Introduction
Laryngeal squamous cell carcinoma (LSCC), a type of
head and neck cancer (HNSCC), is the 11th most common cancer in men
worldwide (1). It is also the only
cancer with a decreased survival rate in the USA (2). Once it metastasizes, the 5-year
survival rate of HNC patients is reduced by 50% (3). The lack of progress has been mainly
attributed to local and regional recurrences particularly in
patients with stages III and IV disease (4). Therefore, further efforts must be made
to improve our understanding of LSCC pathogenesis and prognosis.
Similar to most types of tumor, LSCC can result in a suppressed
immune system with an altered serum cytokine profile and immune
cells that function aberrantly (5).
In recent years, a concept has emerged that peripheral tolerance to
tumors is maintained and enhanced by T cells with their
immunoregulatory function (6).
Regulatory T cells (Tregs) are a subgroup of
CD4+ T cells characterized by expression of CD25 and
forkhead box P3 (Foxp3) (6). To
date, there are 3 main types of CD4+ Treg cells partly
characterized in humans: i)
CD4+CD25-IL-10+Foxp3low
type 1 T regulatory (Tr1) cells, which arise in the periphery in an
IL-10-dependent manner (7); ii)
naturally occurring
CD4+CD25+Foxp3+ T cells (nTregs),
which arise directly in the thymus and have the ability to suppress
responses of both CD4+ and CD8+ T cells in a
contact-dependent, cytokine-independent and antigen non-specific
manner (8–10); and iii) Th3 cells, which are defined
by their production of large amounts of transforming growth factor
(TGF)-β (11).
Tregs can suppress the activation, proliferation and
effector functions of various immune cells in vitro and
in vivo(12), which could
play an important role in the maintenance of immune tolerance.
However, Tregs can also suppress anticancer immune responses, which
is in favor of tumor progression (13). The underlying mechanism of the
enrichment of the Treg subset in tumor mass remains to be fully
elucidated, but may aid in understanding the mechanisms of distinct
Treg subsets in immunosuppression and in improving patient
treatment and quality of life (14). However, the Treg migration and
accumulation in local tissue is the precondition for its full
functionality. Increasing evidence has shown that Tregs express
chemokine receptors, which take part in their migration through
interaction with specific ligands (15,16).
This chemokine system is a superfamily composed of
~50 ligands and 20 receptors, which are directly involved in
trafficking along with lymphocyte activation and homing.
Furthermore, it participates and plays a key role in inflammatory
reactions (17–19). It has been demonstrated that
CD4+CD25+ regulatory T cells can express a
number of chemokine receptors, including CCR6 and CCR7 (20), which are also expressed by several
cancer cells (21). It has been
demonstrated that other chemokine receptors were expressed on
cancer cells and acted at all stages of tumor development and
progression, including neoplastic transformation of cells,
promotion of angiogenesis, clonal expansion and growth (22). Chemokines have been shown to have
quite a multifaceted role in cancer development and progression
(23). Previous reports suggested
that chemokines contribute to protection mechanisms that enable
malignant cancer cells to resist chemotherapy and radiation therapy
(24,25).
Several cancer cells overexpress chemokine receptors
and numerous metastasis sites express the corresponding chemokines.
For example, CXCR4-CXCL12 signaling has been shown to play a role
in breast cancer metastasis to bone, brain and liver (26). Meanwhile, Treg can also migrate to
specific locations (such as tumor sites) via this mechanism. It has
been proved that CCR7 expressing Treg can be chemoattractant to
draining lymph nodes (LNs) where CCR7 ligands (CCL19 and CCL21) are
expressed (27,28). The CCR6 also plays a role in organ
selective liver metastasis of colorectal cancer (29). Therefore, in the present study, we
investigated whether chemokines and their receptors favor the Treg
migration, LSCC cell metastasis and the subsequent LSCC
progression. This study was conducted to analyze the possible role
of CCR6, CCR7 and their ligands CCL20 (also known as MIP-3α, LARC),
CCL19 (also called MIP-3β, ELC) and CCL21 (also called 6Ckine, SLC)
and to explore the possible association between their expression
levels and clinical/pathological characteristics of LSCC.
Materials and methods
Patients and healthy donors
A total of 88 LSCC cases were enrolled from patients
who were diagnosed and underwent surgery in the Otolaryngology Head
and Neck Surgery Department of the Eye, Ear, Nose and Throat
Hospital, Fudan University, between November 2008 and 2009. A total
of 50 tumor specimens and paired adjacent pathologically confirmed
normal mucosa (at least 1 cm from the tumor margin) were collected
from patients undergoing total or partial laryngectomy for LSCC.
These samples included 1, 16, 21 and 12 patients in stages I, II,
III and IV. The detailed clinicopathological characteristics of
these patients are summarized in Table
I. Peripheral blood samples were obtained from another 38
untreated LSCC patients. None of these patients received
chemotherapy or radiotherapy prior to specimen collection. Tumor
stage was determined according to the 2002 International Union
Against Cancer TNM classification system (30). Blood samples were also obtained from
20 healthy volunteers. All specimens were collected under study
protocols approved by the Ethics Committee of Fudan University and
all subjects provided written informed consent prior to their
inclusion in the study (KJ2007-01).
 | Table IClinicopathological characteristics
of LSCC patients. |
Table I
Clinicopathological characteristics
of LSCC patients.
| Variable | Patients (fresh
tissue) (N=50)
n (%) | Patients (blood)
(N=38)
n (%) |
|---|
| Age/years |
| Mean (range) | 60.82 (37–81) | 60.92 (41–82) |
| Gender |
| Male | 47 (94.0) | 37 (97.4) |
| Female | 3 (6.0) | 1 (2.6) |
| Location |
| Supraglottic | 23 (46.0) | 14 (36.8) |
| Glottic | 23 (46.0) | 22 (57.9) |
| Subglottic | 4 (8.0) | 2 (5.3) |
| cT stage |
| T1+T2 | 18 (36.0) | 26 (68.5) |
| T3+T4 | 32 (64.0) | 12 (31.6) |
| pN stage |
| N0 | 34 (68.0) | 34 (89.5) |
| N1+N2 | 16 (32.0) | 4 (10.5) |
| Clinical grade |
| I+II | 17 (34.0) | 24 (63.2) |
| III+IV | 33 (66.0) | 14 (36.8) |
Reagents and kits
The following reagents were used in this study:
TRIzol® (15596-018; Life Technologies, USA),
PrimeScript™ RT-PCR kit (Perfect Real Time) (DRR063A; Takara,
Japan), mouse anti-human CD4-FITC, CD25-PE-Cy5, Foxp3-PE (11-0049,
15-0259, 12-4777; eBioscience, USA), CCR6- and CCR7-Alexa Fluor 647
(BioLegend, USA) monoclonal antibodies (mAb) and their respective
isotypes, anti-Human Foxp3 Staining Set PE (72-5774; eBioscience),
mouse anti-human CCR6 monoclonal antibody (MAB195; R&D Systems,
USA), mouse anti-human CCR7 monoclonal antibody (550937; BD
Pharmingen, USA), EnVision™+ Single Reagents (GK400115; Dako,
Denmark) and human IL-2/IL-4/IL-10/IL-12p70/interferon
(IFN)-γ/TGF-β1 ELISA Ready-SET-Go (eBioscience).
RNA isolation and reverse
transcription
Total RNA was extracted from patient frozen tissues
using TRIzol reagent according to the manufacturer’s instructions.
A total of 1 μg of total RNA was reverse transcribed to cDNA in a
20-μl reaction system using PrimeScript RT reagent kit (Perfect
Real Time) to prepare the template cDNA, which was then diluted
with sterile water and stored at −20°C. The reverse transcription
procedure was performed according to the manufacturer’s
instructions.
Semi-quantitative real-time PCR
Semi-quantitative real-time PCR for chemokine
ligands, receptors and cytokines was performed on an Applied
Biosystems 7500 Fast Real-Time PCR System and the data was analyzed
using the 7500 software. In brief, 2 μl of cDNA was added in a
20-μl reaction mixture containing 10 μl of 2X SYBR Premix Ex Taq,
0.4 μl forward primer (10 μM), 0.4 μl reverse primer (10 μM), 0.4
μl ROX reference dye and 6.8 μl sterile water. All primers were
designed by Primer Premier 5 software, with their specificity
confirmed by BLAST on the NCBI webpage (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Detailed
information of these primers is listed in Table II. The PCR conditions were: 95°C
for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 25
sec. The expression level of target gene mRNA was normalized by
GAPDH and was represented as 100,000×2−ΔCt, in which the
ΔCt represented the difference between the Ct value of the target
gene and GAPDH (Cttarget gene−CtGAPDH). The
real-time PCR products were subjected to 2% (w/v) agarose gel
electrophoresis and were stained with ethidium bromide.
 | Table IIPrimer sequences used for real-time
PCR. |
Table II
Primer sequences used for real-time
PCR.
| Primer name | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) | Product (bp) |
|---|
| CCR6 |
TGCTCTACGCTTTTATTGGG |
TTGTCGTTATCTGCGGTCTC | 163 |
| CCR7 |
GATTACATCGGAGACAACACCA |
AGTACATGATAGGGAGGAACCAG | 106 |
| CCL19 |
GGCACCAATGATGCTGAAGAC |
GCAGCCATCCTTGATGAGAAG | 102 |
| CCL20 |
CAACTTTGACTGCTGTCTTGGAT |
ACTTTTTTACTGAGGAGACGCAC | 195 |
| CCL21 |
CAGCTATCCTGTTCTTGCCC |
TTGGAGCCCTTTCCTTTCTT | 181 |
| IL-2 |
AACTCCTGTCTTGCATTGCAC |
TGCTCCAGTTGTAGCTGTGTTT | 94 |
| IL-10 |
CTTTAAGGGTTACCTGGGTTG |
CACATGCGCCTTGATGTCT | 109 |
| IL-12p40 |
TGGACCTTGGACCAGAGC |
CTCGCCTCCTTTGTGACAG | 108 |
| TGF-β1 |
CCCACAACGAAATCTATGACA |
AGCAACACGGGTTCAGGT | 103 |
| IFN-γ |
TCGGTAACTGACTTGAATGTCCA |
TCCTTTTTCGCTTCCCTGTT | 100 |
| Foxp3 |
TCCCAGAGTTCCTCCACAAC |
ATTGAGTGTCCGCTGCTTCT | 122 |
| GAPDH |
GAAGGTCGGAGTCAACGGAT |
CCTGGAAGATGGTGATGGG | 224 |
Flow cytometry (FCM)
The peripheral blood mononuclear cells (PBMCs) were
isolated from 5 ml of heparinized blood from the patients with LSCC
as well as healthy individuals using Ficoll. Half of these cells
were suspended in TRIzol and were stored at −20°C for future use.
The other half was extracellularly stained with specific antibodies
against human CD4, CD25, CCR6 or CCR7 for 30 min, fixed,
permeabilized with Fixation/Permeabilization solution and
intracellularly stained with anti-Foxp3 according to the
manufacturer’s protocol. Flow cytometry was performed on a BD
FACSCalibur and the results were analyzed by CellQuest Pro
software. To determine the percentage of
CD4+CD25+Foxp3+ Tregs, lymphocytes
were gated by plotting forward vs. side scatter followed by gating
on CD4+ T cells. The gated cells were then analyzed for
CD25, Foxp3, CCR6 or CCR7 expression.
Immunohistochemistry (IHC)
Paraffin-embedded tissue sections of human LSCC were
used for immunostaining followed by standard procedures for the
avidin-biotin-peroxidase method. The color reaction was developed
in diaminobenzidine solution and the cells were counterstained with
hematoxylin solution. Tissue sections were stained using mouse
anti-human CCR6 mAb (1:200) and CCR7 mAb (1:200), followed by
incubations with secondary Abs. Histopathological evaluation was
independently carried out by 2 pathologists. As previously
described (31,32), the evaluation of staining was
performed based on its intensity and the percentage of stained
cells. The staining was ranked as no staining, weak staining,
medium staining and strong staining with the values of 0, 100, 200
and 300 assigned to each staining intensity, respectively. The
final scores were determined by multiplying the staining values by
the percentage of positively stained cells.
Enzyme-linked immunosorbent assay
(ELISA)
The expression level of IL-2, IL-4, IL-10, IL-12p70,
IFN-γ and TGF-β1 was determined with ELISA Ready-SET-Go in the
plasma of LSCC patients and healthy volunteers according to the
manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with SPSS 13.0
for Windows. The data are reported as mean ± SD or mean ± SE.
Statistical significance of the data was assessed using paired or
unpaired t-tests, Mann-Whitney U test and one-way ANOVA, where
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
The mRNA expression of CCR6, CCR7 and their ligands
in the LSCC group and the paired adjacent normal tissue (ANT) were
measured. The CCR6, CCR7, CCL19 and CCL21 mRNA were downregulated
in the LSCC tissue, while the CCL20 mRNA (the sole ligand of CCR6)
was significantly upregulated as compared to the ANT (Fig. 1A). The CCR6,CCR7 and CCL19 mRNA
expression was downregulated in LN(+) subjects (Fig. 1B) in LSCC tissue, while the CCL20
mRNA was increased in LN(+) samples (Table III and Fig. 1C). The CCL20 mRNA expression was
higher in T3+T4 and III+IV groups as compared to that of the T1+T2
and I+II groups, respectively (Fig.
1C). The expression of CCL21 mRNA in LSCC tissue showed no
significant difference within various pT stages, cN stages and
clinical groups (Table III and
Fig. 1B). Our data further
indicated that the age and tumor localization had no correlation
with the expression of CCR6, CCR7 and their ligands.
 | Table IIICorrelation between mRNA expression
in cancer and clinical characteristics. |
Table III
Correlation between mRNA expression
in cancer and clinical characteristics.
| | P- or F-value |
|---|
| |
|
|---|
| Variable | N | CCR6 | CCR7 | CCL19 | CCL20 | CCL21 |
|---|
| Age/years |
| ≤60 | 25 | 0.57 | 0.66 | 0.32 | 0.33 | 0.58 |
| >60 | 25 | | | | | |
| Location |
| Supraglottic | 23 | 0.16 | 0.11 | 0.39 | 0.21 | 0.35 |
| Glottic | 23 | | | | | |
| Subglottic | 4 | | | | | |
| cT stage |
| T1+T2 | 18 | 0.26 | 0.63 | 0.04 | 0.03 | 0.17 |
| T3+T4 | 32 | | | | | |
| pN stage |
| N0 | 34 | 0.04 | 0.04 | 0.04 | 0.01 | 0.08 |
| N1+N2 | 16 | | | | | |
| Clinical grade |
| I+II | 17 | 0.26 | 0.63 | 0.04 | 0.01 | 0.09 |
| III+IV | 33 | | | | | |
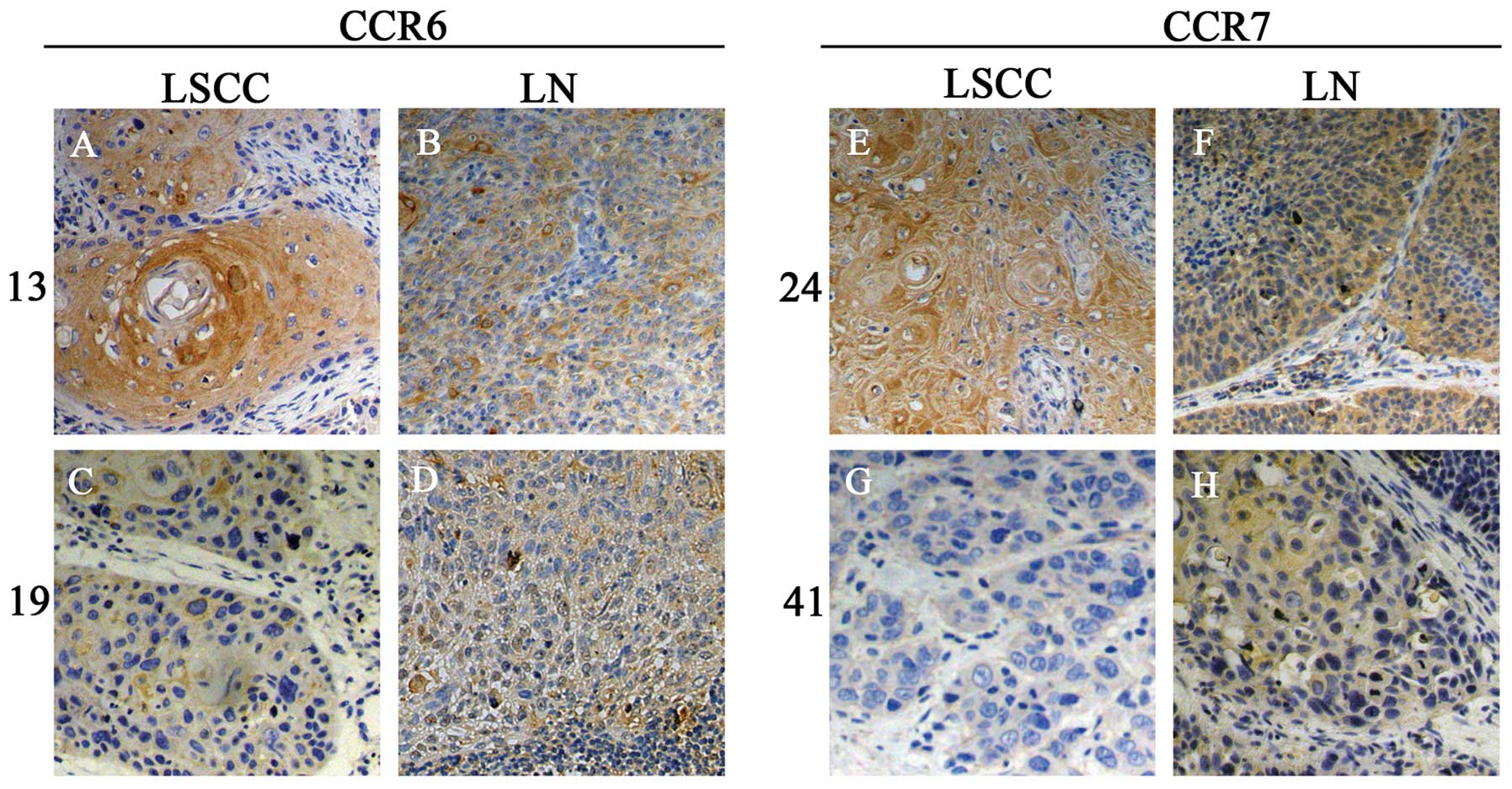
Immunostaining of CCR6 and CCR7 in LSCC
and LN
To study the chemokine receptor expression in
vivo, the paraffin-embedded tissue sections were stained for
CCR6 and CCR7 by IHC (Fig. 2).
Differential CCR6 and CCR7 expressions in LSCC and metastatic LN
were confirmed. The results indicated that the primary and
metastatic cancer cells expressed both CCR6 and CCR7. Co-expression
of CCR6 and CCR7 was found in 40/50 primary and 15/16 lymphatic
metastatic cancer samples. Using semi-quantitative
histopathological evaluation, our results indicated that CCR6 and
CCR7 were upregulated significantly once the metastasis occurred.
Similarly, both CCR6 and CCR7 expression levels were higher in T3 +
T4 stage and III + IV group as compared to those of the T1 + T2
stage and I + II group, respectively (Table IV). Notably, the data from 16 LN(+)
LSCC patients showed that CCR6 and CCR7 scores of primary cancer
were higher (stronger staining) in patients with a higher score
(stronger staining) of CCR6 or CCR7 in their metastatic LN
(Fig. 2).
 | Table IVCorrelation between tumor CCR6, CCR7
IHC scores and clinical characteristics. |
Table IV
Correlation between tumor CCR6, CCR7
IHC scores and clinical characteristics.
| Variable | N | CCR6 score (mean ±
SD) | P-value (mean ±
SD) | CCR7 score (mean ±
SD) | P-value |
|---|
| Age/years |
| ≤60 | 25 | 155.8±28.2 | 0.38 | 178.3±32.1 | 0.41 |
| >60 | 25 | 163.2±29.3 | | 190.4±24.8 | |
| T stage |
| T1+T2 | 18 | 131.8±23.4 | 0.03 | 147.5±23.2 | 0.02 |
| T3+T4 | 32 | 175.1±19.6 | | 205.1±27.6 | |
| N stage |
| N0 | 34 | 152.5±19.7 | 0.02 | 173.7±15.7 | 0.03 |
| N1+N2 | 16 | 174.4±12.8 | | 207.0±12.8 | |
| Clinical grade |
| I+II | 17 | 143.7±16.7 | 0.02 | 158.3±15.6 | 0.03 |
| III+IV | 33 | 167.6±20.2 | | 197.8±17.3 | |
We divided the 16 LN(+) LSCC patients into 2 groups
according to the IHC score of tumor CCR6 or CCR7 respectively, CCR6
high expression group (tumor CCR6 score >174.4) and CCR6 low
expression group (tumor CCR6 score <174.4); CCR7 high expression
group (tumor CCR7 score >207.0) and CCR7 low expression group
(tumor CCR7 score <207.0). CCR6 or CCR7 high expression group
had a higher score of their expression in LN, compared with their
low expression group, respectively (Fig. 2).
CD4+CD25+Foxp3+ Treg analysis and
CCR6, CCR7 expression patterns detected by FACS
In order to understand the role of
CD4+CD25+Foxp3+ Tregs and the
potential function of CCR6 and CCR7 on Tregs, flow cytometry was
used to investigate the proportion of
CD4+CD25+Foxp3+ Tregs in
peripheral blood and the CCR6, CCR7 expression pattern in them in
LSCC patients. The frequency of circulating
CD4+CD25+Foxp3+ Tregs in the LSCC
patients (7.55±2.82% of the CD4+ T population) was
significantly increased as compared to the normal controls (NCs)
(3.91±1.81% of the CD4+ T population). Furthermore, the
frequency of CD4+CD25+Foxp3+ Tregs
was compared among different groups. The distribution of
CD4+CD25+Foxp3+ Tregs was
calculated in 4 clinical groups and is presented in Fig. 3A. The frequency of the Tregs was
increased with the clinical group progression (Fig. 3A). Furthermore, the frequency of
Tregs increased in LN(+) LSCC patients (10.73±0.81% of the
CD4+ T population) as compared to LN(−) LSCC patients
(7.13±2.32% of the CD4+ T population) (Figs. 3B and 4A). Further analysis of the data showed
that the ratio of Foxp3 in the
CD4+CD25+CCR6+ and
CD4+CD25+ T-cell subpopulation was higher in
LSCC patients than in LNs (Figs. 3C,
E and 4B). Meanwhile, the
CD4+CD25+Foxp3+ Tregs of LSCC
patients had a higher CCR6 expression ratio (Fig. 3D).
The Foxp3 gene expression pattern in ANT
and LSCC tissues
Foxp3, a valid marker of regulatory T cells, was
thoroughly investigated by real-time RT-PCR. Our results showed a
significant increase of Foxp3 expression in LSCC tissue (Fig. 1D). As Tregs are known to be able to
suppress the induction of effective antitumor immunity, this result
was consistent with our expectations. However, further analysis
showed that Foxp3 expression of LSCC tissue from LN(+) patients was
downregulated as compared to that of the LN(−) patients. The same
trend was also observed between the early and advanced LSCC
(Fig. 1D).
Cytokine profiles in ANT and LSCC tissues
and plasma
A series of cytokines, including IL-2, IL-10, IL-12,
IFN-γ and TGF-β1, were detected by real-time RT-PCR and ELISA.
Real-time RT-PCR showed that the immune suppressive cytokine IL-10
and TGF-β1 of LSCC tissue were upregulated, while IL-2, IL-12 and
IFN-γ were downregulated, as compared to those of the ANT (Fig. 5A). ELISA was used to detect the
cytokine expression levels at the protein level. Our results also
showed that IL-10 and TGF-β1 expression was increased in the plasma
of LSCC patients, while the IFN-γ expression was decreased
(Fig. 5B).
Discussion
Until the 1980s, total laryngectomy was considered
the most appropriate therapy for patients with locally advanced
laryngeal and hypopharyngeal cancer. Although this strategy did
help to achieve a better disease control, it had a significant
negative impact on patient quality of life due to the presence of a
permanent tracheostomy and the loss of natural voice (33). Therefore, non-surgical treatment of
LSCC became a hot topic in head and neck cancer (34–38).
Cervical LN is the first stop of metastatic laryngeal cancer cells,
which plays a leading role in its prognosis. Therefore, it is
reasonable to identify LN metastases at an early stage in LSCC.
Our data showed that CCR6 and CCR7 mRNA expression
levels were not increased and they were even significantly
decreased in LN(+) patients. Although the CCR6 and CCR7 proteins
were both expressed in ANT (data not shown), their expressions were
shown to have some significance in the development of this cancer
in LSCC patients. The immunostaining results showed that the score
of advanced stage samples (T3 + T4 or III + IV or LN positive) was
higher than that of the early stage samples (T1 + T2 or I + II or
LN negative). Moreover, the strong staining of CCR6 and CCR7 in LN
indicated a more advanced stage of LSCC. This could be used as a
potential marker to assess the condition and prognosis, and may
help in choosing the most suitable treatment for LSCC patients.
Considering the downregulation of CCR6 and CCR7 mRNA expression
levels, we speculated that the expression of CCR6 and CCR7 was more
sensitive in LSCC tissue for the assessment of LSCC. Unlike Wang
et al(21), we found a
heavier staining of CCR7 in metastatic LN as compared to that of
the primary LSCC tissue. However, we did not find a decrease in
CCR6 expression at the protein level. To some extent, this finding
could be the result of the difference in composition of the
subjects.
Real-time RT-PCR showed an increased expression of
CCR6, CCR7, CCL19 and CCL21 and a decreased expression of CCL20 in
ANT, which may have indicated that CCR7 played a more significant
role in local infiltration and metastasis as compared to CCR6. The
distinction of CCR7 was reinforced by its expression pattern in
LN(+) and LN(−) LSCC tissue. Our results further showed that the
CCL20 expression was elevated in cancer tissues with a higher level
in metastatic and advanced cancer patients. It has been reported
that CCL20 stimulates the cell proliferation and their adhesion to
collagen in various tumor cells. Furthermore, overexpression of
CCL20 in tumor cells promoted the growth and adhesion in
vitro and increased tumor growth and invasiveness in
vivo. Moreover, neutralizing antibodies to CCL20 inhibited the
in vivo growth of tumors that either overexpressed CCL20 or
naturally expressed CCL20 (39,40).
Previous studies have showed that LN, spleen, tonsil T zone and
lymphatic endothelial cells, which expressed CCL19 and CCL21
attracted the CCR7+ cells (17). Increasing evidence has demonstrated
the role of CCR7 in LN metastasis, in oral, gastric, esophageal and
lung cancer (41–43). Although CCR6 has been reported to be
involved in hepatocellular carcinoma metastasis (44), its role in LSCC was not the same.
Therefore, we speculated that CCR6 and CCR7 may have a different
effect on the progression and metastasis of LSCC, where CCR6 could
conduct the proliferation of LSCC cells, while CCR7 could mediate
the migration and metastasis.
Previous studies indicated that CCR2, CCR4, CCR5,
CCR6, CCR7, CCR8 and CXCR4 are expressed in
CD4+CD25+ Treg (20,45–47),
and they may participate in the process of
CD4+CD25+ Treg migration, homing and
selective immune response. The frequency of Treg cells in this
study was significantly elevated in CD4+ T cells in LSCC
patients as compared to the healthy controls, and was positively
correlated with the disease progression or the tumor burden.
Further analysis demonstrated that the percentage of Foxp3 positive
CD4+CD25+ Treg and
CD4+CD25+CCR6+ Treg was elevated
in LSCC, which indicated that
CD4+CD25+Foxp3+ Tregs may be
induced and expanded in LSCC patients. The
CD4+CD25+Foxp3+ Tregs suppressed
the activation, proliferation and effector functions such as
cytokine production in a wide range of immune cells, including
CD4+ and CD8+ T cells, natural killer (NK)
and NKT cells, B cells and antigen-presenting cells (APCs) in
vitro and in vivo(12).
These results clearly showed that
CD4+CD25+Foxp3+ Tregs were
involved in the LSCC progression and metastasis. Continuous
proliferation of Tregs gradually strengthened the suppression of
immune system and induced an immune tolerance status, which favored
the LSCC progression and metastasis. Similar results were
demonstrated in several other types of cancer (48–50).
Furthermore, 82.70±15.08% of the
CD4+CD25+Foxp3+ Tregs expressed
CCR6 in LSCC, while this percentage was 65.43±22.71% in NCs. There
was no difference in CCR7 expression on
CD4+CD25+Foxp3+ Tregs between the
LSCC and NC groups, although >50% of the
CD4+CD25+Foxp3+ Tregs expressed
CCR7 (data not shown). Xu et al(14) demonstrated, in a mouse breast cancer
model, that dendritic cells (DCs) in the tumor masses induced the
proliferation of CCR6+ Tregs through TGF-β. This finding
was in line with our results reporting a high percentage of CCR6
expression on CD4+CD25+Foxp3+
Tregs in LSCC patients. As CCL20 mRNA was highly expressed in the
LSCC tissue, the CCR6+ Tregs may have been attracted and
cumulated in the center of LSCC, which may have formed a more
suppressive microenvironment. However, this requires further
investigation to confirm whether the CCL20 had an effect on the
proliferation of CCR6+ Tregs.
Several mechanisms of
CD4+CD25+Foxp3+ Treg-mediated
suppression have been proposed. It is believed that there are 2
main types of mechanisms for contact-dependent suppression
including i) downregulation of APC co-stimulatory function,
interaction with CD80 and CD86 on conventional T cells and
conventional T cell lysis, and ii) cytokine-mediated suppression
including attenuation of DC function, conversion of conventional T
cells to Tr1 cells, cell cycle arrest and apoptosis in conventional
T cells (13). In this study, IL-2,
IL-12 and IFN-γ mRNA levels were decreased in cancer tissue, while
IL-10 and TGF-β1 mRNA levels were increased as compared to those of
the ANT. The ELISA assays showed that IFN-γ protein level was
reduced to 25.32 pg/ml in the plasma of LSCC patients, while the
IL-10 and TGF-β1 protein levels were increased to 27.38 and
1,527.00 pg/ml, respectively, and IL-2, IL-4 and IL-12p70 were not
detectable. The results of this study showed that the Th1/Th2
cytokine responses were skewed toward a Th2 bias in the plasma of
patients with LSCC as compared to the healthy controls.
Furthermore, suppressive cytokines (IL-10 and TGF-β1) played a key
role in forming an immunosuppressive status for LSCC patients.
These results were consistent with Strauss et al(51).
Transcription factor Foxp3 was thought to be a
special marker of Tregs (52);
however, recent studies demonstrated that Foxp3 was also expressed
in cancer cells (53,54). Our study showed a high expression
level of Foxp3 in LSCC tissue at the genomic level, but a decreased
expression in LN(+) and advanced LSCC patients. Although protein
expression level and cellular localization of Foxp3 in LSCC remain
to be identified, Ladoire et al(55) demonstrated that the Foxp3
upregulation was closely related to a better prognosis in breast
cancer, which indicated a high predictive value for Foxp3. We
speculated that the Foxp3 gene may be involved in certain
tumor-suppressing mechanisms in association with tumor
metastasis.
Although the chemokine system-based research of
tumor immunology has made some progress, the results from different
tumors are not fully compatible (21,56–58).
This illustrated not only the complexity of the chemokine system,
but also the unique characteristics of different types of cancer.
In this study, several methods were used to detect the expression
of CCR6, CCR7 and their ligands, the expression patterns of CCR6
and CCR7 on CD4+CD25+Foxp3+ Tregs,
as well as the cytokine profiles of LSCC patients. The present
study revealed that CCR6 and CCR7 may directly mediate the
migration of cancer cells and induce immune tolerance by recruiting
CD4+CD25+Foxp3+ Tregs to cancer
sites in order to form a particular cancer microenvironment in
favor of LSCC initiation, invasion and metastasis. These results
could function as a foundation to further explore a chemokine
system-based cancer intervention strategy.
Since Müller et al(26) reported the involvement of chemokine
receptor in tumor growth and progression in 2001 and the
identification of regulatory T cells by Sakaguchi et al in
1995 (59), significant progress
has been made in the field of cancer pathogenesis, prevention and
treatment (60). The CCR6, CCR7,
their paired ligands and the ligand-receptor interaction bridged
the gap between LSCC cells and
CD4+CD25+Foxp3+ Tregs. Therefore,
they may directly or indirectly be involved in tumor progression
and should be evaluated as novel candidate target molecules for
specific treatment interventions as well as prognosis assessments
in LSCC treatment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30801283, 30972691), the Shanghai
Science and Technology Development Funds (09QA1401000,
10QA1405900), the Training Program of the Excellent Young Talents
of the Shanghai Municipal Health System (XYQ2011055, XYQ2011015)
and the Shanghai Municipal Science and Technology Foundation
(11JC1410802).
References
|
1
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffman HT, Porter K, Karnell LH, et al:
Laryngeal cancer in the United States: changes in demographics,
patterns of care, and survival. Laryngoscope. 116:1–13. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah JP, Karnell LH, Hoffman HT, et al:
Patterns of care for cancer of the larynx in the United States.
Arch Otolaryngol Head Neck Surg. 123:475–483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alhamarneh O, Agada F, Madden L, Stafford
N and Greenman J: Serum IL10 and circulating CD4(+) CD25(high)
regulatory T cell numbers as predictors of clinical outcome and
survival in patients with head and neck squamous cell carcinoma.
Head Neck. 33:415–423. 2011.
|
|
5
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roncarolo MG, Bacchetta R, Bordignon C,
Narula S and Levings MK: Type 1 T regulatory cells. Immunol Rev.
182:68–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dieckmann D, Plottner H, Berchtold S,
Berger T and Schuler G: Ex vivo isolation and characterization of
CD4(+)CD25(+) T cells with regulatory properties from human blood.
J Exp Med. 193:1303–1310. 2001.
|
|
9
|
Jonuleit H, Schmitt E, Stassen M,
Tuettenberg A, Knop J and Enk AH: Identification and functional
characterization of human CD4(+)CD25(+) T cells with regulatory
properties isolated from peripheral blood. J Exp Med.
193:1285–1294. 2001.
|
|
10
|
Levings MK, Sangregorio R and Roncarolo
MG: Human cd25(+)cd4(+) T regulatory cells suppress naive and
memory T cell proliferation and can be expanded in vitro without
loss of function. J Exp Med. 193:1295–1302. 2001.
|
|
11
|
Chen Y, Kuchroo VK, Inobe J, Hafler DA and
Weiner HL: Regulatory T cell clones induced by oral tolerance:
suppression of autoimmune encephalomyelitis. Science.
265:1237–1240. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: Foxp3+ regulatory T cells in the human immune
system. Nat Rev Immunol. 10:490–500. 2010.
|
|
14
|
Xu L, Xu W, Wen Z and Xiong S: In situ
prior proliferation of CD4+ CCR6+ regulatory
T cells facilitated by TGF-β secreting DCs is crucial for their
enrichment and suppression in tumor immunity. PLoS One.
6:e202822011.PubMed/NCBI
|
|
15
|
Wei S, Kryczek I and Zou W: Regulatory
T-cell compartmentalization and trafficking. Blood. 108:426–431.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J and Paul WE: CD4 T cells: fates,
functions, and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mburu YK, Wang J, Wood MA, Walker WH and
Ferris RL: CCR7 mediates inflammation-associated tumor progression.
Immunol Res. 36:61–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schutyser E, Struyf S and Van Damme J: The
CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor
Rev. 14:409–426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirahara K, Liu L, Clark RA, Yamanaka K,
Fuhlbrigge RC and Kupper TS: The majority of human peripheral blood
CD4+CD25highFoxp3+ regulatory T
cells bear functional skin-homing receptors. J Immunol.
177:4488–4494. 2006.PubMed/NCBI
|
|
21
|
Wang J, Xi L, Hunt JL, et al: Expression
pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell
carcinoma of the head and neck identifies a novel metastatic
phenotype. Cancer Res. 64:1861–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arya M, Patel HR and Williamson M:
Chemokines: key players in cancer. Curr Med Res Opin. 19:557–564.
2003. View Article : Google Scholar
|
|
23
|
Vinader V and Afarinkia K: A beginner’s
guide to chemokines. Future Med Chem. 4:845–852. 2012.
|
|
24
|
Tetu B, Popa I, Bairati I, et al:
Immunohistochemical analysis of possible chemoresistance markers
identified by micro-arrays on serous ovarian carcinomas. Mod
Pathol. 21:1002–1010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Müller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001.PubMed/NCBI
|
|
27
|
Forster R, Davalos-Misslitz AC and Rot A:
CCR7 and its ligands: balancing immunity and tolerance. Nat Rev
Immunol. 8:362–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mantovani A, Savino B, Locati M, Zammataro
L, Allavena P and Bonecchi R: The chemokine system in cancer
biology and therapy. Cytokine Growth Factor Rev. 21:27–39. 2010.
View Article : Google Scholar
|
|
29
|
Ghadjar P, Coupland SE, Na IK, et al:
Chemokine receptor CCR6 expression level and liver metastases in
colorectal cancer. J Clin Oncol. 24:1910–1916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wittekind C, Compton CC, Greene FL and
Sobin LH: TNM residual tumor classification revisited. Cancer.
94:2511–2516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pavelic ZP, Pavelic K, Carter CP and
Pavelic L: Heterogeneity of c-myc expression in histologically
similar infiltrating ductal carcinomas of the breast. J Cancer Res
Clin Oncol. 118:16–22. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tao L, Zhou L, Zheng L and Yao M: Elemene
displays anti-cancer ability on laryngeal cancer cells in vitro and
in vivo. Cancer Chemother Pharmacol. 58:24–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lefebvre JL and Ang KK: Larynx
preservation clinical trial design: key issues and recommendations
- a consensus panel summary. Head Neck. 31:429–441. 2009.
View Article : Google Scholar
|
|
34
|
Chen AY, Schrag N, Hao Y, et al: Changes
in treatment of advanced laryngeal cancer 1985–2001. Otolaryngol
Head Neck Surg. 135:831–837. 2006.
|
|
35
|
Holsinger FC and Weber RS: Swing of the
surgical pendulum: a return to surgery for treatment of head and
neck cancer in the 21st century? Int J Radiat Oncol Biol Phys.
69(Suppl 2): S129–S131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laccourreye O, Brasnu D, Bassot V, Menard
M, Khayat D and Laccourreye H: Cisplatin-fluorouracil exclusive
chemotherapy for T1-T3N0 glottic squamous cell carcinoma complete
clinical responders: five-year results. J Clin Oncol. 14:2331–2336.
1996.
|
|
37
|
Lefebvre JL, Rolland F, Tesselaar M, et
al: Phase 3 randomized trial on larynx preservation comparing
sequential vs alternating chemotherapy and radiotherapy. J Natl
Cancer Inst. 101:142–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stenson KM, Maccracken E, Kunnavakkam R,
et al: Chemoradiation for patients with large-volume laryngeal
cancers. Head Neck. 34:1162–1167. 2012.PubMed/NCBI
|
|
39
|
Beider K, Abraham M, Begin M, et al:
Interaction between CXCR4 and CCL20 pathways regulates tumor
growth. PLoS One. 4:e51252009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bonnotte B, Crittenden M, Larmonier N,
Gough M and Vile RG: MIP-3alpha transfection into a rodent tumor
cell line increases intratumoral dendritic cell infiltration but
enhances (facilitates) tumor growth and decreases immunogenicity. J
Immunol. 173:4929–4935. 2004. View Article : Google Scholar
|
|
41
|
Cabioglu N, Yazici MS, Arun B, et al: CCR7
and CXCR4 as novel biomarkers predicting axillary lymph node
metastasis in T1 breast cancer. Clin Cancer Res. 11:5686–5693.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shang ZJ, Liu K and Shao Z: Expression of
chemokine receptor CCR7 is associated with cervical lymph node
metastasis of oral squamous cell carcinoma. Oral Oncol. 45:480–485.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takanami I: Overexpression of CCR7 mRNA in
nonsmall cell lung cancer: correlation with lymph node metastasis.
Int J Cancer. 105:186–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen KJ, Lin SZ, Zhou L, et al: Selective
recruitment of regulatory T cell through CCR6-CCL20 in
hepatocellular carcinoma fosters tumor progression and predicts
poor prognosis. PLoS One. 6:e246712011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Annunziato F, Cosmi L, Liotta F, et al:
Phenotype, localization, and mechanism of suppression of
CD4(+)CD25(+) human thymocytes. J Exp Med. 196:379–387. 2002.
|
|
46
|
Iellem A, Mariani M, Lang R, et al: Unique
chemotactic response profile and specific expression of chemokine
receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp
Med. 194:847–853. 2001.PubMed/NCBI
|
|
47
|
Xu L, Xu W, Qiu S and Xiong S: Enrichment
of CCR6+Foxp3+ regulatory T cells in the
tumor mass correlates with impaired CD8+ T cell function
and poor prognosis of breast cancer. Clin Immunol. 135:466–475.
2010.PubMed/NCBI
|
|
48
|
Kosmaczewska A, Ciszak L, Potoczek S and
Frydecka I: The significance of Treg cells in defective tumor
immunity. Arch Immunol Ther Exp (Warsz). 56:181–191. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mao C, Wang S, Jiang Q, et al: Increased
CD4+CD25+Foxp3+ regulatory T cells
in cancer patients from conversion of
CD4+CD25− T cells through tumor-derived
factors. Onkologie. 31:243–248. 2008.
|
|
50
|
Pakravan N, Hassan AT and Hassan ZM:
Naturally occurring self-reactive CD4+CD25+
regulatory T cells: universal immune code. Cell Mol Immunol.
4:197–201. 2007.
|
|
51
|
Strauss L, Bergmann C, Szczepanski M,
Gooding W, Johnson JT and Whiteside TL: A unique subset of
CD4+CD25highFoxp3+ T cells
secreting interleukin-10 and transforming growth factor-beta1
mediates suppression in the tumor microenvironment. Clin Cancer
Res. 13:4345–4354. 2007.PubMed/NCBI
|
|
52
|
Sakaguchi S: Naturally arising
Foxp3-expressing CD25+CD4+ regulatory T cells
in immunological tolerance to self and non-self. Nat Immunol.
6:345–352. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ebert LM, Tan BS, Browning J, et al: The
regulatory T cell-associated transcription factor FoxP3 is
expressed by tumor cells. Cancer Res. 68:3001–3009. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Karanikas V, Speletas M, Zamanakou M, et
al: Foxp3 expression in human cancer cells. J Transl Med. 6:192008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ladoire S, Arnould L, Mignot G, et al:
Presence of Foxp3 expression in tumor cells predicts better
survival in HER2-overexpressing breast cancer patients treated with
neoadjuvant chemotherapy. Breast Cancer Res Treat. 125:65–72. 2011.
View Article : Google Scholar
|
|
56
|
O’Hayre M, Salanga CL, Handel TM and Allen
SJ: Chemokines and cancer: migration, intracellular signalling and
intercellular communication in the microenvironment. Biochem J.
409:635–649. 2008.PubMed/NCBI
|
|
57
|
Ito S, Ozawa S, Ikoma T, Yajima N, Kiyono
T and Hata R: Expression of a chemokine BRAK/CXCL14 in oral floor
carcinoma cells reduces the settlement rate of the cells and
suppresses their proliferation in vivo. Biomed Res. 31:199–206.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Silva TA, Ribeiro FL, Oliveira-Neto HH, et
al: Dual role of CCL3/CCR1 in oral squamous cell carcinoma:
Implications in tumor metastasis and local host defense. Oncol Rep.
18:1107–1113. 2007.PubMed/NCBI
|
|
59
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor α-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.
|
|
60
|
DiPersio JF, Micallef IN, Stiff PJ, et al:
Phase III prospective randomized double-blind placebo-controlled
trial of plerixafor plus granulocyte colony-stimulating factor
compared with placebo plus granulocyte colony-stimulating factor
for autologous stem-cell mobilization and transplantation for
patients with non-Hodgkin’s lymphoma. J Clin Oncol. 27:4767–4773.
2009.PubMed/NCBI
|