TK Renilla luciferase DNA using FuGENE® 6 (both from
Promega, Madison, WI, USA) and incubated for 2 days. The
transcriptional activity of PAX3(+/−Q)-FOXO1 was measured by the
dual-luciferase assay (Promega) and was normalized by transfection
efficiency control (pRL-TK Renilla luciferase).
RNA extraction and microarray data
analysis
For microarray analysis, total RNA was extracted
using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). For other
studies including qRT-PCR and RT-PCR, total RNA was extracted using
RNA STAT-60 (Tel-Test, Inc., Friendswood, TX, USA). Microarray
analysis of RNA isolated from RMS tumors and RD cell culture
systems expressing inducible PAX3(+/−Q)-FOXO1-ER in pB was
performed on Affymetrix arrays. Microarray analysis of 31 RNA tumor
samples (15 ERMS and 16 P3F-positive ARMS) was described in our
previous publication (15).
For microarray analysis of inducible cell culture
systems, three RD cell populations were independently transduced
with each construct [PAX3(+Q)-FOXO1-ER in pB; PAX3(−Q)-FOXO1-ER in
pB; pB vector alone] and treated without or with 30 nM Tmf for 24 h
to generate a total of 18 samples. RNA isolated from these cells
was hybridized onto Affymetrix GeneChip Human Genome U133 Plus
v.2.0 (HG U133+ v.2.0). After the expression levels were determined
using Affymetrix Microarray Suite 5.0, the expression levels were
normalized in GeneSpring (Agilent Technology). The probe-sets,
whose expression levels were detected in at least 2 of the 18
samples (in inducible cell culture system) or 1 of the 31 samples
(in tumors), were selected for further analysis. To compare the
gene expression profiles from tumors with those from the inducible
cell culture systems, the probes on the HG U133A array were used
for GeneSpring analysis.
The Significance Analysis of Microarrays (SAM)
(23) with two-class paired
condition was used as the common method to identify differentially
expressed genes between 16 P3F positive-ARMS and 15 fusion-negative
ERMS tumors as well as between the RD cells expressing
PAX3(+/−Q)-FOXO1-ER in pB (treated with Tmf; 6 samples) and the RD
cells expressing PAX3(+/−Q)-FOXO1-ER in pB (not treated with Tmf; 6
samples). Data was filtered by a false discovery rate (FDR) at
<5% (FDR 4.94% for cells; FDR 4.91% for tumors) and ≥1.5
fold-change. The PAX3(+Q)-FOXO1-ER and PAX3(−Q)-FOXO1-ER gene
expression profiles were pooled since SAM did not reveal
significant differentially expressed genes between these two
groups. The genes that were differentially expressed between
transcriptionally active PAX3(+/−Q)-FOXO1-ER (Tmf-treated) and
inactive PAX3(+/−Q)-FOXO1-ER (untreated control) were then
examined.
Finally, common genes that were present in both
tumors and the inducible PAX3(+/−Q)-FOXO1-ER cell culture system
were identified by Venn Diagram analysis. After this analysis, the
probe-sets that encoded the same gene with similar expression
profiles and showed raw expression values at <150 were removed.
Classifications and functional annotations of genes were analyzed
via web-accessible programs: Expression Analysis Systematic
Explorer (EASE) 2010 and Database for Annotation, Visualization and
Integrated Discovery (DAVID) 2010 (24).
Quantitative reverse transcription-PCR
(qRT-PCR) analysis
The qRT-PCR assay was performed as described
previously (15,16). Test gene assays were normalized to
the expression of 18S rRNA. Taqman gene expression assays used
(Applied Biosystems) were: DAPK1 (assay ID# Hs00234489_m1),
GREM1 (assay ID# Hs00171951_m1) and MYOD1 (assay ID#
Hs00159528_m1). The sequences of forward and reverse primers and
probes of PAX3-FOXO1 and HEY1 are available upon request.
Extraction of cellular proteins and
isolation of secreted proteins from the medium of cultured cells
and immunoblot analysis
The cells were seeded at 106/100-mm dish
in phenol red-free DMEM medium containing 10% FBS, 1% P/S and 1% AM
for 24 h prior to Tmf treatment. The cells expressing inducible
PAX3(+/−Q)-FOXO1-ER were then treated with 30 nM Tmf for various
times. Cellular proteins were lysed as described previously
(20). To assay the secreted GREM1
protein, the medium containing 10% FBS was replaced with FBS-free
DMEM after 24 h of Tmf treatment, and cells were cultured for an
additional 24 or 48 h with Tmf treatment. The medium of the
cultured cells was collected and concentrated to <1,000 μl using
Amicon Ultra-4 with Ultracel-10K (UFC80-1024; Millipore, Billerica,
MA, USA). Protein was quantified using Coomassie Plus Protein Assay
(Pierce Biotechnology, Inc., Rockford, IL, USA).
Immunoblot analysis was performed as described
previously (25) with
modifications. The primary antibodies used were: anti-PAX3 rabbit
polyclonal, DAPK1 (ab10443; both from Abcam, Cambridge, MA, USA)
and GREM1 (cat# AP6133a; Abgent, San Diego, CA, USA).
Statistical analysis
The qRT-PCR data of tumors (20 fusion-negative ERMS
and 17 P3(+Q)F-positive ARMS) were analyzed by the Mann-Whitney
Test (Wilcoxon Rank Sum Test) and differences between the two tumor
groups were considered significant at a P-value <0.05.
Statistical analysis of gene expression profiles are described in
‘RNA extraction and microarray data analysis’.
Results
Development of inducible PAX3(+/−Q)-FOXO1
cell culture systems
RD ERMS cell line was transduced with
PAX3(+/−Q)-FOXO1-ER in pB [pB-P3(+/−Q)F-ER] or pB alone and
selected with puromycin for 2 weeks. The transduction of RD cells
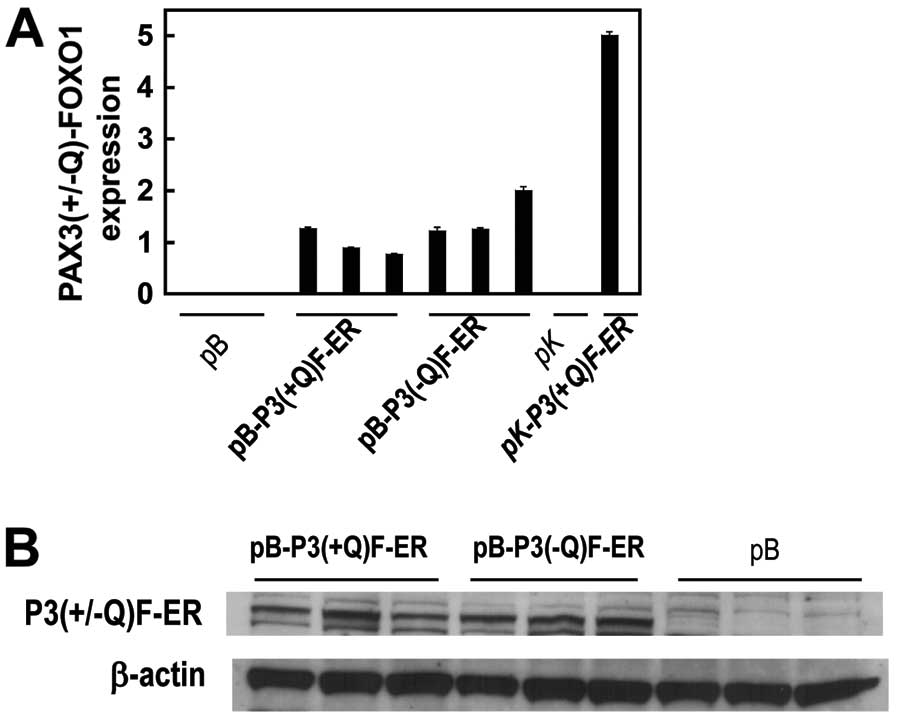
with these pB-P3(+/−Q)F-ER constructs resulted in the expression of
PAX3(+/−Q)-FOXO1-ER at both the mRNA (Fig. 1A) and protein levels (Fig. 1B).
In order to determine and confirm the inducible
PAX3(+/−Q)-FOXO1 function, the RD-derived cells transduced with
pB-P3(+/−Q)F-ER or pB were transiently transfected with a
luciferase gene reporter construct containing PAX3 DNA binding
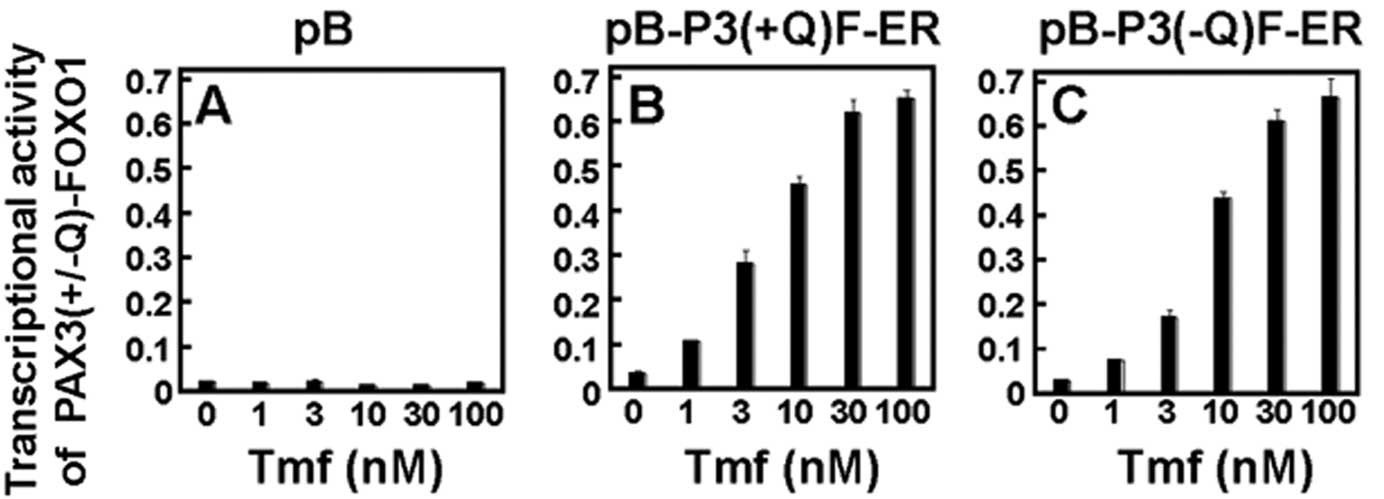
sites and were treated with tamoxifen at various concentrations (0,
1, 3, 10, 30 and 100 nM) for 24 h (Fig.
2). The RD cells transduced with an empty vector pB alone
(Fig. 2A) showed a very low (~0.02)
transcriptional activity indicating only an endogenous PAX3
transcriptional activity. In contrast, RD cells transduced with
pB-P3(+Q)F-ER (Fig. 2B) or
pB-P3(−Q)F-ER (Fig. 2C)
demonstrated much higher transcriptional activities as Tmf
concentrations increased. The maximal transcriptional activity in
RD-derived cells with pB-P3(+/−Q)F-ER was observed at 30 nM Tmf and
showed a ~46- to 47-fold difference in luciferase gene
transactivation between RD-P3(+/−Q)F-ER cells and RD-pB cells
(Fig. 2B and C).
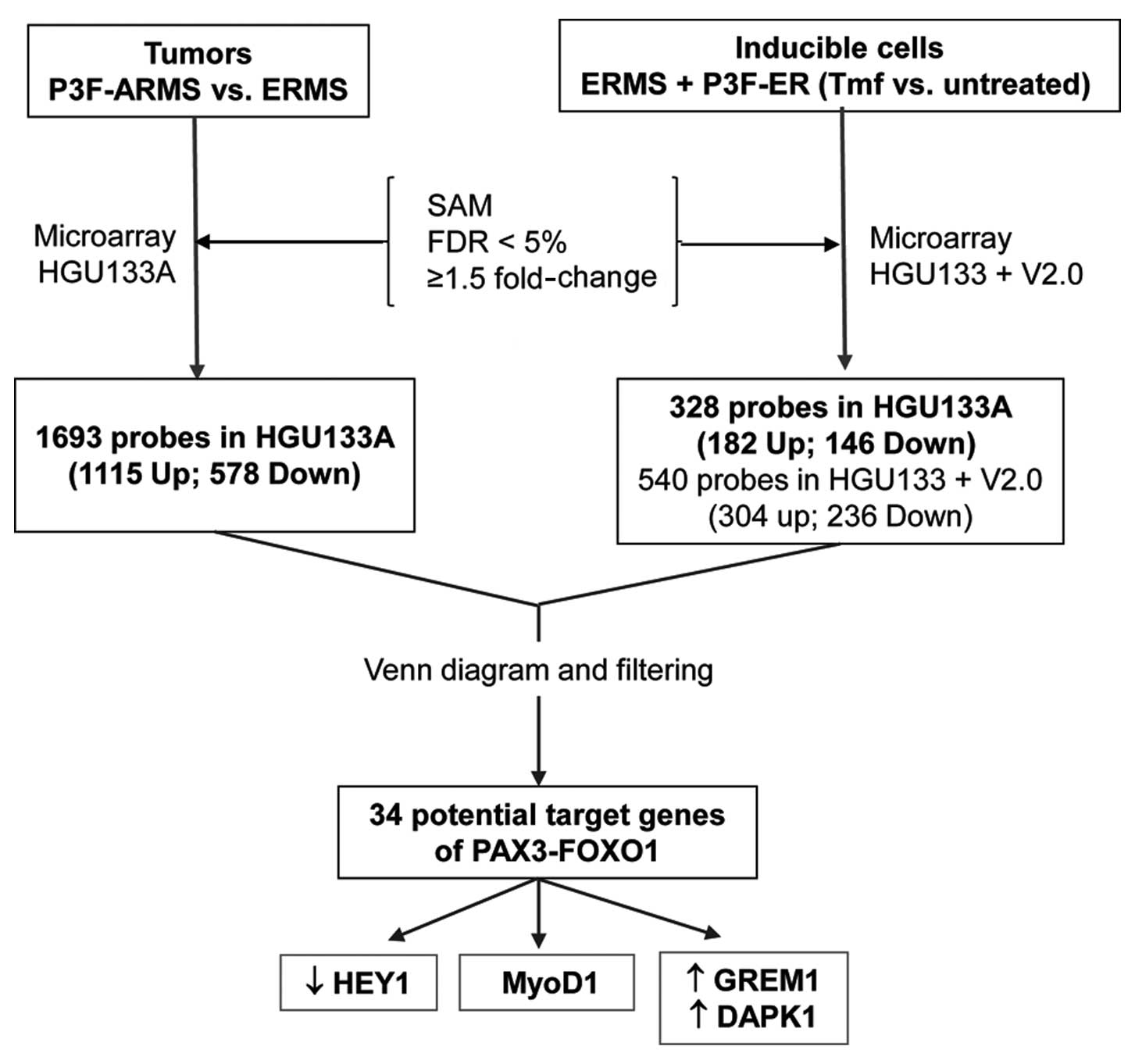
Microarray data analysis of tumors and
inducible cell culture system identifies 34 potential target genes
of P3F
To identify target genes of P3F in the present
study, we utilized RD cells expressing inducible
PAX3(+/−Q)-FOXO1-ER constructs in the pB vector (Figs. 1 and 2) based on the following rationale and
significance. First, because the expression of PAX3(+/−Q)-FOXO1 in
ARMS tumors varies, the utilization of cell culture models having
various levels of PAX3(+/−Q)-FOXO1 can provide an unbiased approach
for identifying potential target genes of PAX3(+/−Q)-FOXO1. Our
previous gene expression profiling study investigated RD cells
transduced with an inducible PAX3(+Q)-FOXO1-ER construct in the pK
vector that enforces high expression of PAX3(+Q)-FOXO1-ER (16). In contrast, our present expression
profiling study used the pB vector to express lower levels of
inducible PAX3(+/−Q)-FOXO1-ER in RD cells. Second, a previous
expression profiling analysis of the inducible cell system
(16) was complicated by the
confounding factor of a treatment-day effect. Thus, microarray data
from inducible cells with PAX3(+Q)-FOXO1-ER in pK were analyzed by
Mixed ANOVA, whereas primary RMS tumors were analyzed by SAM. In
comparison, no such confounding factors were present in the present
study and thus microarray data from both primary RMS tumors and
inducible cells were both analyzed by SAM.
After analyzing microarray data from primary RMS
tumors as well as inducible cells [PAX3(+/−Q)-FOXO1-ER in pB] by
SAM, data were filtered for expression values, FDR, and fold-change
(FDR at <5% and ≥1.5 fold-change). In the tumor microarray data,
1,693 probe-sets (1,115 upregulated and 578 downregulated) on the
HG U133A platform were either significantly upregulated or
downregulated in the PAX3(+/−Q)-FOXO1-positive ARMS tumors (n=16)
compared to the fusion-negative ERMS tumors (n=15). Analysis of
genes differentially expressed between induced (transcriptionally
active) PAX3(+/−Q)-FOXO1-ER in pB (Tmf treated, n=6) and uninduced
PAX3(+/−Q)-FOXO1-ER in pB (untreated control, n=6) revealed 540
probe sets (304 upregulated and 236 downregulated) on the HG U133+
v.2.0 array. Of these 540 probe sets, 328 were present on the
HGU133A array (182 upregulated and 146 downregulated). Finally, 34
potential target genes (27 upregulated and 7 downregulated) were
differentially expressed both in tumors and in the inducible cell
culture system, using probes from the HG U133A array (Table I).
 | Table IPotential target genes of PAX3-FOXO1
as identified by microarray analysis. |
Table I
Potential target genes of PAX3-FOXO1
as identified by microarray analysis.
| | | Fold-change |
|---|
| | |
|
|---|
| Affymetrix ID | Symbol | Gene name | Tumors (n=31) | Inducible cells
(n=12) |
|---|
| Downregulated genes
in P3F-positive categories of tumors and cells |
| 218974_at | FLJ10159 | Hypothetical
protein FLJ10159 | 2.85 | 1.82 |
|
218839_at | HEY1 |
Hairy/enhancer-of-split related with
YRPW motif 1 | 6.21 | 1.72 |
| 209030_s_at | IGSF4 | Cell adhesion
molecule 1 (Immunoglobulin superfamily, member 4) | 2.86 | 1.53 |
| 203708_at | PDE4B | Phosphodiesterase
4B, cAMP-specific (phosphodiesterase E4 dunce homolog,
Drosophila) | 4.80 | 3.77 |
| 202732_at | PKIG | Protein kinase
(cAMP-dependent, catalytic) inhibitor γ | 1.85 | 1.69 |
| 209875_s_at | SPP1 | Secreted
phosphoprotein 1 (osteopontin, bone sialoprotein I, early
T-lymphocyte activation 1) | 6.43 | 2.14 |
| 201368_at | ZFP36L2 | Human Tis11d gene,
complete cds. | 2.60 | 1.56 |
| Upregulated genes
in P3F-positive categories of tumors and cells |
| 209459_s_at | ABAT | 4-Aminobutyrate
aminotransferase | 10.52 | 5.84 |
| 206704_at | CLCN5 | Chloride channel 5
(nephrolithiasis 2, X-linked, Dent disease) | 2.68 | 2.01 |
|
203139_at | DAPK1 | Death-associated
protein kinase 1 | 2.51 | 2.40 |
| 213712_at | ELOVL2 | Catenin
(cadherin-associated protein), α-like 1 | 4.16 | 1.92 |
|
218469_at | GREM1 | Gremlin1,
cysteine knot superfamily 1, BMP antagonist 1 | 5.34 | 2.13 |
| 203233_at | IL4R | Interleukin 4
receptor | 2.03 | 1.74 |
| 203126_at | IMPA2 | Inositol(myo)-1(or
4)-monophosphatase 2 | 2.90 | 1.92 |
| 202794_at | INPP1 | Inositol
polyphosphate-1-phosphatase | 1.89 | 1.51 |
| 205902_at | KCNN3 | Potassium
intermediate/small conductance calcium-activated channel, subfamily
N, member 3 | 6.40 | 1.97 |
| 204094_s_at | KIAA0669 | TSC22D2, TSC22
domain family, member 2 | 4.63 | 2.18 |
| 218829_s_at | KIAA1416 | CHD7, chromodomain
helicase DNA binding protein 7 | 2.26 | 2.31 |
| 211042_x_at | MCAM | Melanoma cell
adhesion molecule | 2.14 | 1.73 |
| 213256_at | MGC48332 | Hypothetical
protein MGC48332 | 4.35 | 1.52 |
|
206657_s_at | MYOD1 | Myogenic
differentiation 1 | 2.59 | 2.28 |
| 209106_at | NCOA1 | Nuclear receptor
coactivator 1 | 3.15 | 1.66 |
| 209289_at | NFIB | Nuclear factor
I/B | 1.97 | 1.51 |
| 205858_at | NGFR | Nerve growth factor
receptor (TNFR superfamily, member 16) | 4.03 | 1.77 |
| 204105_s_at | NRCAM | Neuronal cell
adhesion molecule | 6.62 | 2.36 |
| 209123_at | QDPR | Quinoid
dihydropteridine reductase | 5.04 | 1.84 |
| 203217_s_at | SIAT9 | Sialyltransferase 9
(CMP-NeuAc:lactosylceramide α-2,3-sialyltransferase; GM3
synthase) | 2.43 | 1.84 |
| 203625_x_at | SKP2 | S-phase
kinase-associated protein 2 (p45) | 2.64 | 1.91 |
| 213624_at | SMPDL3A | Sphingomyelin
phosphodiesterase, acid-like 3A | 2.05 | 1.76 |
| 212761_at | TCF7L2 | Transcription
factor 7-like 2 (T-cell specific, HMG-box) | 2.68 | 1.59 |
| 209656_s_at | TM4SF10 | Transmembrane 4
superfamily member 10 | 2.36 | 3.11 |
| 215389_s_at | TNNT2 | Troponin T2,
cardiac | 5.39 | 1.86 |
| 219038_at | ZCWCC2 | Zinc finger,
CW-type with coiled-coil domain 2 | 3.52 | 2.02 |
| 49111_at | DKFZp762M127 | MRNA; cDNA
DKFZp762M127 (from clone DKFZp762M127) | 6.60 | 8.23 |
Many of the differentially expressed
genes in the PAX3(+/−Q)-FOXO1-ER cell systems are related to
apoptosis and development
To investigate biological consequences of the
PAX3(+/−Q)-FOXO1 expression signature, the 540 significantly
upregulated or downregulated probes identified in RD cells
expressing the inducible PAX3(+/−Q)-FOXO1-ER in pB on the HG-U133+
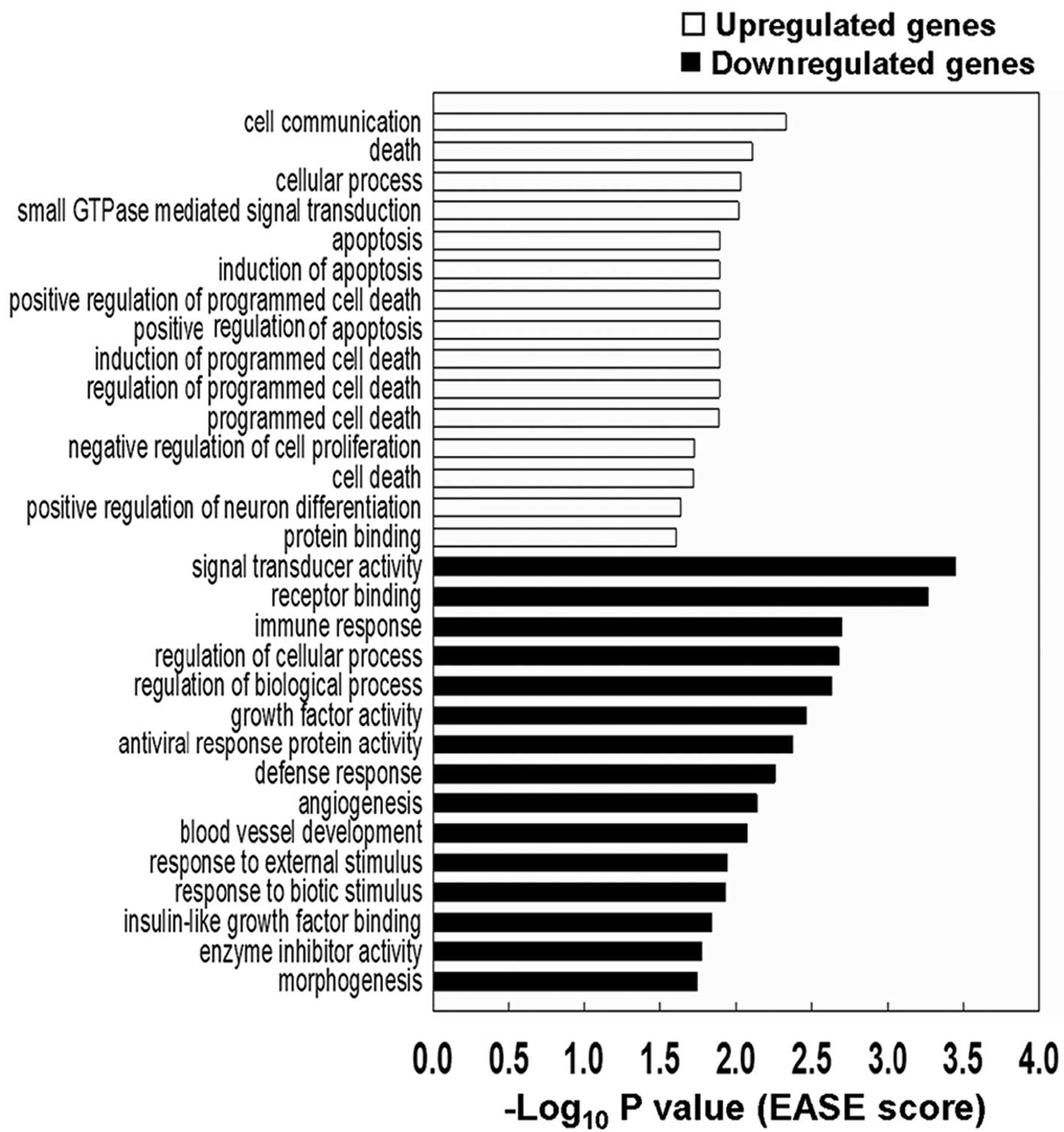
v.2.0 platform were analyzed using EASE and DAVID (24). Notably, 10 out of the 15 most
significant functional categories analyzed by EASE for the
upregulated genes were apoptosis, cell death, or negative
regulation of cell proliferation (Fig.
3).
The most significant 20 overrepresented functional
groups in the RD cells carrying inducible PAX3(+/−Q)-FOXO1-ER in pB
identified by DAVID are listed in Table II. Among these 20 groups, five
groups were commonly found in both upregulated and downregulated
genes. Four out of these 5 functional groups were related to
development (developmental process, multicellular organismal
development, anatomical structure development, system development)
and one group to signal transduction. MYOD1 (an upregulated
gene) and HEY1 (a downregulated gene) were found in all of
the 5 common functional groups.
 | Table IIDAVID analysis of the microarray data
of the inducible PAX3-FOXO1 cell culture system. |
Table II
DAVID analysis of the microarray data
of the inducible PAX3-FOXO1 cell culture system.
| Gene category | Counts (no. of
genes) | No. of probes | P-value |
|---|
| Upregulated genes
in transcriptionally active PAX3(+/−Q)-FOXO1 cells |
| Protein
binding | 112 | 163 | 1.08E-08 |
| Developmental
process | 62 | 91 | 3.04E-06 |
| Multicellular
organismal development | 44 | 63 | 2.60E-04 |
| Ras GTPase
binding | 6 | 7 | 4.32E-04 |
| Enzyme
binding | 11 | 19 | 5.41E-04 |
| Anatomical
structure development | 40 | 65 | 6.75E-04 |
| Small GTPase
binding | 6 | 7 | 7.56E-04 |
| GTPase
binding | 6 | 7 | 1.24E-03 |
| Regulation of
cellular process | 67 | 96 | 1.48E-03 |
| Binding | 151 | 209 | 1.49E-03 |
| Cell
communication | 63 | 94 | 1.88E-03 |
| Central nervous
system development | 10 | 14 | 2.09E-03 |
| System
development | 33 | 50 | 2.13E-03 |
| Splice
variant | 62 | 96 | 3.27E-03 |
| Glycolipid
metabolic process | 4 | 5 | 3.68E-03 |
| Regulation of
biological process | 69 | 99 | 3.94E-03 |
| Transcription
factor binding | 12 | 19 | 4.11E-03 |
| Signal
transduction | 57 | 84 | 4.36E-03 |
| Apoptosis | 10 | 12 | 4.43E-03 |
| Transcription
cofactor activity | 10 | 14 | 4.88E-03 |
| Downregulated genes
in transcriptionally active PAX3(+/−Q)-FOXO1 cells |
| Immune system
process | 26 | 36 | 5.41E-05 |
| Glycoprotein | 57 | 77 | 5.48E-05 |
| Anatomical
structure morphogenesis | 25 | 35 | 9.55E-05 |
| Developmental
process | 50 | 67 | 1.39E-04 |
| Anatomical
structure development | 37 | 50 | 1.97E-04 |
| Multicellular
organismal development | 39 | 53 | 2.46E-04 |
| Response to
virus | 7 | 11 | 2.99E-04 |
| von Willebrand
factor, type C | 5 | 8 | 3.21E-04 |
| Immune
response | 21 | 31 | 3.59E-04 |
| 2-5-Oligoadenylate
synthetase | 3 | 5 | 4.45E-04 |
| Negative
regulation of biological process | 24 | 35 | 4.58E-04 |
| Signal | 44 | 60 | 5.68E-04 |
| Signal
transduction | 53 | 68 | 6.54E-04 |
| 2-5-Oligoadenylate
synthetase, ubiquitin like region | 3 | 5 | 7.37E-04 |
| VWC (von
Willebrand factor (vWF) type C domain) | 5 | 8 | 8.24E-04 |
| Response to
external stimulus | 16 | 23 | 8.50E-04 |
|
PIRSF005680:Interferon-induced 56K
protein | 3 | 5 | 9.61E-04 |
| Morphogenesis of
an epithelium | 6 | 6 | 1.05E-03 |
| System
development | 30 | 39 | 1.21E-03 |
| Multicellular
organismal process | 52 | 70 | 1.22E-03 |
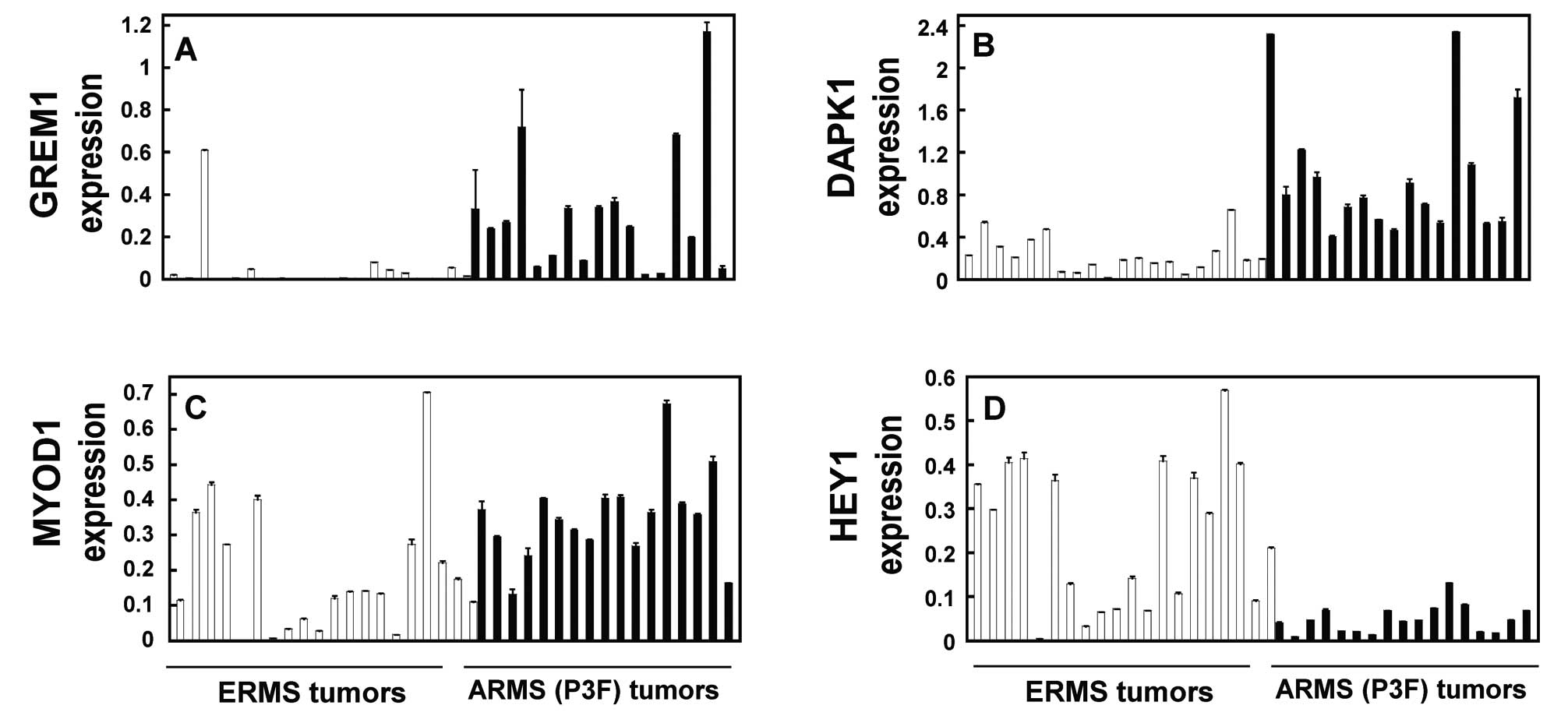
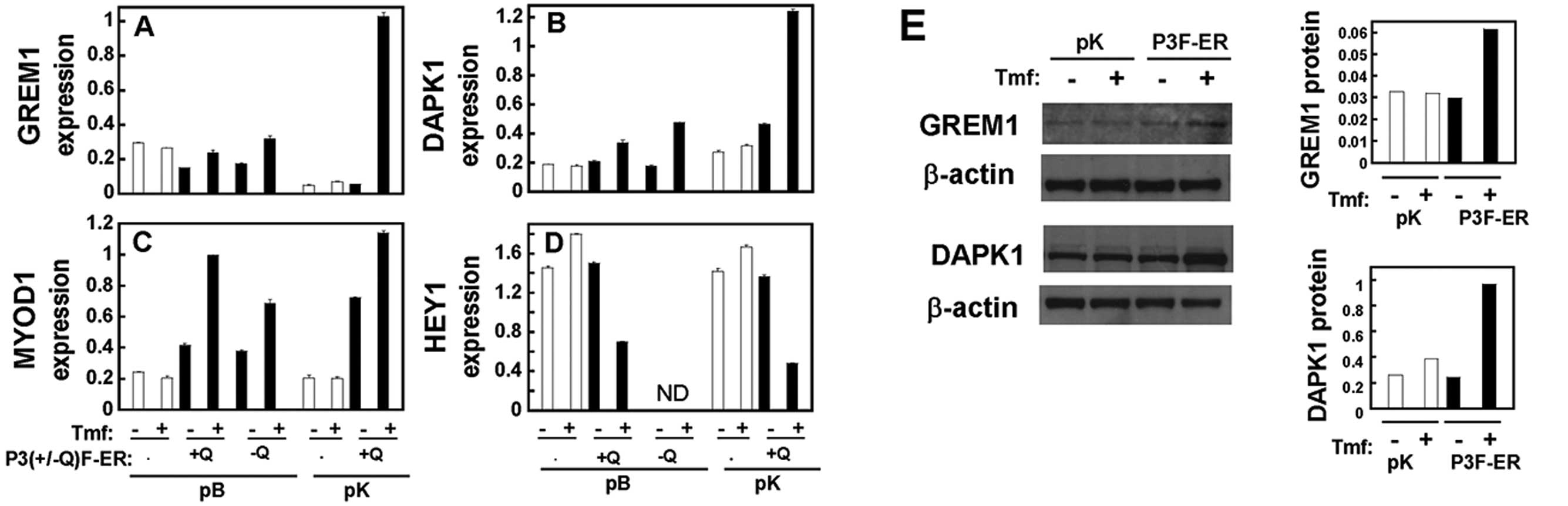
GREM1, DAPK1, MYOD1 and HEY1 are
significantly and differentially expressed in both independent
tumors and inducible PAX3-FOXO1-ER cell systems
Since gene ontology analysis EASE or DAVID indicated
apoptosis (Fig. 3) and development
(Table II) as significant
functional categories, we validated the expression of
apoptosis-related genes (GREM1, DAPK1) and
development-related genes (MYOD1, HEY1) that were identified
among the 34 potential target genes (Table I) differentially expressed in both
primary tumors and the inducible cell culture system. These genes
were analyzed in an independent panel of primary tumors (20
fusion-negative ERMS and 17 PAX3-FOXO1-positive ARMS) (Fig. 4; Table
III) and RD cells carrying inducible PAX3(+/−Q)-FOXO1-ER in pB
or pK (Tmf-treated versus untreated control) (Fig. 5; Table
III). The validation was carried out at the RNA level using
qRT-PCR for the expression of GREM1 (Figs. 4A and 5A), DAPK1 (Figs. 4B and 5B), MYOD1 (Figs. 4C and 5C) and HEY1 (Figs. 4D and 5D). Furthermore, the upregulation of GREM1
and DAPK1 at the protein level was validated using immunoblot
analysis (Fig. 5E). These
validation studies of GREM1, DAPK1, MYOD1 and HEY1 (Figs. 4 and 5) consistently demonstrated significant
differential expression, which was comparable to that noted in the
microarray expression signature (Table III).
 | Table IIIValidation of the expression of 4
target genes (GREM1, DAPK1, MYOD1 and HEY1). |
Table III
Validation of the expression of 4
target genes (GREM1, DAPK1, MYOD1 and HEY1).
| Fold-change |
|---|
|
|
|---|
| Microarray | qRT-PCR |
|---|
|
|
|
|---|
| Tumors | Inducible
cells | Tumors | P-values
(tumors) | Inducible
cells |
|---|
| Downregulated
genes |
| HEY1 | 6.21 | 1.72 | 4.83 | 0.0000 | 2.14 |
| Upregulated
genes |
| GREM1 | 5.34 | 2.13 | 6.82 | 0.0000 | 1.76 |
| DAPK1 | 2.51 | 2.40 | 4.26 | 0.0000 | 2.17 |
| MYOD1 | 2.59 | 2.28 | 1.86 | 0.0033 | 2.11 |
Discussion
In the present study, we developed the RD ERMS cell
culture system expressing the inducible PAX3(+/−Q)-FOXO1-ER in pB,
which is useful for determining the early short-term effects of
PAX3(+/−Q)-FOXO1 and for regulating the strength and duration of
transcriptional activity of PAX3-FOXO1. We analyzed two independent
sets of gene expression profiles: primary RMS tumors and RD ERMS
cells transduced with inducible PAX3(+/−Q)-FOXO1 constructs. We
found 34 potential target genes (27 upregulated and 7
downregulated) that were significantly and differentially expressed
between PAX3(+/−Q)-FOXO1-positive and fusion-negative categories,
in both primary tumors and the inducible PAX3(+/−Q)-FOXO1 cell
culture system. Among the 34 genes, we investigated cell death or
apoptosis-related (GREM1, DAPK1) and
development-related genes (MYOD1, HEY1).
Target genes of PAX3-FOXO1 and PAX7-FOXO1, or
PAX3-FOXO1 only, were previously reported, based on gene expression
profiling in both primary tumors and cells transduced with
constitutive PAX3/PAX7-FOXO1 (13)
or inducible PAX3-FOXO1-ER (16)
constructs. Constitutive PAX3-FOXO1 cell system represents
relatively longer (later) effects of PAX3-FOXO1 compared to the
inducible PAX3-FOXO1-ER cell systems. These two studies (13,16)
reported 81 and 39 target genes, respectively. Compared with these
two previous studies, 11 and 12 target genes, respectively, were
found in common with the present study (Table IV). Six genes (KCNN3, MCAM,
MYOD1, TCF7L2, TM4SF10, ZFP36L2) were identified as targets in
all three studies (Table IV)
(13,16, present study).
 | Table IVComparison of the potential target
genes of PAX3-FOXO1 in three studies (13,16, present study). |
Table IV
Comparison of the potential target
genes of PAX3-FOXO1 in three studies (13,16, present study).
| 6 common genes in
all three studies [Davicioni et al(13), Mercado et al(16) and present study] | 12 common genes in
present study and Mercado et al(16) | 11 common genes in
present study and Davicioni et al(13) | 16 common genes in
Davicioni et al(13) and
Mercado et al(16) |
|---|
| KCNN3 | DAPK1 | ABAT | DCX |
| MCAM | DKFZp762M127 | IL4R | DUSP4 (↓) |
| MYOD1 | GREM1 | INPP1 | GADD45A |
| TCF7L2 | HEY1 (↓) | KCNN3 | IGFBP3 (↓) |
| TM4SF10 | KCNN3 | MCAM | KCNN3 |
| ZFP36L2 (↓) | MCAM | MYOD1 | MARCH3 |
| MYOD1 | NRCAM | MCAM |
| NCOA1 | SKP2 | MEG3 |
| QDPR | TCF7L2 | MET |
| TCF7L2 | TM4SF10 | MYCN |
| TM4SF10 | ZFP36L2 (↓) | MYOD1 |
| ZFP36L2 (↓) | | NEBL |
| | | PRKAR2B |
| | | TCF7L2 |
| | | TM4SF10 |
| | | ZFP36L2 (↓) |
We demonstrated that higher expression of
MYOD1 and lower expression of HEY1 were consistently
observed in all PAX3-FOXO1-expressing ARMS primary tumors and
cells, in comparison to fusion-negative ERMS primary tumors and
cells. These findings of MYOD1 upregulation are in accord
with results reported in previous studies of MYOD1 expression in
human ARMS (26,27), PAX3/PAX7-FOXO1-positive cells
(13), mesenchymal stem cells
transfected with PAX3-FOXO1 (28),
and NIH3T3 fibroblasts transduced with PAX3-FOXO1 (10,29).
In a previous study (30), HEY1
overexpression was found to inhibit MYOD1, an early myogenic
differentiation marker, which indicates that HEY1 downregulation
found in our study is consistent with this PAX3-FOXO1-induced
myogenic developmental program including MYOD1 upregulation. It is
hypothesized that PAX3-FOXO1 simultaneously reinforces myogenic
determination by upregulating MYOD1, while suppressing terminal
myogenic differentiation (31,32).
The oncogenic activity of PAX3-FOXO1 is
characterized in part by its stimulatory effects on cell
proliferation (33–35) and cell survival/anti-apoptosis
(36,37) as well as its inhibitory role on
terminal myogenic differentiation (31,32).
However, in addition to these effects, PAX3-FOXO1 can also cause
growth suppression and cell death in other settings (20,21,38).
These paradoxical features of PAX3-FOXO1 being both oncogenic and
growth-suppressive were demonstrated in previous studies with
immortalized murine fibroblasts (20,21)
and human myoblasts (38). We
identified and validated that tumor-suppressor genes, GREM1
(39) and DAPK1 (40), are upregulated at both RNA and
protein levels in PAX3-FOXO1-positive ARMS tumors and PAX3-FOXO1-ER
inducible cell culture systems. Our study suggests that GREM1 and
DAPK1 tumor suppressor genes can be potential target genes
contributing to this growth-suppressive activity of high PAX3-FOXO1
expression.
In conclusion, we identified 34 potential downstream
target genes of PAX3(+/−Q)-FOXO1 by analyzing two independent sets
of gene expression profiles: primary RMS tumors and RD ERMS cells
transduced with inducible PAX3-FOXO1 constructs. Our study can
serve as a basis to propose the 4 genes (GREM1, DAPK1, MYOD1
and HEY1) as targets that function in growth suppression or
myogenic differentiation downstream of PAX3-FOXO1 in ARMS (Fig. 6).
Acknowledgements
This research was supported by NIH grants
CA064202-13 (F.G. Barr), CA104896-03 (F.G. Barr), CA087812-06 (F.G.
Barr, G.E. Mercado), and CA106450 (M. Ladanyi). We thank Dr
Frederic G. Barr for his support and advice; Donna M. Gustafson for
technical assistance; Dr Shujuan J. Xia for technical advice;
Juseong Lee for editorial assistance; and Dr Lawrence A. Loeb
(CA077852-14) for encouragement and comment.
Abbreviations:
|
RMS
|
rhabdomyosarcoma
|
|
Q
|
glutamine
|
|
ARMS
|
alveolar rhabdomyosarcoma
|
|
ERMS
|
embryonal rhabdomyosarcoma
|
|
ER
|
estrogen receptor
|
|
pK
|
pK1
|
|
P3F
|
PAX3-FOXO1
|
|
P3F-ER
|
PAX3-FOXO1-ER
|
|
Tmf
|
4-hydroxytamoxifen
|
References
|
1
|
Linardic CM: PAX3-FOXO1 fusion gene in
rhabdomyosarcoma. Cancer Lett. 270:10–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorensen PH, Lynch JC, Qualman SJ, et al:
PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in
alveolar rhabdomyosarcoma: a report from the Children’s Oncology
Group. J Clin Oncol. 20:2672–2679. 2002.PubMed/NCBI
|
|
3
|
Stevens MC: Treatment for childhood
rhabdomyosarcoma: the cost of cure. Lancet Oncol. 6:77–84. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scrable HJ, Witte DP, Lampkin BC and
Cavenee WK: Chromosomal localization of the human rhabdomyosarcoma
locus by mitotic recombination mapping. Nature. 329:645–647. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visser M, Sijmons C, Bras J, et al:
Allelotype of pediatric rhabdomyosarcoma. Oncogene. 15:1309–1314.
1997. View Article : Google Scholar
|
|
6
|
Davis RJ and Barr FG: Fusion genes
resulting from alternative chromosomal translocations are
overexpressed by gene-specific mechanisms in alveolar
rhabdomyosarcoma. Proc Natl Acad Sci USA. 94:8047–8051. 1997.
View Article : Google Scholar
|
|
7
|
Du S, Lawrence EJ, Strzelecki D, Rajput P,
Xia SJ, Gottesman DM and Barr FG: Co-expression of alternatively
spliced forms of PAX3, PAX7, PAX3-FKHR and PAX7-FKHR with distinct
DNA binding and transactivation properties in rhabdomyosarcoma. Int
J Cancer. 115:85–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelly KM, Womer RB, Sorensen PHB, Xiong QB
and Barr FG: Common and variant gene fusions predict distinct
clinical phenotypes in rhabdomyosarcoma. J Clin Oncol.
15:1831–1836. 1997.PubMed/NCBI
|
|
9
|
Anderson J, Gordon T, McManus A, et al:
Detection of the PAX3-FKHR fusion gene in paediatric
rhabdomyosarcoma: a reproducible predictor of outcome? Br J Cancer.
85:831–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan J, Bittner ML, Saal LH, et al: cDNA
microarrays detect activation of a myogenic transcription program
by the PAX3-FKHR fusion oncogene. Proc Natl Acad Sci USA.
96:13264–13269. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wachtel M, Dettling M, Koscielniak E, et
al: Gene expression signatures identify rhabdomyosarcoma subtypes
and detect a novel t(2;2)(q35;q23) translocation fusing PAX3 to
NCOA1. Cancer Res. 64:5539–5545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wachtel M, Runge T, Leuschner I, et al:
Subtype and prognostic classification of rhabdomyosarcoma by
immunohistochemistry. J Clin Oncol. 24:816–822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davicioni E, Finckenstein FG, Shahbazian
V, Buckley JD, Triche TJ and Anderson MJ: Identification of a
PAX-FKHR gene expression signature that defines molecular classes
and determines the prognosis of alveolar rhabdomyosarcoma. Cancer
Res. 66:6936–6946. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Pittà C, Tombolan L, Albiero G, et al:
Gene expression profiling identifies potential relevant genes in
alveolar rhabdomyosarcoma pathogenesis and discriminates PAX3-FKHR
positive and negative tumors. Int J Cancer. 118:2772–2781.
2006.
|
|
15
|
Laé M, Ahn EH, Mercado GE, et al: Global
gene expression profiling of PAX-FKHR fusion-positive alveolar and
PAX-FOXO1 fusion-negative embryonal rhabdomyosarcomas. J Pathol.
212:143–151. 2007.PubMed/NCBI
|
|
16
|
Mercado GE, Xia SJ, Zhang C, et al:
Identification of PAX3-FKHR-regulated genes differentially
expressed between alveolar and embryonal rhabdomyosarcoma: focus on
MYCN as a biologically relevant target. Genes Chromosomes Cancer.
47:510–520. 2008. View Article : Google Scholar
|
|
17
|
Barr FG, Smith LM, Lynch JC, Strzelecki D,
Parham DM, Qualman SJ and Breitfeld PP: Examination of gene fusion
status in archival samples of alveolar rhabdomyosarcoma entered on
the Intergroup Rhabdomyosarcoma Study-III trial: a report from the
Children’s Oncology Group. J Mol Diagn. 8:202–208. 2006.PubMed/NCBI
|
|
18
|
Bennicelli JL, Edwards RH and Barr FG:
Mechanism for transcriptional gain of function resulting from
chromosomal translocation in alveolar rhabdomyosarcoma. Proc Natl
Acad Sci USA. 93:5455–5459. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomescu O, Xia SJ, Strezlecki D,
Bennicelli JL, Ginsberg J, Pawel B and Barr FG: Inducible
short-term and stable long-term cell culture systems reveal that
the PAX3-FKHR fusion oncoprotein regulates CXCR4, PAX3, and PAX7
expression. Lab Invest. 84:1060–1070. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia SJ and Barr FG: Analysis of the
transforming and growth suppressive activities of the PAX3-FKHR
oncoprotein. Oncogene. 23:6864–6871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia SJ, Rajput P, Strzelecki DM and Barr
FG: Analysis of genetic events that modulate the oncogenic and
growth suppressive activities of the PAX3-FKHR fusion oncoprotein.
Lab Invest. 87:318–325. 2007.PubMed/NCBI
|
|
22
|
Littlewood TD, Hancock DC, Danielian PS,
Parker MG and Evan GI: A modified oestrogen receptor ligand-binding
domain as an improved switch for the regulation of heterologous
proteins. Nucleic Acids Res. 23:1686–1690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tusher VG, Tibshirani R and Chu G:
Significance analysis of microarrays applied to the ionizing
radiation response. Proc Natl Acad Sci USA. 98:5116–5121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009.PubMed/NCBI
|
|
25
|
Ahn EH and Schroeder JJ: Sphinganine
causes early activation of JNK and p38 MAPK and inhibition of AKT
in HT-29 human colon cancer cells. Anticancer Res. 26:121–127.
2006.PubMed/NCBI
|
|
26
|
Tonin PN, Scrable H, Shimada H and Cavenee
WK: Muscle-specific gene expression in rhabdomyosarcomas and stages
of human fetal skeletal muscle development. Cancer Res.
51:5100–5106. 1991.PubMed/NCBI
|
|
27
|
Tapscott SJ, Thayer MJ and Weintraub H:
Deficiency in rhabdomyosarcomas of a factor required for MyoD
activity and myogenesis. Science. 259:1450–1453. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren YX, Finckenstein FG, Abdueva DA, et
al: Mouse mesenchymal stem cells expressing PAX-FKHR form alveolar
rhabdomyosarcomas by cooperating with secondary mutations. Cancer
Res. 68:6587–6597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Graf Finckenstein F, Shahbazian V,
Davicioni E, Ren YX and Anderson MJ: PAX-FKHR function as pangenes
by simultaneously inducing and inhibiting myogenesis. Oncogene.
27:2004–2014. 2008.PubMed/NCBI
|
|
30
|
Sun J, Kamei CN, Layne MD, Jain MK, Liao
JK, Lee ME and Chin MT: Regulation of myogenic terminal
differentiation by the hairy-related transcription factor CHF2. J
Biol Chem. 276:18591–18596. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Epstein JA, Lam P, Jepeal L, Maas RL and
Shapiro DN: Pax3 inhibits myogenic differentiation of cultured
myoblast cells. J Biol Chem. 270:11719–11722. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ebauer M, Wachtel M, Niggli FK and Schäfer
BW: Comparative expression profiling identifies an in vivo target
gene signature with TFAP2B as a mediator of the survival function
of PAX3/FKHR. Oncogene. 26:7267–7281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kikuchi K, Tsuchiya K, Otabe O, et al:
Effects of PAX3-FKHR on malignant phenotypes in alveolar
rhabdomyosarcoma. Biochem Biophys Res Commun. 365:568–574. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Collins MH, Zhao H, Womer RB and Barr FG:
Proliferative and apoptotic differences between alveolar
rhabdomyosarcoma subtypes: a comparative study of tumors containing
PAX3-FKHR or PAX7-FKHR gene fusions. Med Pediatr Oncol. 37:83–89.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anderson J, Ramsay A, Gould S and
Pritchard-Jones K: PAX3-FKHR induces morphological change and
enhances cellular proliferation and invasion in rhabdomyosarcoma.
Am J Pathol. 159:1089–1096. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bernasconi M, Remppis A, Fredericks WJ,
Rauscher FJ III and Schäfer BW: Induction of apoptosis in
rhabdomyosarcoma cells through down-regulation of PAX proteins.
Proc Natl Acad Sci USA. 93:13164–13169. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ayyanathan K, Fredericks WJ, Berking C,
Herlyn M, Balakrishnan C, Gunther E and Rauscher FJ III:
Hormone-dependent tumor regression in vivo by an inducible
transcriptional repressor directed at the PAX3-FKHR oncogene.
Cancer Res. 60:5803–5814. 2000.PubMed/NCBI
|
|
38
|
Xia SJ, Holder DD, Pawel BR, Zhang C and
Barr FG: High expression of the PAX3-FKHR oncoprotein is required
to promote tumorigenesis of human myoblasts. Am J Pathol.
175:2600–2608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen B, Athanasiou M, Gu Q and Blair DG:
Drm/Gremlin transcriptionally activates p21(Cip1) via a novel
mechanism and inhibits neoplastic transformation. Biochem Biophys
Res Commun. 295:1135–1141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bialik S and Kimchi A: DAP-kinase as a
target for drug design in cancer and diseases associated with
accelerated cell death. Semin Cancer Biol. 14:283–294. 2004.
View Article : Google Scholar : PubMed/NCBI
|