Introduction
Osteosarcoma, the most common type of primary bone
cancer, occurs mostly in young age with more than 60% of cases
noted in patients between 10 and 20 years of age and is most
frequently present in lower long bones. The five-year survival of
these patients is over 60% and varies with regard to age, gender,
race and location of tumours (1–3).
Treatments for osteosarcoma include surgery, chemotherapy (before
and after surgery) and radiotherapy, although the latter is less
effective when compared with surgery and chemotherapy. Other
alternative therapies have been explored but are presently limited
(4–6).
In a previous study, we reported that the
traditional Chinese medical formula, Yangzheng Xiaoji, has a
marked effect on angiogenesis The extract from Yangzheng
Xiaoji, DME25, inhibited tubule formation in human endothelial
cells (7). We demonstrated that
this effect was through inhibition of focal adhesion kinase (FAK).
Furthermore, we demonstrated that the same extract had a direct
effect on a diverse range of human cancer cell lines including
breast, prostate, gastric and colorectal cancer and other cancer
cell lines (8), of which human
osteosarcoma was found to be one of the most sensitive cell
types.
Yangzheng Xiaoji has been shown, in clinical
trials, to have anticancer activity in patients with certain solid
tumours. For example, in patients with primary liver cancer, those
who received conventional chemotherapy combined with Yangzheng
Xiaoji (n=304) showed a significantly increased rate of disease
remission (complete and partial remissions) compared with patients
who received chemotherapy alone. Patients who received
combinational therapy also had improved quality of life, based on
the Karnofsky method (9,10), and had improved immune
functions.
There have been no reports on the antitumour effect
of this formula in in vivo models, and no studies have been
conducted in human osteosarcoma. In light of our recent study
showing that osteosarcoma is sensitive to DME25, we carried out the
present study in which we tested the biological impact of
Yangzheng Xiaoji on osteosarcoma in vitro and tested
the effect on tumour growth using an in vivo tumour
model.
Materials and methods
Human osteosarcoma cell line, MG63 (ATCC-CRL1427™)
was purchased from ATCC (LGC, UK). The cells were maintained in
Dubecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, Poole,
Dorset, UK) supplemented with penicillin, streptomycin and 10%
fetal calf serum (Sigma-Aldrich). The cells were incubated at 37°C
in 5% CO2 and 95% humidity. Matrigel (reconstituted
basement membrane) was purchased from Collaborative Research
Products (Bedford, MA, USA). A selective small inhibitor to FAK
(FP573228) was from Tocris (Bristol, UK). The antibody to paxillin
was from Transduction Laboratories, anti-integrin-β1 was obtained
from R&D Europe, and anti-FAK and phospho-specific antibodies
(anti-pFAK and anti-pPaxillin) were from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA).
Preparation of extract DME25 from
Yangzheng Xiaoji for experimental use
The extract of Yangzheng Xiaoji (Yiling
Pharmaceutical, Shijiazhuang, Hebei, China), DME25, was prepared as
previously reported (11). The
extract was standardised by quantifying the optical density of the
preparation using a spectrophotometer at a wavelength of 405 nm. A
master preparation of the extract which exhibited an OD of 0.25 was
stocked as the master stock and so named as DME25 for the
experiments. Preparations from different batches of the formula
were found to be consistent by way of chemical finger printing.
In vitro cell growth assay
MG63 cells were seeded into 96-well plates at a
density of 3,000 cells/well. Triplicate plates were set up for
overnight, 3- and 5-day incubation periods. Following sufficient
incubation, the plates were removed from the incubator, fixed in 4%
formaldehyde (v/v) and stained with 0.5% (w/v) crystal violet. The
crystal violet stain was extracted using 10% acetic acid (v/v). The
absorbance was determined using a spectrophotomer (Bio Tek ELx800;
Bio Tek Instruments, Inc., Winooski, VT, USA).
Cell-matrix adhesion assay
The cell-motility adhesion assay was based on an
established method (12). Briefly,
solution containing 5 μg Matrigel was added to each well of a
96-well tissue culture plate. This was allowed to air dry, after
which the gel was rehydrated. MG63 cells (4,000) were added to each
well, in the presence of DME25, FAK inhibitor or the DME25/FAK
inhibitor combination or medium as control. After incubating at
37°C for 40 min, the non-adherent cells were gently washed off
using a multi-pipette. The adherent cells were fixed using a 4%
formalin solution for 30 min and then stained with 0.5% crystal
violet. The number of adherent cells were then counted under a
microscope and expressed as the cell number per high power
field.
Electric cell-substrate impedance sensing
(ECIS)-based cellular adhesion assays
An ECIS-Zθ instrument with a 96-well station
(Applied Biophysics Inc., Troy, NJ, USA) was used for the cell
adhesion assays (13,14). Cell modelling was carried out using
the ECIS RbA modelling software, supplied by the manufacturer. The
96W1E ECIS arrays were used. ECIS measures the interaction between
cells and the substrate to which they are attached via gold-film
electrodes placed on the surface of culture dishes. Following
treatment of the array surface with a cysteine solution, the arrays
were incubated with complete medium for 1 h. The adhesion was
tracked immediately after adding the cells into the arrays.
Impedance and resistance of the cell layer were immediately
recorded for a period of up to 4 h. For signalling transduction
inhibitor assays, the respective inhibitors were included in the
assay wells. Adhesion was also modelled using the ECIS RbA cell
modelling software as recently reported (14,15).
In vivo development of osteosarcoma
Athymic female nude mice (nude CD-1), 4–6 weeks of
age, were purchased from Charles River, UK, and maintained in
filter-topped units. One hundred microliters of cell suspension
(0.5 million MG63 cells in 0.5 mg/ml Matrigel) was subcutaneously
injected at the scapula area. Each tumour group was divided into
groups receiving, on alternate days: i) control buffer, ii) i.p.
injection of DME25, iii) oral delivery (by way of gavage) of DME25,
iv) FAK inhibitor or v) the combination of DME25 and the FAK
inhibitor. Mice were weighed, and tumour sizes were measured twice
weekly for 5 weeks. Mice with weight loss >25% and tumour size
>1 cm in any dimension were sacrificed according to the UK Home
Office and UKCCCR guideline. The volume of the tumour was
determined using the formula: Tumour volume = 0.523 ×
width2 × length (16).
At the conclusion of the experiment, animals were terminally
sacrificed, primary tumours were dissected, weighed and frozen at
−80°C. Part of the primary tumour was used for frozen sections for
histological and immunohistological examinations.
Immunofluorescent staining of FAK and
paxillin
Frozen sections of osteosarcoma tissues were cut at
a thickness of 6 μm using a cryostat (17). The sections were mounted on Super
Frost Plus microscope slides, air-dried and then fixed in a mixture
of 50% acetone and 50% methanol. The sections were then placed in
Optimax wash buffer for 5–10 min to rehydrate. Sections were
incubated for 20 min in 10% horse serum of blocking solution and
probed with the primary antibody. Following extensive washings,
sections were probed with specific antibodies to FAK and
phospho-FAK (SC-1688 and SC-11766 respectively, from Santa Cruz
Biotechnology, Inc.), paxillin and phospho-paxillin and
integrin-β1. Primary antibodies were made up in Tris buffer with 3%
milk at a 1:100 concentration for 1 h. The primary antibody was
then completely removed by washing the cells 5 times in the same
buffer. FITC-conjugated secondary antibodies (Sigma-Aldrich) were
subsequently added to the cells, and the slides were incubated on a
shaker platform in the dark for 1 h. The slides were finally washed
3 times to remove the unbound secondary antibody, mounted with
FluorSave™ (Calbiochem-Novabiochem Ltd., Nottingham, UK) and
visualized under an Olympus BX51 fluorescence microscope at ×100
objective magnification.
Similarly, immunofluorescent staining was also
carried out on MG63 cells. Cells were placed in a chamber slide to
adhere for 24 h before being treated with DME25 or FAK inhibitors.
After the treatment, cells were first fixed with 4% formalin for 30
min, before gently being permeabilized with Triton X-100 (0.1%) for
5 min. The rest of the procedure was similar to that used on
tissues.
Statistical analysis was conducted using Sigma Plot
(version 11).
Results
YZXJ has a direct effect on the adhesion
of osteosarcoma cells
DME25 had a significant inhibitory effect on the
adhesion of MG63 cells to a Matrigel-coated surface (Fig. 1A–a and B). The FAK inhibitor
similarly inhibited the adhesion, although a weaker effect was
noted when compared with that of DME25 at the concentration tested
(100 nM) (Fig. 1A–c and B). The
combination of DME25 and the FAK inhibitor markedly potentiated the
inhibitory effect on cell adhesiveness (Fig. 1A–d and B).
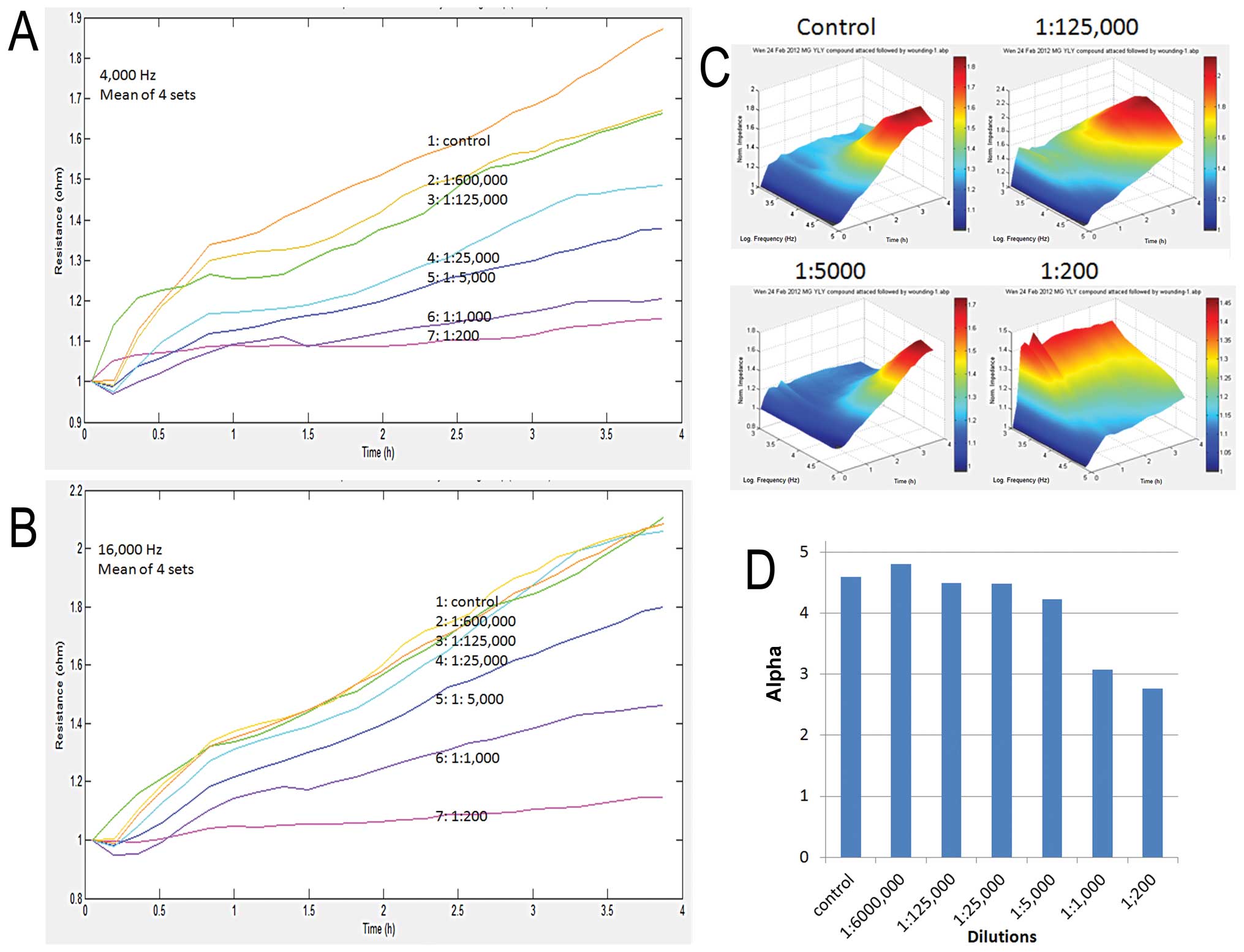
Using an automated ECIS method, we first
demonstrated that DME25 had a marked effect on the adhesion of MG63
cells (Fig. 2). As shown in
Fig. 2A, DME25 at a concentration
as low as 1:125,000 dilution already demonstrated an inhibitory
effect on cell adhesion, although a more profound inhibitory effect
was noted after treatment at a concentration higher than 1:25,000.
A similar trend was noted at both low and high frequencies in the
experimental settings (Fig. 2A and
B). This inhibitory effect was noted across all of the
frequencies tested as demonstrated in the 3D graph (Fig. 2C).
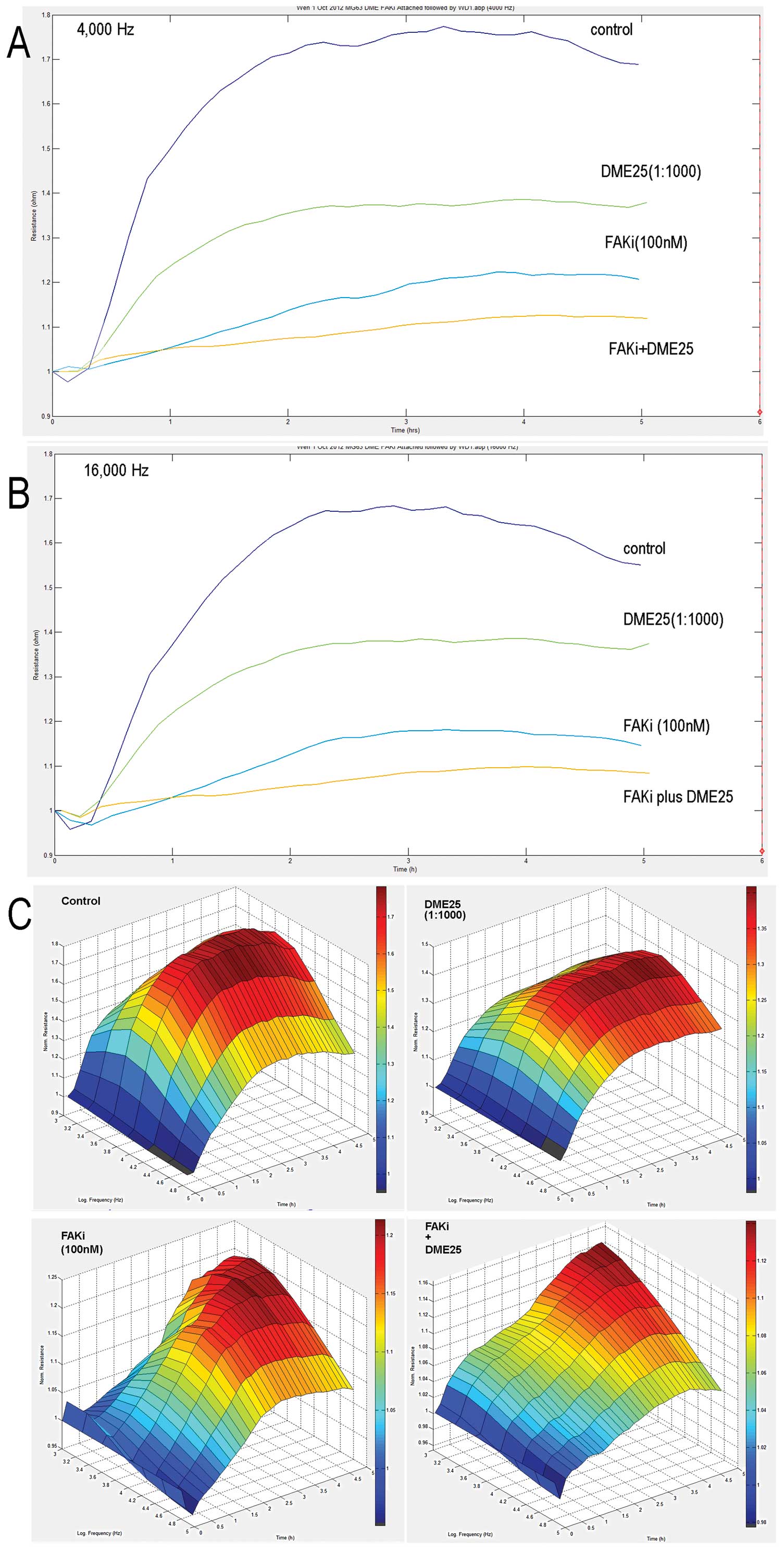
Inhibitory effects of the FAK inhibitor
and anti-FnR on cell-matrix adhesion potentiated by YZXJ
As already shown in Fig.
1, the FAK inhibitor had an effect on cell-matrix adhesion.
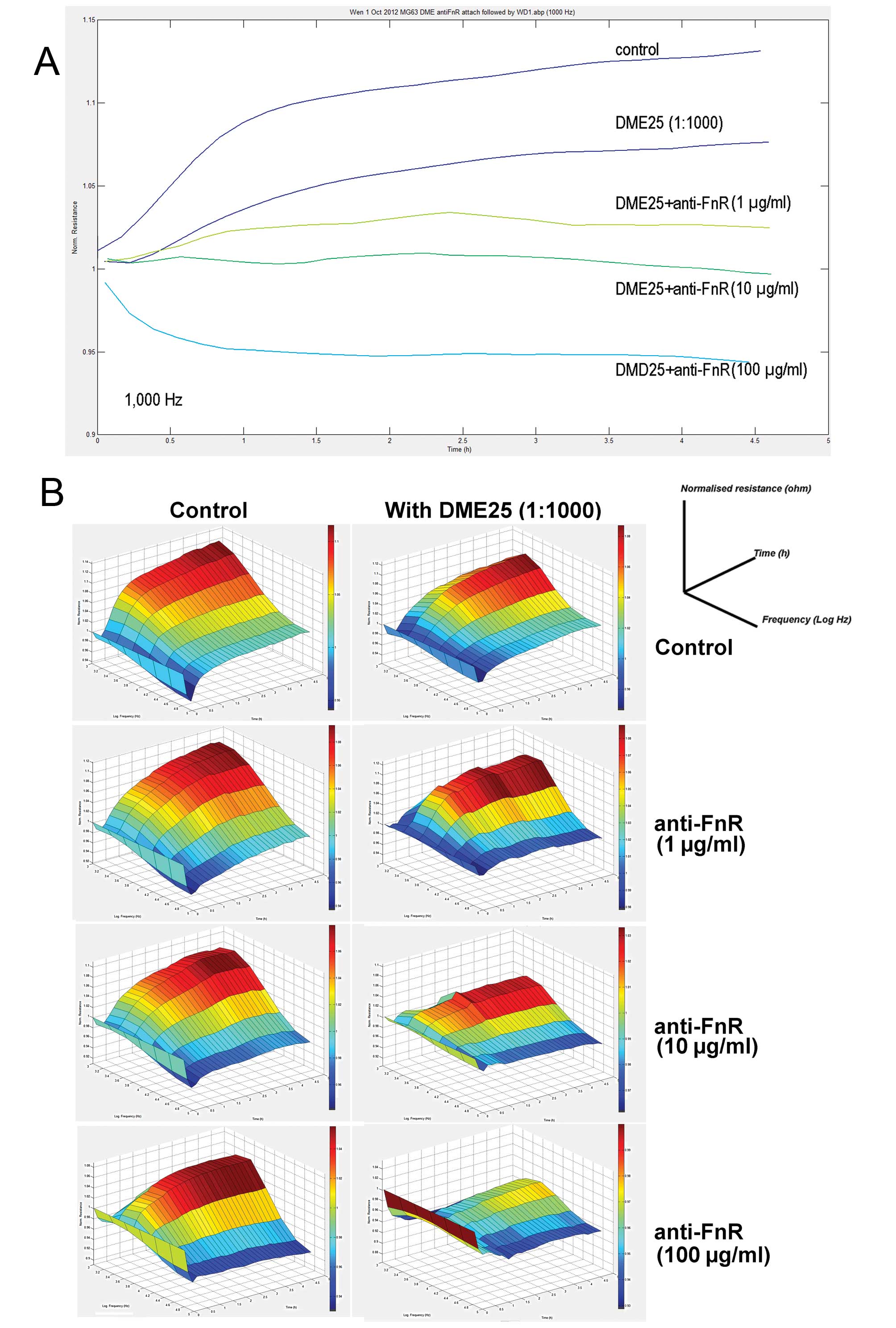
This was further demonstrated using the ECIS model (Fig. 3). We attempted to block the
fibronectin receptor (FnR) by using a neutralising antibody. As
shown in Fig. 4, the antibody
reduced the cell adhesion. Again, the inhibition of cell adhesion
by DME25 was further enhanced by anti-FnR.
YZXJ exhibits no significant effect on
the growth of osteosarcoma cells
We recently reported that DME25 has an extremely
small effect on the growth of a range of human cancer cell lines
(8). In the present study,
YZXJ at high concentrations did not show any toxic effect on
cancer cells (data not shown).
Inhibition of the activation of FAK and
paxillin by YZXJ in osteosarcoma cells
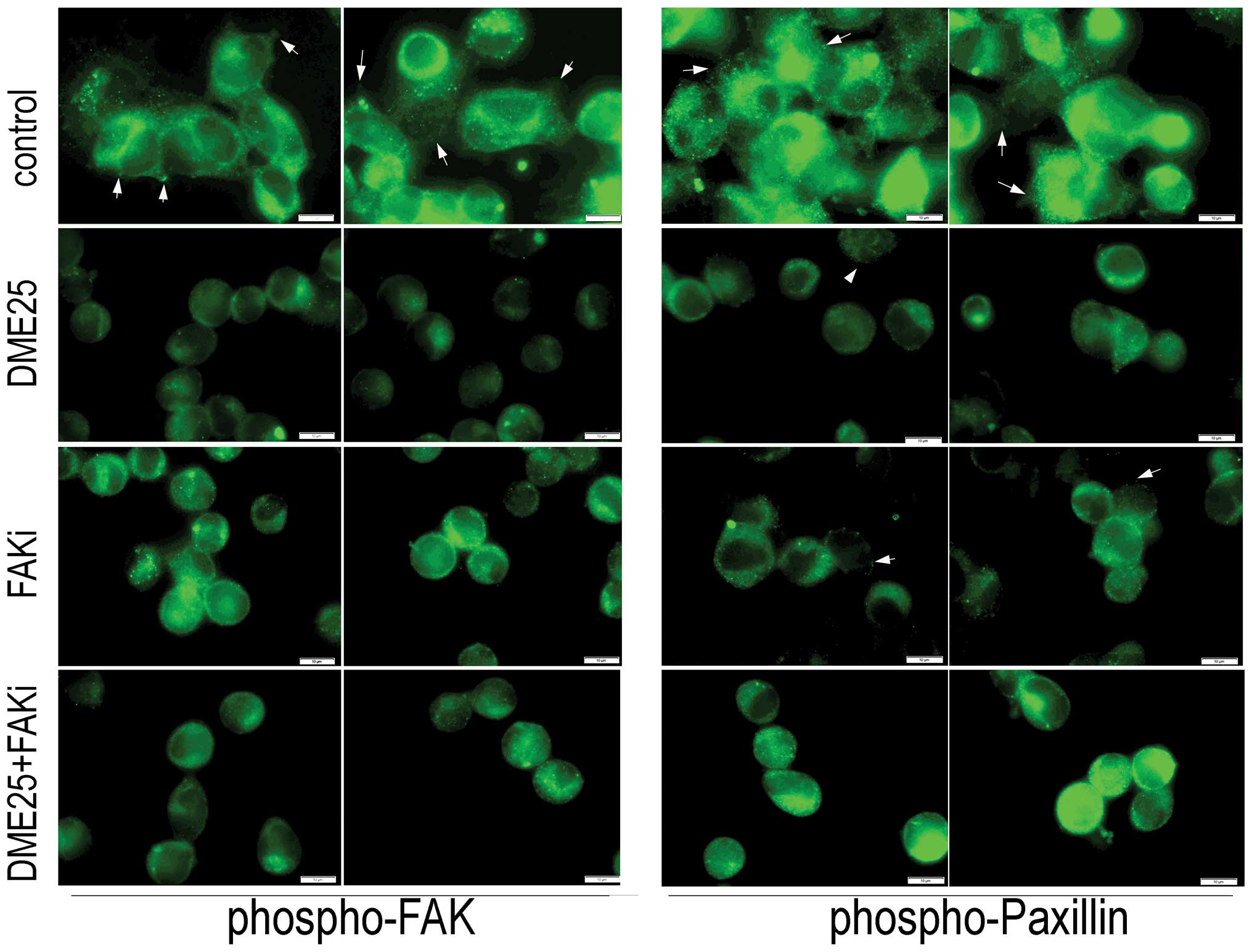
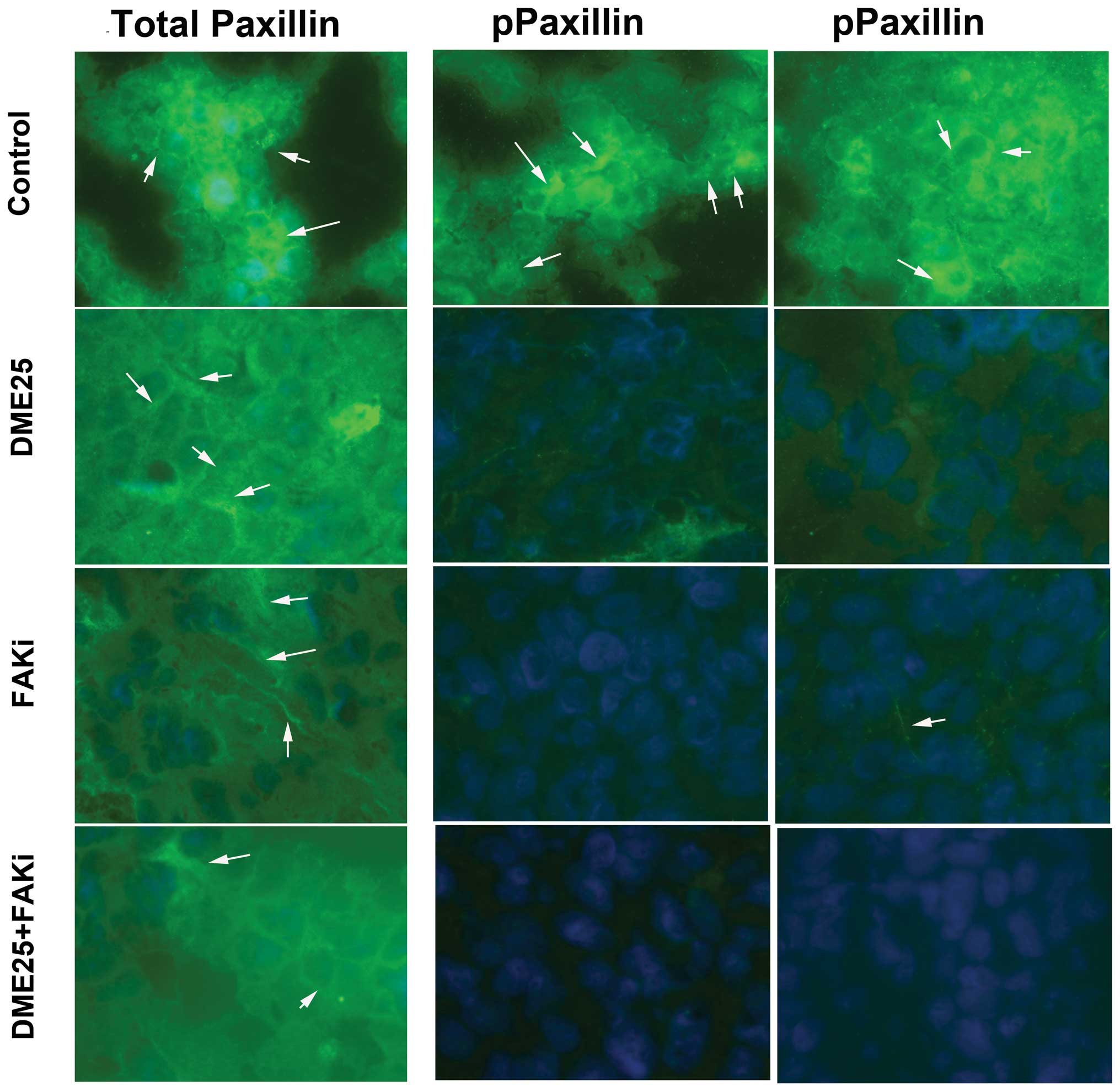
To investigate the effect of YZXJ on the
activation of FAK and paxillin, we used an immunofluorescence
method to visualize phosphorylated FAK and paxillin in MG63 cells.
As shown in Fig. 5, control cells,
which spread well over the matrix-coated surface, exhibited a high
level of staining of phosphorylated FAK (Fig. 5, left panel, indicated by arrows)
and phosphorylated paxillin (Fig.
5, right panel, indicated by arrows). The staining of both
phosphorylated FAK and paxillin in the DME25-treated cells as well
as in the FAK inhibitor-treated cells were markedly reduced,
accompanied by morphological changes similar to that shown in
Fig. 1. Likewise, the staining of
both activated proteins was reduced in cells treated with the
combination of DME25 and the FAK inhibitor.
YZXJ inhibits the growth of osteosarcoma
in vivo
Using athymic nude mice, we first established
subcutaneous osteosarcomas. The tumours became measurable after 1
week, when treatment began. As shown in Fig. 6A, delivery of DME25 via both the
oral route and via intraperitoneal (i.p.) injection reduced the
rate of growth, an effect which achieved statistical significance
after 3 weeks. The i.p. injection resulted in sustained inhibition
after 1 week whereas the oral route took longer than the i.p. route
to reach a significant inhibitory effect. We also tested the effect
of the FAK inhibitor and the combination of the FAK inhibitor and
DME25. As shown in Fig. 6B, the FAK
inhibitor at the concentration used (10 nM) showed little effect on
tumour growth. However, the combination of DME25 and the FAK
inhibitor exhibited a significant effect after 3 weeks. No obvious
side-effects were observe throughout the study.
YZXJ and the activation of FAK and
paxillin in the in vivo tumour models
We examined the staining pattern of FAK and paxillin
by comparing the total proteins and phosphorylated forms using
antibodies that recognized total FAK/paxillin or the
phospho-specific proteins. Osteosarcoma tumour cells stained
strongly for total FAK and also phospho-FAK (Fig. 7, indicated by arrows). Tumours from
mice which received DME25, the FAK inhibitor and the DME25/FAK
inhibitor combination showed a marked reduction in phospho-FAK
staining, but to a lesser degree for the staining of total FAK.
Similar to total FAK, a reduction in integrin staining was noted in
tumours from mice that received treatment.
Fig. 8 shows a
similar reduction in phospho-paxillin in the treatment group,
except that the reduction was more profound that that noted with
phospho-FAK (Fig. 7, left
panel).
Discussion
In the present study, we demonstrated that DME25,
previously shown to have an inhibitory effect on the adhesion and
migration of human tumour cells, inhibited the cell-matrix adhesion
of human osteosarcoma cells, possibly via an inhibitory effect on
the activation of FAK and paxillin. This effect was noted together
with a reduction in in vivo tumour growth and a reduction in
the phosphorylation of FAK and paxillin in the osteosarcoma
tumours.
The most significant finding of the present study
was the antitumour effect of DME25, in vivo. Delivery of
DME25 via both an oral route and an intraperitoneal route resulted
in reduction in tumour growth. It is clear that the intraperitoneal
route was more effective in the early days compared with the oral
route. A method to monitor the absorption of DME25 in the body is
yet to be developed. However, the different effects of oral and
i.p. delivery may reflect the absorption and availability of DME25
to the tumours, as it is anticipated that an i.p. route may be more
effective in regards to absorption. It is noteworthy that no
side-effects were observed following the treatment regimes,
suggesting that treatment under the present condition is safe.
Osteosarcoma cells markedly reduced their ability to
adhere to matrix in the presence of DME25 at a broad range of
concentrations. This was accompanied by a reduction in phospho-FAK
and phospho-paxillin in the treated cells. This observation was
similar to that noted in human endothelial cells with which DME25
reduced the phosphorylation of FAK (7). Together with a reduction in
phospho-FAK and phospho-paxillin in osteosarcoma tumours, it is
clear that the FAK pathway is a common target for DME25, both in
endothelial and in tumour cells.
FAK plays an important role in cell-matrix adhesion,
cellular migration and mediating intracellular and extracellular
signals (18–20). Upon interaction between integrin and
the extracellular matrix, the FAK pathway is activated which
consequently activates downstream events including PI3K-Akt,
Grb2-Erk, Crk-CAS. The finding of the present study that DME25
inhibits the activation of FAK and paxillin is in agreement with
our previous study that DME25 inhibits the Akt pathway in other
types of cancer cells including breast cancer and colorectal cancer
cells (8); namely DME25 inhibits
the early event in the FAK signalling chain of events.
FAK inhibitors are currently being tested in several
clinical trials for treating patients with solid tumours (21–23).
Here, we found that the FAK inhibitor only had a marginal effect on
cell adhesion and tumour growth. This was primarily due to the
choice of concentrations, namely below 100 nM, well below the
recommended clinical dose. The concentrations were chosen in order
to test whether DME25 may potentiate the effect of the inhibitor,
which was indeed demonstrated both in the in vitro and in
vivo models here. The pivotal role of the FAK pathway in the
action of DME25 was further supported by the finding that blockage
of the fibronectin receptor with neutralizing antibody also
enhanced the effect of DME25. Together with the anti-angiogenic
effect of DME25 (7), it can be
argued that DME25 exerts antitumour effects by acting directly on
tumour and endothelial cells, in which the FAK pathway is
inhibited.
Osteosarcoma is an aggressive tumour mostly seen in
young patients. Treatment includes surgery and chemotherapy.
Surgery includes complete removal of the tumour when possible
(24). Chemotherapy mostly involves
a combination of multiple drugs, including adriamycin, ifosfamide,
methotrexate and cisplatin. In a recent meta-analysis, it was
indicated that a three-drug combination conferred a better survival
benefit than a combination of less than three drugs (25,26).
Other types of therapies are also being explored including
anti-angiogenesis therapy, interferons, and small-molecule
inhibitors. The present study indicates that combination of DME25
and the FAK inhibitor may be a useful choice for treating patients
with osteosarcoma. However, the present study was only based on one
human osteosarcoma cell line, which happens to be highly sensitive
to DME25. Thus, one should interpret the nature of tumour
specificity with caution until more studies using other
osteosarcoma cells are tested.
In conclusion, DME25, an extract from Yangzheng
Xiaoji, has a profound inhibitory effect on the adhesion of
osteosarcoma cells and on in vivo tumour growth. This is
likely achieved by action on the focal adhesion kinase pathway in
the cells.
Acknowledgements
The authors wish to thank Cancer Research Wales, the
Albert Hung Foundation for supporting their study.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dorfman HA and Czerniak B: Bone cancers.
Cancer. 75(Suppl 1): S203–S210. 1995. View Article : Google Scholar
|
|
3
|
Hayden JB and Hoang BH: Osteosarcoma:
basic science and clinical implications. Orthop Clin North Am.
37:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wachtel M and Schäfer BW: Targets for
cancer therapy in childhood sarcomas. Cancer Treat Rev. 36:318–327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubo T, Piperdi S, Rosenblum J, Antonescu
CR, Chen W, Kim HS, Huvos AG, Sowers R, Meyers PA, Healey JH and
Gorlick R: Platelet-derived growth factor receptor as a prognostic
marker and a therapeutic target for imatinib mesylate therapy in
osteosarcoma. Cancer. 112:2119–2129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari S and Palmerini E: Adjuvant and
neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr
Opin Oncol. 19:341–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang WG, Ye L, Ji K, Frewer N, Ji J and
Mason MD: Inhibitory effects of Yangzheng Xiaoji on
angiogenesis and the role of the focal adhesion kinase pathway. Int
J Oncol. 41:1635–1642. 2012.
|
|
8
|
Ye L, Ji K, Frewer N, Ji J and Jiang WG:
Impact of Yangzheng Xiaoji on the adhesion and migration of
human cancer cells: the role of the AKT signalling pathway.
Anticancer Res. 32:2537–2543. 2012.
|
|
9
|
Zhang SY, Gu CH, Gao XD and Wu YL: A
random, double-blinded and multicentre study of chemotherapy
assisted Yangzheng Xiaoji capsule on treating primary hepatic
carcinoma. Chin J Diffic Compl Case. 8:461–464. 2009.
|
|
10
|
Wang QL, Xuo CM, Wu XP, Li YX and Bi XJ:
Treatment of atypical gastric dysplasia using Yangzheng Xiaoji.
Chin J Diffic Compl Case. 7:38–39. 2009.
|
|
11
|
Ye Li, Ji K, Ji JF, Hargest R and Jiang
WG: Application of electric cell-substrate impedance sensing in
evaluation of traditional medicine on the cellular functions of
gastric and colorectal cancer cells. Cancer Metastasis Biol Treat.
17:195–202. 2012.
|
|
12
|
Jiang WG, Hiscox S, Hallett MB, Scott C,
Horrobin DF and Puntis MC: Inhibition of hepatocyte growth
factor-induced motility and in vitro invasion and motility of human
colon cancer cells by gamma-linolenic acid. Br J Cancer.
71:744–752. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giaever I and Keese CR: Micromotion of
mammalian cells measured electrically. Proc Natl Acad Sci USA.
88:7896–7900. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keese CR, Wegener J, Walker SR and Giaever
I: Electrical wound-healing assay for cells in vitro. Proc Natl
Acad Sci USA. 101:1554–1559. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang WG, Martin TA, Lewis-Russell JM,
Douglas-Jones A, Ye L and Mansel RE: Eplin-alpha expression in
human breast cancer, the impact on cellular migration and clinical
outcome. Mol Cancer. 7:712008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davies G, Mason MD, Martin TA, Parr C,
Watkins G, Lane J, Matsumoto K, Nakamura T and Jiang WG: The HGF/SF
antagonist NK4 reverses fibroblast- and HGF-induced prostate tumor
growth and angiogenesis in vivo. Int J Cancer. 106:348–354. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye L, Martin TA, Parr C, Harrison GM,
Mansel RE and Jiang WG: Biphasic effects of 17-β-estradiol on
expression of occludin and transendothelial resistance and
paracellular permeability in human vascular endothelial cells. J
Cell Physiol. 196:362–369. 2003.
|
|
18
|
Gilmore AP and Romer LH: Inhibition of
focal adhesion kinase (FAK) signaling in focal adhesions decreases
cell motility and proliferation. Mol Biol Cell. 7:1209–1224. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen JY, Tang YA, Huang SM, Juan HF, Wu
LW, Sun YC, Wang SC, Wu KW, Balraj G, Chang TT, Li WS, Cheng HC and
Wang YC: A novel sialyltransferase inhibitor suppresses
FAK/paxillin signaling and cancer angiogenesis and metastasis
pathways. Cancer Res. 71:473–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stokes JB, Adair SJ, Slack-Davis JK,
Walters DM, Tilghman RW, Hershey ED, Lowrey B, Thomas KS, Bouton
AH, Hwang RF, Stelow EB, Parsons JT and Bauer TW: Inhibition of
focal adhesion kinase by PF-562,271 inhibits the growth and
metastasis of pancreatic cancer concomitant with altering the tumor
microenvironment. Mol Cancer Ther. 10:2135–2145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halder J, Lin YG, Merritt WM, Spannuth WA,
Nick AM, Honda T, Kamat AA, Han LY, Kim TJ, Lu C, Tari AM, Bornmann
W, Fernandez A, Lopez-Berestein G and Sood AK: Therapeutic efficacy
of a novel focal adhesion kinase inhibitor TAE226 in ovarian
carcinoma. Cancer Res. 67:10976–10983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Infante JR, Camidge DR, Mileshkin LR, Chen
EX, Hicks RJ, Rischin D, Fingert H, Pierce KJ, Xu H, Roberts WG,
Shreeve SM, Burris HA and Siu LL: Safety, pharmacokinetic, and
pharmacodynamic phase I dose-escalation trial of PF-00562271, an
inhibitor of focal adhesion kinase, in advanced solid tumors. J
Clin Oncol. 30:1527–1533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soria JC, Plummer R, Ranson M, Gan H,
Arkenau HT, Zalcman G, Blagden S, Evans TRJ, Peddareddigari V,
Mazumdar J, Murray S, Gidson D, Fleming RA, Auger K and Millward M:
Loss of the tumor suppressor merlin as a potential predictive
biomarker of clinical activity for the oral, focal adhesion kinase
(FAK) inhibitor GSK2256098 in pts with recurrent
mesothelioma. Eur J Cancer. 48(Suppl 6): S1882012. View Article : Google Scholar
|
|
24
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21(Suppl 7): vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaffe N: Osteosarcoma: review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar : PubMed/NCBI
|