Introduction
Upper urinary tract urothelial carcinoma (UUTUC) is
an aggressive urologic malignancy associated with high morbidity
and mortality although it is relatively rare, accounting for
approximately 5–10% of all urothelial tumors (1). The natural history of upper tract UC
is different from UC of the bladder with higher incidence of
high-grade deeply invasive disease in the upper urinary tract than
in the bladder (2). UUTUCCs that
invade the muscle wall generally have a very poor prognosis with
5-year specific survival less than 50% for pT2/pT3 and less than
10% for pT4 (1,3). The median survival of pT4 patients is
only 7–9 months even with radical nephroureterectomy and
chemotherapy (3,4). Therefore, UUTUC presents a serious
public health problem and a challenge for clinical physicians and
basic scientists to find more effective systemic adjuvant therapy
to improve the outcome of UUTUC patients.
UUTUC is a male-dominant disease with a male to
female ratio of 2:1 to 2.5:1 (5,6).
Survival of UUTUC patients was significantly influenced by the male
gender, age over 80 years, a two-incision operation, location in
both the pelviocaliceal system and the ureter, grade III, and stage
T3 and T4 with adjusting for gender and age (7). Gender and stage of UUTUC patients were
the only independent prognostic factors predictive of overall
survival and female gender was associated with a better survival
(7). These studies suggest that
gender plays an important role in the development and progression
of UUTUC. However, gender differences in malignant diseases,
including UUTUC, are not fully understood and, therefore, more
studies are required to elucidate their pathological mechanisms.
Androgens and androgen receptor (AR) have been demonstrated to play
an important role in male-dominant cancers, including liver and
bladder cancer, by affecting tumor initiation and progression
(8–10). Therefore, in the present study, we
hypothesized that AR also plays a role in the progression of UUTUC
and accounts for the high incidence and poor prognosis of UUTUC in
males.
During the progression of tumors from primary sites
to metastasis, specific proteins and signals are activated to
enable cancer cells to detach from neighboring cells, re-orientate
their polarity, invade, migrate, survive and proliferate in foreign
microenvironments. These proteins include ECM-degrading enzymes,
such as matrix metalloproteinases (MMPs) and cathepsins, which help
the degradation of the basement membrane and extracellular matrix
(ECM) (11,12). There are also signal molecules
capable of inducing stress-fiber assembly and contraction for
mobility, such as small G-protein Rho and its important downstream
effector, the Rho-associated serine/threonine kinase (ROCK), which
are also involved in tumor cell migration and invasion (13–16).
In UUTUC, the mRNA levels of RhoA and RhoA protein were higher in
tumors and metastatic lymph node tissues than in non-tumor tissues,
suggesting that RhoA-ROCK 1 signaling is involved in the invasion
and metastasis of UUTUC (17). The
expression of MMP-2, MMP-9 and TIMP-1 was an independent predictor
of high pT stage (18) and elevated
expression levels of MMP-9 and MMP-2 were associated with poor
prognosis (19).
AR has been shown to regulate MMP-2 and MMP-9 in the
prostate (20) and in prostate
cancer (21) and AR action in
migration is mediated by RhoA-ROCK signaling axis that controls
cell motility in prostate cancer (22). The upregulation of cyclooxygenase 2
(COX-2) expression occurs frequently in a variety of different
tumors and COX-2, which is also shown to be an important signaling
molecule that regulates cell motility (23,24).
The AR agonist, dihydrotestosterone (DHT), was shown to increase
levels of the vascular inflammatory mediator COX-2 (25).
However, how AR affects the above signals related to
the progress of UUTUC remains unclear. In the present study, we
investigated this issue by adding AR overexpression in UUTUC cells
to observe the role of AR in cell migration and invasion as well as
to examine possible genes involved in the regulatory role of AR in
the migration and invasion in UUTUCs.
Materials and methods
Cell lines and chemicals
The UUTUC cell line BFTC 909 cells (from a UUTUC of
a renal pelvis patient) were a generous gift from Dr C.C. Tzeng
(26) and were cultured in
Dulbecco's modified Eagle's medium, containing 10% heat-inactivated
fetal bovine serum (FBS) at 37°C in an atmosphere of 5%
CO2. SV-HUC cells (ureter cells immortalized by SV40,
from an 11-year-old male accident victim) were obtained from ATCC
and were cultured in Ham's F-12 medium, containing 10%
heat-inactivated FBS at 37°C in an atmosphere of 5% CO2.
To exogenously express AR in cells, a recombinant lentiviral vector
containing wild-type AR (pWPI hAR) (27) and a control lentiviral vector
expressing the enhanced green fluorescent protein (pWPI) were used
to overexpress AR. Lentiviral PWPI-AR/PWPI-control with pMD2.G
packaging and psPAX2 envelope plasmids
(lentivirus:packaging:envelopeZ 2:1:1) were co-transfected into
293T cells. After 48 h of transfection, target cells were cultured
in the presence of viral supernatant containing 8 mg/ml polybrene
(Millipore, Billerica, MA, USA) for 6 h.
MMP-9 inhibitor I was purchased from Calbiochem
(Frankfurt, Germany) and celecoxib and casodex were purchased from
Sigma-Aldrich (Buchs SG, Switzerland).
Wound-healing migration assay
Cells were seeded onto 35-mm plates until
confluence. The plates were scratched using a sterile pipette tip
to generate a wound through the confluent monolayer. Cells were
analyzed and photographed with a microscope. Images of the cell
wound were captured at 0 and 24 h of migration. The relative
migration was calculated by setting the percentage of wound closure
in control cells after 24 h as 1.
Transwell migration assay
Cells were first harvested from the culture dish and
1.0×104 cells in 200 μl of serum-free medium were
transferred to the Transwell inserts (the top compartment, 8-μm
pore size) and 750 μl of medium was placed in the lower chamber.
Following incubation at 37°C for 4 h in a cell culture incubator,
cells on the upper surface of the filters were removed with cotton
swabs, filters were washed, fixed, and stained with crystal violet.
Cells that had moved to the lower surface of the filter were
counted under the microscope. Migrated cells in each field were
quantified. Results are presented as relative migration by setting
the migrating cell number of control cells as 1.
Transwell invasion assay
Cell invasion through a three-dimensional ECM was
assessed by a Matrigel invasion assay using BD Matrigel
coated-Transwell with 8.0-μm filter membranes. Cells resuspended in
200 μl of serum-free medium were plated onto each filter, and 750
μl of DMEM containing 10% FBS were added into the lower compartment
of the invasion chambers. After 24 h, cells on the upper surface of
the filters were removed with cotton swabs, filters were washed in
4% paraformaldehyde and stained with 1% crystal violet. Cells that
had invaded to the lower surface of the filter were counted under
the microscope.
In vitro adhesion to fibronectin
assay
Twenty-four-well culture dishes were pre-coated with
80 μl of fibronectin (2.5 mg/ml) adhesion buffer (0.25% bovine
serum albumin in Hank's balanced salt solution; HBSS) for 30 min at
37°C. Cancer cells (1×105) were added to each well.
After 30 min at 37°C in a CO2 incubator, non-adherent
cells were removed by gentle wash with HBSS. Then, the cells were
fixed with 2% paraformaldehyde in 1X PBS and the adhesive cancer
cells were stained with 0.05% crystal solution. The staining
intensity was quantitated with a spectrometer.
Quantitative real-time RT-PCR
Total RNA was extracted from cells using TRIzol
(Invitrogen, Carlsbad, CA, USA) and used for first-strand cDNA
synthesis. The mRNA levels were measured using CFX96™ Real-Time
system (Bio-Rad Laboratories) using KAPA SYBR® Fast qPCR
kits (Kapa Biosystems, Inc., Woburn, MA, USA). Specific primers for
MMP-2, MMP-9, RhoA, Rock1, COX-2 and β-actin were: MMP-2, F:
5′-CCCCAGACAGGTGATCTTGAC-3′ and R: 5′-GCTTGCGAGGGAAGAAGTTG-3′;
MMP-9, F: 5′-CGC TGGGCTTAGATCATTCC-3′ and R: 5′-AGGTTGGATAC
ATCACTGCATTAGG-3′; RhoA, F: 5′-TCAAGCCGGAGGT CAACAAC-3′ and R:
5′-ACGAGCTGCCCATAGCAGAA-3′; Rock1, F:
5′-ATGAGTTTATTCCTACACTCTACCACT TTC-3′ and R:
5′-TAACATGGCATCTTCGACAC TCTAG-3′; COX-2, F:
5′-CCCTTGGGTGTCAAAGGTAA-3′ and R: 5′-GCCCTCGCTTATGATCTGTC-3′; and
β-actin, F: 5′-TCA CCCACACTGTGCCCATCTACGA-3′ and R: 5′-CAG
CGGAACCGCTCATTGCCAATGG-3′. PCR cycling conditions were: 3 min at
95°C for 1 cycle followed by 40 amplification cycles at 95°C for 10
sec and 52°C (MMP-2, MMP-9, Rock-1, COX-2 and β-actin), or 62°C
(RhoA) for 30 sec. Expression levels were normalized to β-actin
mRNA level determined by the 2−ΔΔCT method.
Western blot analysis
Cell lysates were resolved by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred
to a nitrocellulose membrane and incubated with specific primary
antibodies. Protein bands were visualized using horseradish
peroxidase (HRP)-conjugated secondary antibodies and enhanced
chemiluminescence reagent (Millipore, Bedford, MA, USA) with the
Bio-Rad imaging system.
Statistical analysis
Experiments were repeated at least three independent
times. Results are expressed as the means ± SD. Two-tailed unpaired
t-test was used to compare the results between the two groups. A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Addition of AR in BFTC 909 and SV-HUC
cells increases cell migration
To determine the role of AR in the migration of
UUTUC cells, we used different UUT urothelial cells, including
established UUTUC cells, BFTC 909, and transformed UUTUC cells,
SV-HUC. Since these cells express a very low amount of AR, we
exogenously expressed AR with viral infection into these cells to
examine how AR affects cell migration. As shown in Fig. 1A, contrast-phase images show that
the wound area was significantly reduced in BFTC 909-hAR cells with
the addition of AR compared to BFTC 909-pWPI cells. Transwell
migration assay also showed that SV-HUC-hAR cells migrated more
than SV-HUC-pWPI cells (Fig. 1B).
These results indicate that AR stimulates UUTUC cell migration
either in cancer cells or transformed UC cells.
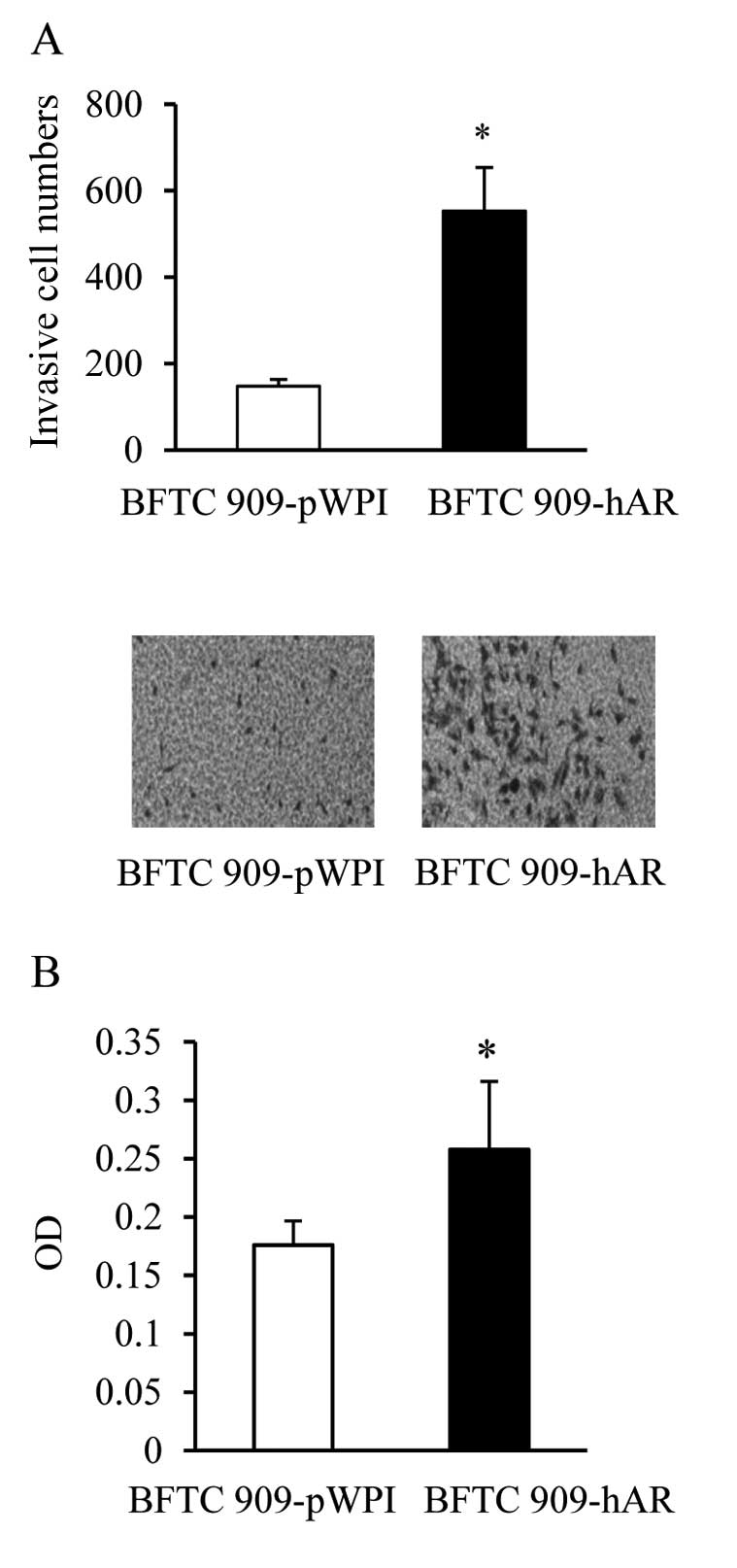
Addition of AR in BFTC 909 cells
increases cell migration
The metastatic process involves several critical
steps such as invasion and adhesion (28). We further determined the ability of
AR to enhance invasion of UUTUC cells. Consistent with findings in
Fig. 1, the addition of AR in BFTC
909-hAR cells increased the number of cells invading through
Matrigel-coated Transwell filters (Fig.
2A). Adhesion to ECM is an important ability of cancer cells to
anchor on the matrix for invasion. Therefore, we also examined the
adhesion ability of BFTC 909 cells with or without AR addition. We
coated fibronectin, an important component of ECM, on culture
plates to investigate the effect of AR on BFTC 909 cell
adhesiveness to fibronectin. The addition of AR significantly
enhanced the adhesion of BFTC 909-hAR cells compared to BFTC
909-pWPI cells (Fig. 2B).
Collectively, our results suggest that AR not only stimulates the
invading ability of urothelial cancer cells but also the ability of
cells to adhere to cell matrix to promote cell invasion.
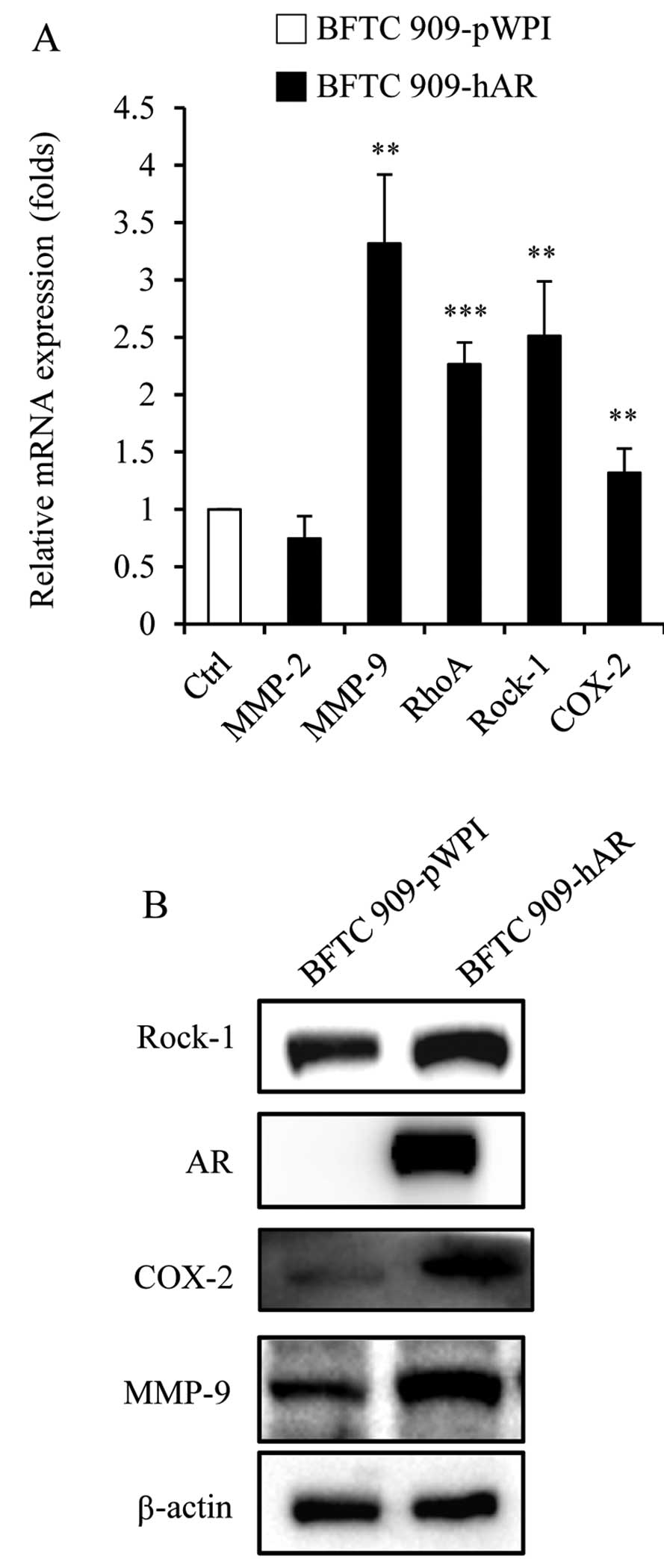
Genes related to migration and invasion
expression changed by AR
To investigate which signaling pathways are
activated by AR to increase cell migration and invasion, we
examined the several genes which are involved in the migration and
invasion of tumor cells. Previous studies have shown that AR
stimulates MMP-2 expression in human prostate cancer, which is
involved in cell migration (29).
AR action in migration is mediated by RhoA-ROCK signaling axis that
controls cell motility in prostate cancer (22), which regulates the cytoskeleton and
cell migration and is frequently overexpressed in tumors (15). COX-2 is another important signal
molecule which regulates cell motility, and AR agonist DHT was
shown to increase levels of the vascular inflammatory mediator
COX-2 (25). Therefore, we assessed
the expression of these genes by qRT-PCR and immunoblot analysis in
BFTC 909 cells with or without AR addition. In mRNA levels, the
expression of MMP-9, RhoA and Rock-1 was increased, while the
expression of MMP-2 and COX-2 was not changed in BFTC 909-hAR
cells, when compared with BFTC 909-pWPI cells (Fig. 3A). Furthermore, in protein levels,
BFTC 909-hAR cells had higher expression of MMP-9, Rock-1 and COX-2
than BFTC 909-pWPI cells (Fig. 3B).
These results suggest that AR may upregulate these genes at the RNA
and protein level to enhance cell migration and invasion.
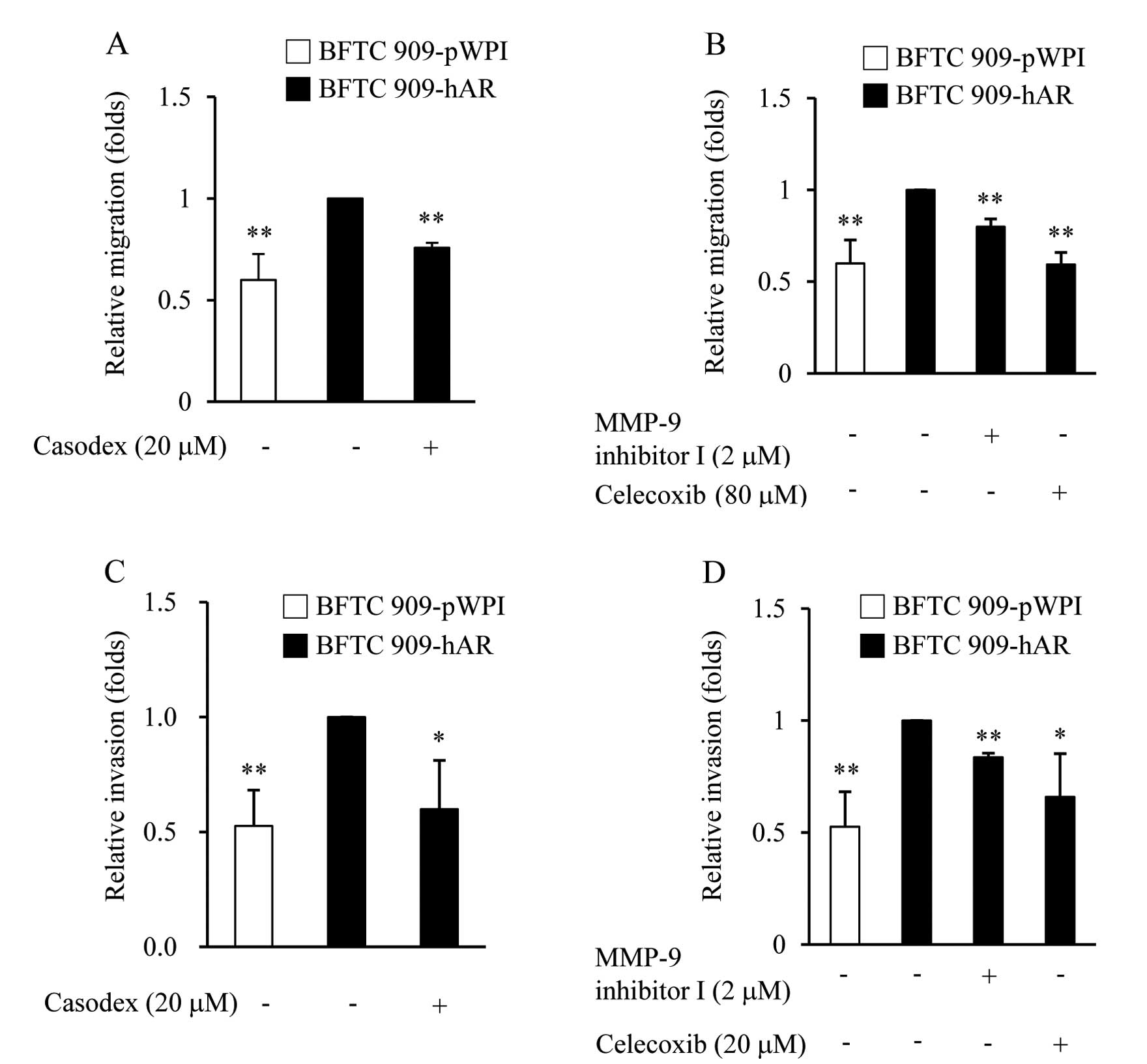
Inhibitor effects on AR-enhanced cell
migration and invasion in UUTUC cells
To further determine the role of MMP-9 and COX-2 in
AR-enhanced migration and invasion, we tested whether MMP-9 and
COX-2 activities were required for enhancing migration and invasion
of BFTC 909-hAR cells by performing the same experiments as above,
but in the presence of MMP-9 inhibitor I, a cell-permeable, potent,
selective, and reversible MMP-9 inhibitor, or selective COX-2
inhibitor (celecoxib). In the migration assay, as expected,
AR-enhanced cell migration in BFTC 909-hAR cells was suppressed by
the anti-androgen, casodex (Fig.
4A). Furthermore, MMP-9 and COX inhibitors also showed the
ability to suppress AR-enhanced cell migration (Fig. 4B). In the invasion assay, BFTC
909-hAR cells that invaded Matrigel to the lower surface of the
filter were decreased by casodex (Fig.
4C) and MMP-9 and COX-2 inhibitors also had the same effect
(Fig. 4D). The finding that both
migration and invasion were markedly reduced in the presence of
casodex, MMP-9 inhibitor and celecoxib, indicate that AR, MMP-9,
COX-2 signaling are involved in the ability of AR to promote
migration and invasion of BFTC 909 cells.
Discussion
The present study indicated that AR plays a role in
the migration and invasiveness of UUTUC cells, based on the results
that there is increased cell migration and invasion following AR
addition in UUTUC cells (Figs. 1
and 2). Our findings may aid in
clarifying the clinical implications of androgen and AR signaling
in the progression of UUTUC, which may explain the higher male
ratio in invasive UUTUC. Although our previous study showed that
approximately 40% of invasive UUTUCs were AR positive (30), the correlation of AR status with
relapse and metastasis following radical nephroureterectomy, and
survival of patients with invasive UUTUC, has not been
investigated. Whether AR is critical in influencing UUTUC progress
and survival outcome requires further investigation. In the present
study, we provided evidence that AR promotes UUTUC metastasis
involving induction of MMP-9 and COX-2 in UUTUC cells, all of which
have well established roles in cancer metastasis (6,11,12).
AR expression levels in the tumor and/or its
microenvironment affect prostate cancer metastasis (31,32).
Exogenous expression of AR in AR-negative PC-3 prostate cancer
cells decreased their invasive properties, and treatment with
androgen further reduced invasion of these cells (33), but AR functions in prostate stromal
cells as a promoter for prostate cancer proliferation and
metastasis (34). Although the role
of AR in UUTUC has not been investigated, in UC of bladder, AR was
shown to promote BBN-induced bladder cancer in mice (9) and bladder cancer cell migration and
invasion (35), suggesting AR also
plays an important role in urothelial carcinoma. Our study is the
first to show the role of AR in increasing UUTUC cell migration and
invasion, which is in contrast to the effect of AR on prostate
cancer, although others also reported that AR promotes the
invasiveness of prostate cancer cells (21,29).
These studies indicate that AR may have diverse effects on
molecules involved in cancer invasion and metastasis by suppressing
or stimulating cancer cell migration and invasion, which may be due
to the tumor microenvironment, coregulators of AR and alterations
of growth factors and their receptors in tumors (36). The exact mechanisms by which AR
exerts its effects in different cancer cells requires further
investigation in order to dissect the multiple roles of AR in
cancer progression and metastasis.
In delineating the molecules regulated by AR in
affecting UUTUC metastasis, we examined the genes including MMPs
involved in the degradation of the ECM, which is a key step in the
process of cancer invasion and metastasis. Our results showed that
among different MMPs, MMP-2 and MMP-9, AR increased MMP-9
expression both at the mRNA and protein level, but not MMP-2 in
UUTUC cells (Fig. 3A). In prostate
cancer cells, regulation of MMP-2 or MMP-9 by AR signaling has
different results from different groups. Some studies have reported
that androgen stimulates pro-MMP-2 expression but not pro-MMP-9 in
LNCaP cells (29) and both MMP-2
and MMP-9 are stimulated by AR signaling in MDA-I cells (21). However, Miyamoto et al
reported that androgen decreases MMP-9 secretion in PC-3 cells
stably expressing AR (37).
Therefore, the molecular mechanism on the upregulation of MMP-9
expression of AR requires further analysis, but the inhibitors of
MMP-9 were shown able to block AR-enhanced cell migration and
invasion. Since MMP inhibitors have been proposed as promising
targets for cancer therapy (38),
the combination of AR antagonists is likely to increase treatment
efficacy in AR positive UUTUC cells.
To metastasize, tumor cells need to increase
motility by remodelling the cytoskeletons and cell contacts with
the ECM, which is regulated by RhoA and ROCK-1 kinase (15). Although we have demonstrated the
increase of RhoA and ROCK-1 expression in BFTC 909 cells with
addition of AR, the inhibitors of ROCK-1 failed to block
AR-enhanced migration and invasion (data not shown), suggesting
that RhoA and ROCK-1 may not significantly affect AR function. The
higher expression level of COX-2 has been shown to increase
invasiveness in colon cancer and prostate cancer cells (24,39).
For UUTUC, overexpressed COX-2 was also found in patients and was
associated with the pathologic stage and grade, indicating that
COX-2 may be involved in UUTUC carcinogenesis and development
(40,41). The effect of the specific COX-2
inhibitor on AR-enhanced migration and invasion clearly
demonstrated the essential role of COX-2 in cell migration and
invasion (Fig. 4B and D).
Inhibition of COX-2 and MMP-9 suppressed AR-enhanced
cell migration and invasion in UUTUC (Fig. 4B and D). This finding provides the
rationale to develop new therapeutic treatment by combining AR
blockade and chemotherapy or target therapy, which may produce
better efficacy. Since COX-2 and MMP-9 are also associated with
tumor progression, inhibition of the COX-2 and MMP-9 pathways could
be an effective therapeutic approach for advanced UUTUCs. The
combined therapy of androgen deprivation and other anti-AR
therapies with inhibition of the COX-2 and MMP-9 pathways may have
improved therapeutic efficacy on advanced UUTUCs. Therefore, our
study has several important clinical implications; first, our data
proved that the expression of AR is linked to tumor cell migration
and invasion, although whether AR overexpression in UUTUCs is
associated with higher clinical stage and poor clinical outcome
requires further investigation. Second, our study suggested that
the addition of AR blockade in therapeutic regimens combined with
targeted drugs may have better responses in UUTUC patients who have
AR positive tumors. Adjuvant chemotherapy following surgery is
currently used in UUTUC patients to prevent cancer relapse and
metastasis, but adjuvant chemotherapy only achieves a 5-year
recurrence-free rate of up to 50 % with minimal impact on survival
(42,43). Since AR blockade has been commonly
used and has been shown to improve survival of men with locally
advanced and high-grade prostate cancer (44), the combination of AR blockade with
either traditional chemotherapy or target therapy may increase the
efficacy of therapeutic regimens on advanced UUTUCs.
Acknowledgements
This study was supported by the grant (TTCRD-10110)
from Buddhist Tzu Chi General Hospital, Taichung, Taiwan. This
study was also supported in part by Taiwan Department of Health
Clinical Trial and Research Center of Excellence
(DOH100-TD-B-111-004).
References
|
1
|
Roupret M, Zigeuner R, Palou J, et al:
European guidelines for the diagnosis and management of upper
urinary tract urothelial cell carcinomas: 2011 update. Eur Urol.
59:584–594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart GD, Bariol SV, Grigor KM, Tolley
DA and McNeill SA: A comparison of the pathology of transitional
cell carcinoma of the bladder and upper urinary tract. BJU Int.
95:791–793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li CC, Chang TH, Wu WJ, et al: Significant
predictive factors for prognosis of primary upper urinary tract
cancer after radical nephroureterectomy in Taiwanese patients. Eur
Urol. 54:1127–1134. 2008. View Article : Google Scholar
|
|
4
|
Hall MC, Womack JS, Roehrborn CG, Carmody
T and Sagalowsky AI: Advanced transitional cell carcinoma of the
upper urinary tract: patterns of failure, survival and impact of
postoperative adjuvant radiotherapy. J Urol. 160:703–706. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munoz JJ and Ellison LM: Upper tract
urothelial neoplasms: incidence and survival during the last 2
decades. J Urol. 164:1523–1525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozsahin M, Zouhair A, Villa S, et al:
Prognostic factors in urothelial renal pelvis and ureter tumours: a
multicentre Rare Cancer Network study. Eur J Cancer. 35:738–743.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papatsoris AG, Chrisofos M, Skolarikos A,
et al: Upper urinary tract transitional cell carcinoma. A 10-year
experience. Tumori. 94:75–78. 2008.PubMed/NCBI
|
|
8
|
Wu MH, Ma WL, Hsu CL, et al: Androgen
receptor promotes hepatitis B virus-induced hepatocarcinogenesis
through modulation of hepatitis B virus RNA transcription. Sci
Transl Med. 2:32ra352010.PubMed/NCBI
|
|
9
|
Miyamoto H, Yang Z, Chen YT, et al:
Promotion of bladder cancer development and progression by androgen
receptor signals. J Natl Cancer Inst. 99:558–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma WL, Hsu CL, Wu MH, et al: Androgen
receptor is a new potential therapeutic target for the treatment of
hepatocellular carcinoma. Gastroenterology. 135:947–955. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Himelstein BP, Canete-Soler R, Bernhard
EJ, Dilks DW and Muschel RJ: Metalloproteinases in tumor
progression: the contribution of MMP-9. Invasion Metastasis.
14:246–258. 1994.PubMed/NCBI
|
|
12
|
Kupferman ME, Fini ME, Muller WJ, Weber R,
Cheng Y and Muschel RJ: Matrix metalloproteinase 9 promoter
activity is induced coincident with invasion during tumor
progression. Am J Pathol. 157:1777–1783. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitz AA, Govek EE, Bottner B and Van
Aelst L: Rho GTPases: signaling, migration, and invasion. Exp Cell
Res. 261:1–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahai E and Marshall CJ: Differing modes
of tumour cell invasion have distinct requirements for Rho/ROCK
signalling and extracellular proteolysis. Nat Cell Biol. 5:711–719.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Itoh K, Yoshioka K, Akedo H, Uehata M,
Ishizaki T and Narumiya S: An essential part for Rho-associated
kinase in the transcellular invasion of tumor cells. Nat Med.
5:221–225. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamai T, Kawakami S, Koga F, et al: RhoA
is associated with invasion and lymph node metastasis in upper
urinary tract cancer. BJU Int. 91:234–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyata Y, Kanda S, Nomata K, Hayashida Y
and Kanetake H: Expression of metalloproteinase-2,
metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in
transitional cell carcinoma of upper urinary tract: correlation
with tumor stage and survival. Urology. 63:602–608. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inoue K, Kamada M, Slaton JW, et al: The
prognostic value of angiogenesis and metastasis-related genes for
progression of transitional cell carcinoma of the renal pelvis and
ureter. Clin Cancer Res. 8:1863–1870. 2002.PubMed/NCBI
|
|
20
|
Delella FK, Justulin LA Jr and Felisbino
SL: Finasteride treatment alters MMP-2 and −9 gene expression and
activity in the rat ventral prostate. Int J Androl. 33:e114–e122.
2010.PubMed/NCBI
|
|
21
|
Hara T, Miyazaki H, Lee A, Tran CP and
Reiter RE: Androgen receptor and invasion in prostate cancer.
Cancer Res. 68:1128–1135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidt LJ, Duncan K, Yadav N, et al: RhoA
as a mediator of clinically relevant androgen action in prostate
cancer cells. Mol Endocrinol. 26:716–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolff H, Saukkonen K, Anttila S,
Karjalainen A, Vainio H and Ristimaki A: Expression of
cyclooxygenase-2 in human lung carcinoma. Cancer Res. 58:4997–5001.
1998.PubMed/NCBI
|
|
24
|
Tsujii M, Kawano S and DuBois RN:
Cyclooxygenase-2 expression in human colon cancer cells increases
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Osterlund KL, Handa RJ and Gonzales RJ:
Dihydrotestosterone alters cyclooxygenase-2 levels in human
coronary artery smooth muscle cells. Am J Physiol Endocrinol Metab.
298:E838–E845. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tzeng CC, Liu HS, Li C, et al:
Characterization of two urothelium cancer cell lines derived from a
blackfoot disease endemic area in Taiwan. Anticancer Res.
16:1797–1804. 1996.PubMed/NCBI
|
|
27
|
Ma WL, Hsu CL, Yeh CC, et al: Hepatic
androgen receptor suppresses hepatocellular carcinoma metastasis
through modulation of cell migration and anoikis. Hepatology.
56:176–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited - the role of tumor-stroma interactions
in metastasis to different organs. Int J Cancer. 128:2527–2535.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao X, Thrasher JB, Pelling J,
Holzbeierlein J, Sang QX and Li B: Androgen stimulates matrix
metalloproteinase-2 expression in human prostate cancer.
Endocrinology. 144:1656–1663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shyr CR, Chen CC, Hsieh TF, et al: The
expression and actions of androgen receptor in upper urinary tract
urothelial carcinoma (UUTUC) tissues and the primary cultured
cells. Endocrine. 43:191–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niu Y, Altuwaijri S, Lai KP, et al:
Androgen receptor is a tumor suppressor and proliferator in
prostate cancer. Proc Natl Acad Sci USA. 105:12182–12187. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonaccorsi L, Carloni V, Muratori M, et
al: Androgen receptor expression in prostate carcinoma cells
suppresses alpha6beta4 integrin-mediated invasive phenotype.
Endocrinology. 141:3172–3182. 2000.PubMed/NCBI
|
|
34
|
Niu Y, Altuwaijri S, Yeh S, et al:
Targeting the stromal androgen receptor in primary prostate tumors
at earlier stages. Proc Natl Acad Sci USA. 105:12188–12193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu JT, Han BM, Yu SQ, Wang HP and Xia SJ:
Androgen receptor is a potential therapeutic target for bladder
cancer. Urology. 75:820–827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baldi E, Bonaccorsi L and Forti G:
Androgen receptor: good guy or bad guy in prostate cancer invasion?
Endocrinology. 144:1653–1655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyamoto H, Altuwaijri S, Cai Y, Messing
EM and Chang C: Inhibition of the Akt, cyclooxygenase-2, and matrix
metalloproteinase-9 pathways in combination with androgen
deprivation therapy: potential therapeutic approaches for prostate
cancer. Mol Carcinog. 44:1–10. 2005. View
Article : Google Scholar
|
|
38
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kirschenbaum A, Liu X-H, Yao S and Levine
AC: The role of cyclooxygenase-2 in prostate cancer. Urology.
58:127–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oku S, Higashi M, Imazono Y, et al:
Overexpression of cyclooxygenase-2 in high-grade human transitional
cell carcinoma of the upper urinary tract. BJU Int. 91:109–114.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeon HG, Jeong IG, Bae J, et al:
Expression of Ki-67 and COX-2 in patients with upper urinary tract
urothelial carcinoma. Urology. 76:513.e7–513.e12. 2010.PubMed/NCBI
|
|
42
|
Vassilakopoulou M, de La Motte Rouge T,
Colin P, et al: Outcomes after adjuvant chemotherapy in the
treatment of high-risk urothelial carcinoma of the upper urinary
tract (UUT-UC): results from a large multicenter collaborative
study. Cancer. 117:5500–5508. 2011. View Article : Google Scholar
|
|
43
|
Hellenthal NJ, Shariat SF, Margulis V, et
al: Adjuvant chemotherapy for high risk upper tract urothelial
carcinoma: results from the Upper Tract Urothelial Carcinoma
Collaboration. J Urol. 182:900–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyamoto H, Messing EM and Chang C:
Androgen deprivation therapy for prostate cancer: current status
and future prospects. Prostate. 61:332–353. 2004. View Article : Google Scholar : PubMed/NCBI
|