Introduction
Thomsen-Friedenreich (TF) antigen is a
tumor-associated carbohydrate structure which is defined as the
carbohydrate epitope (glycotope) sequence Galβ1-3GalNAcα1-R
(1). TF was assigned as CD176
during the 7th Conference on Human Leucocyte Differentiation
Antigens (2). In adult human normal
and benign tissues, CD176 is masked by terminal sialylation
(3), but it is exposed during
tumorigenesis as a tumor-associated antigen (4). Approximately 70–80% of carcinomas
carry CD176 on their cell surface (5). CD176 is also expressed in leukemia and
lymphoma cells (4,6), and may be a promising prognostic
marker in T-cell acute lymphoblastic leukemia (7,8). In
addition, CD176 is expressed in some cancer stem cells (9), and it is functionally involved in the
liver metastasis process of tumors (1,10), the
adhesion of human breast cancer cells to the endothelium (11,12)
and the apoptosis of leukemic cells (13). Therefore, CD176 may be a promising
target for cancer immunotherapy.
Immunotherapy using a CD176 vaccine has been studied
on patients with advanced breast carcinoma (4,5),
ovarian carcinoma (14) and
prostate carcinoma (15). These
studies demonstrated that the CD176 (TF) vaccine was effective and
safe for the patients. In the patients immunized with CD176
antigen, CD176-specific delayed type hypersensitivity (DTH; 4,5)
and high-titer CD176 IgM and IgG antibodies (15) were observed, indicating that both
responses of cellular and humoral immunity are involved in this
immunotherapeutic approach.
Antitumor antibodies applied in cancer immunotherapy
are effective for a variety of hematological malignancies (16). In previous studies, we observed that
CD176 antibody can induced apoptosis of CD176+ leukemic
cells through activation of the CD95 pathway and caspase-3
(13,17). Liver colonization of CD176
(TF)-positive syngeneic tumor cells was suppressed by pretreatment
with a CD176 (TF) antibody (18).
CD176 (TF) antibody markedly inhibited the adhesion of human breast
cancer cells to the endothelium and caused an extension of survival
time in mice carrying a 4T1 metastatic breast tumor (12). Based on these observations, we
hypothesized that CD176 antibody might have a therapeutic effect on
patients with CD176+ cancer. In the present study, we
investigated: i) whether the therapeutic effect could be observed
by immunization with the CD176 antigen; and ii) whether treatment
with the CD176 antibody treatment alone leads to a therapeutic
response in mice inoculated with CD176+ leukemic cells.
The present study aims to open new strategies for the development
of a CD176 antibody drug.
Materials and methods
Mice and cell lines
Eight to ten-week-old BALB/c mice (Experimental
Animal Center, Kunming Medical College, China) were housed at the
Animal Facility, Kunming Institute of Zoology, Chinese Academy of
Science. Both male and female mice were used. All experiments were
performed in accordance with the regulation for animal
experimentation and approved by the responsible authorities.
Cell lines of WEHI-3 (murine myelomonocytic
leukemia) and KG-1 (human acute myelogenous leukemia) were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). WEHI-3 and KG-1 cells were cultured in DMEM and
RPMI-1640 medium (Gibco-Invitrogen, Grand Island, NY, USA),
supplemented with 10 and 20% fetal bovine serum, respectively, and
penicillin and streptomycin. For the generation of
CD176+ WEHI-3, WEHI-3 cells were pretreated with VCN
(sialidase from Vibrio cholerae; Sigma, St. Louis, MO, USA)
for 1 h to remove NeuAc in 2–3, 2–6 and 2–8 linkages.
Reagents and antibodies
Asialoglycophorin A (aGP) and TF
(CD176)-polyacrylamide (TF-PAA) were purchased from International
Laboratory Ltd., (San Francisco, CA, USA) and Glyco Tech Corp.
(Gaithersburg, MD, USA), respectively.
CD176 antiserum was generated by subcutaneously
injecting mice with 40 μg aGP together with incomplete Freund’s
adjuvant (Sigma). Following two-boosting immunization every two
weeks, 100 μl of blood were collected by tail bleeding. An indirect
solid-phase enzyme linked immunosorbent assay (ELISA) was used as
described below to assess the titer of CD176 antibody in the sera.
The specific reaction of the CD176 antiserum was determined by
inhibition experiments using the inhibitor TF-PAA as described
below. When titers of the CD176-specific antibodies of IgM and IgG
isotype reached 1:1,000, the sera were collected and filtered.
ELISA assay
TF-PAA or aGP were coated on microplates, and then
blocked with 2% bovine serum albumin (BSA). Following incubation
with sera from the immunized mice, the wells were treated with
peroxidase-labeled goat anti-mouse IgM (μ-chain specific; Southern
Biotech, Birmingham, AL, USA) or peroxidase-labeled goat anti-mouse
IgG (H+L). The color reaction was developed with a solution of
o-phenylenediamine dihydrochloride. Negative controls were
performed using the normal mouse serum instead of the CD176
antiserum.
Inhibition experiments based on ELISA,
cell-ELISA and immunofluorescence staining
The WEHI-3 cells were treated with VCN before
harvesting. The cells were lysed with cell lysis buffer and
centrifuged. The supernatants were coated on microplates, and then
blocked with 2% BSA. Following incubation with the CD176 antiserum
or the CD176 antiserum plus TF-PAA, the wells were supplemented
with peroxidase-labeled goat anti-mouse immunoglobulin. The color
development was previously described.
For cell-ELISA, WEHI-3 cells were cultured in the
96-well plate for 2 days. The cells were treated with VCN, and were
then fixed with 4% paraformaldehyde. After blocking with 2% BSA,
the cells were incubated with the CD176 antiserum or the CD176
antiserum plus TF-PAA. Subsequently, the cells were treated with
peroxidase-labeled goat anti-mouse immunoglobulin. The color
reaction was previously described.
For immunofluorescence staining, WEHI-3 cells grown
on cover slips coated with poly-L-lysine were treated with VCN and
then fixed in acetone. After blocking with 1% BSA, the cells were
treated with the CD176 antiserum or the CD176 antiserum plus
TF-PAA. Then, the cells were incubated with FITC-labeled goat
anti-mouse immunoglobulin and counterstained with DAPI (Beyotime
Biotechnology Co., Ltd., Shanghai, China). The cover slips were
mounted with glycerol and the images were viewed and captured.
Induction of apoptosis with CD176
antiserum in vitro
WEHI-3 cells were treated with VCN, and were then
incubated with the CD176 antiserum for 22 and 26 h, respectively.
Apoptotic cells were assessed by using the Annexin V-FITC Apoptosis
Detection kit (KGA107; KeyGen, Nanjing, China) and propidium iodide
(PI) according to the manufacturer’s instructions using a FACScan
flow cytometer (BD Biosciences, Rockville, MD, USA). WEHI-3 cells
were treated with VCN and normal mouse serum instead of the CD176
antiserum as negative controls. KG-1 was used as positive control
(13).
Initiative immunotherapy using the CD176
antigen
In initiative immunotherapy experiments, we
investigated the effect of aGP immunization on the survival time of
CD176+ leukemia mice. Forty mice were randomly divided
into 2 groups (20 mice per group). The first group was immunized
subcutaneously at flanks and base of backs with 40 μg aGP together
with incomplete Freund’s adjuvant (Sigma) for three times. ELISA
was used to estimate titers of the CD176 antibody in the sera. When
the antibody titers reached 1:1,000, the mice were intravenously
(i.v.) injected with CD176+ WEHI-3 (1×107
cells). The second group was only i.v. injected with
CD176+ WEHI-3 (1×107 cells) as a control.
Mice were weighed and observed on a daily basis. The survival time
was recorded and analyzed.
Passive immunotherapy using the CD176
antiserum
In the first experiments of antibody treatment, we
studied effects of the CD176 antibody treatment on the survival
time of CD176+ leukemia mice using passive transfer of
the CD176 antiserum. Sixty mice were randomly divided into 3 groups
with twenty mice in each group. Group 1 was injected i.v. with
1×107 CD176+ WEHI-3 cells; group 2 was
injected i.v. with 1×107 CD176+ WEHI-3 cells,
and then treated i.v. with normal mouse serum three times (2nd
day/700 μl, 5th day/600 μl and 9th day/500 μl) after cell
injection. Group 3 was injected i.v. with 1×107
CD176+ WEHI-3 cells, and then treated i.v. with the
CD176 antiserum three times (2nd day/700 μl, 5th day/600 μl and 9th
day/500 μl) after cell injection. The analysis of specificity and
titer determination of CD176 antibody in the sera were previously
described. The survival was monitored daily by two investigators.
Clinical symptoms, such as rapid and labored breathing, paralyzed
hind limbs and rough coat were recorded and used as indicators of
morbidity. Mice that survived for more than 65 days were
sacrificed.
In the second set of experiments of antibody
treatment, we studied effects of the CD176 antibody treatment on
the growth and spreading of CD176+ cancer cells in
vivo. Eighty mice were used (20 mice in each group). The design
and method applied were identical for group 1, 2 and 3 as mentioned
above. Group 4 consisted of normal mice without treatments. All
mice of the 4 groups were sacrificed on the 19th day. The organs,
such as lung, liver, spleen and kidney, were isolated, weighed,
observed and photographed. The number of the liver and lung cancer
spreading nodules in each group was counted. The tissues and bone
marrow samples were used for further histopathological
analysis.
Histopathology and
immunohistochemistry
All mice (control and experimental groups) were
weighed before being sacrificed. Liver, spleen, lung and kidney
from each group were weighed, observed and photographed, and then
fixed in the 4% formaldehyde and embedded in the paraffin. Paraffin
sections were stained with hematoxylin and eosin (H&E).
For analysis of apoptotic cells in tissue sections
using anti- single stranded DNA (ssDNA) monoclonal antibody (mAb),
tissues were fixed in cold 4% paraformaldehyde and cut into
sections. The slides were immersed in methanol. Subsequently, the
slides were treated with 10 μg/ml proteinase K at 56°C and then
with 50% formamide. The slides were then treated with 3%
H2O2 and blocked with 3% BSA. Anti-ssDNA mAb
(Chemicon, Temecula, CA, USA) and peroxidase-labeled goat
anti-mouse immunoglobulin were used as the primary and secondary
antibody, respectively. Color was developed with the peroxidase
substrate 3,3′-diaminobenzidine. Counterstaining was performed with
hematoxylin. Negative controls were performed with an irrelevant
IgM from a mouse plasmocytoma (Sigma) at a comparable dilution
instead of the mAb. Tissue sections which were not treated with
proteinase K and heated formamide were used for the detection of
CD176. CD176 mAb (19) was applied
as the first antibody.
Bone marrow smears
The bone marrow was washed out from the femur of
sacrificed mice treated with the CD176 antiserum on the 19th day
after the inoculation of CD176+ WEHI-3. The bone marrow
smear was carried out by pushing two slides and then Giemsa
staining was applied. Leukemia cell numbers were counted by
microscopy. The proportion of leukemia cells to total bone marrow
cells was estimated in ten optical fields.
Statistical analysis
Data (number of liver tumor metastasis nodules,
swelling degree of liver and spleen, the proportion of leukemia
cells to total bone marrow cells) were analyzed using the paired
t-test. Incidences of liver and lung tumor metastasis were compared
with the Fisher’s exact test (two-tailed). The Kaplan-Meier curves
were used to determine survival differences between the CD176
antiserum treatment group or CD176 antigen vaccination group and
their control groups. Log-rank test was used to determine the
differences between curves. A value of P<0.05 was considered to
indicate a statistically significant result.
Results
Establishment of CD176+
leukemic transplanted mice models
The mice injected with CD176+ WEHI-3 were
investigated histologically and immunohistologically. Under
histopathological observation, large numbers of scattered cancer
cells and cancerous nodules were observed in lung, spleen, liver
and bone marrow from the mice injected i.v with CD176+
WEHI-3 cells but not in those left untreated mice. CD176 was
strongly expressed in the cancer cells as observed by
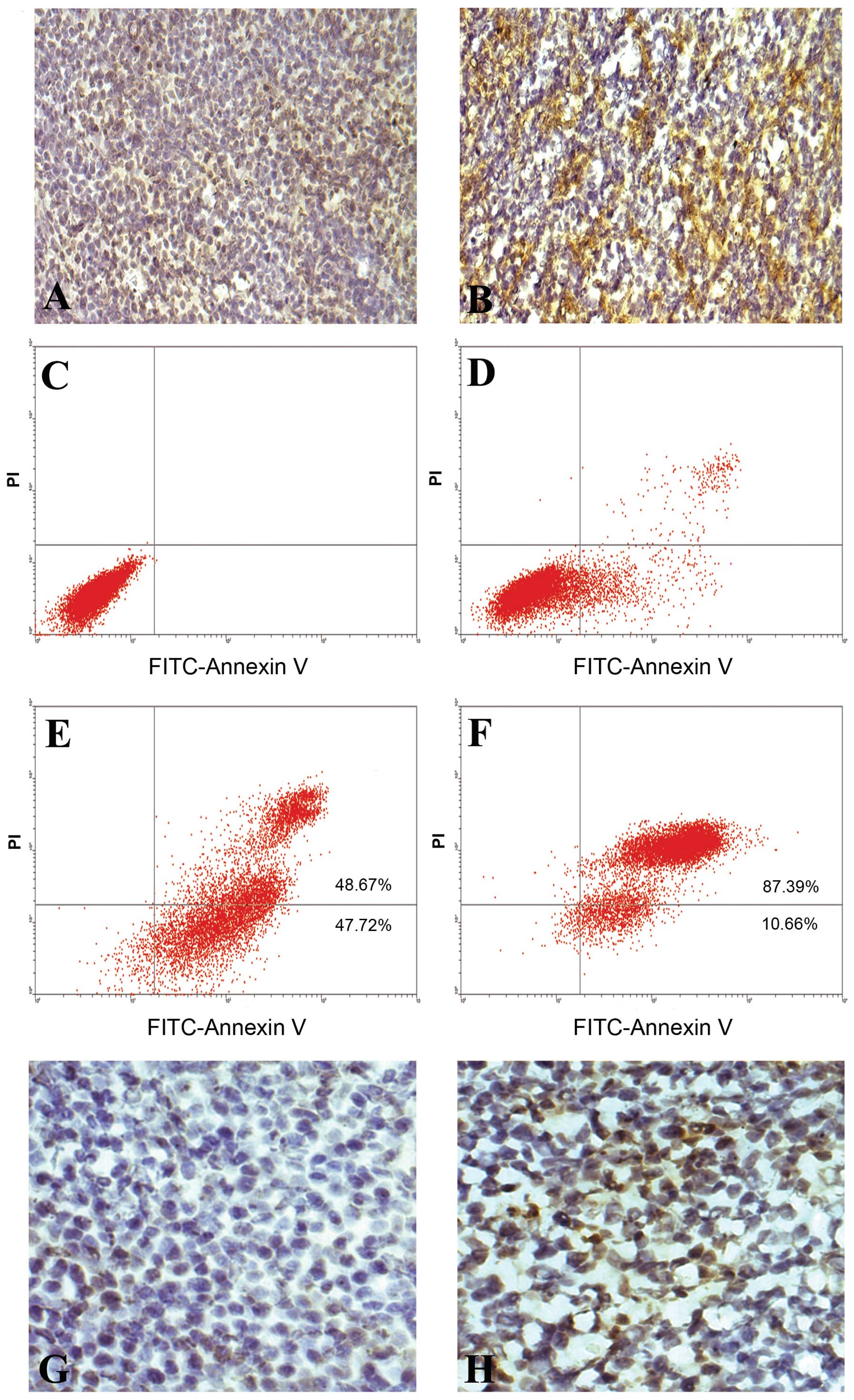
immunohistological analysis (Fig. 1A
and B). A mouse model of CD176+ leukemia transplants
was established successfully.
Apoptosis of CD176+ cancer
cells induced by the CD176 antiserum treatment in vitro and in
vivo
The CD176 antiserum induced apoptosis of
CD176+ WEHI-3 in vitro and in vivo by flow
cytometry and histochemistry examination (Fig. 1C–H), indicating that the CD176
antiserum could kill CD176+ leukemia cells by inducing
the apoptosis of CD176+ leukemia cells.
Specific reaction of the CD176 antiserum
with the tumor-associated CD176
The reaction of the CD176 antiserum with aGP was
partially inhibited in ELISA using the inhibitor TF-PAA (data not
shown). However, the reaction of the CD176 antiserum with the
tumor-associated CD176 was almost completely inhibited in
cell-ELISA and ELISA (data not shown). Furthermore, the staining of
the CD176 antiserum at the surface of cancer cells was also clearly
reduced using the inhibitor TF-PAA as seen in immunofluorescence
staining (data not shown). Although the CD176 antiserum prepared
from aGP also binds to peptides of aGP, the antibody binding
peptides of aGP in the serum do not react with cancer cells, due to
the absence of aGP peptides in cancer cells. These data
demonstrated that the CD176 antiserum prepared from aGP reacted
only with the tumor-associated CD176 in cancer cells.
The survival time of leukemic mice is
prolonged by immunization using CD176 antigen
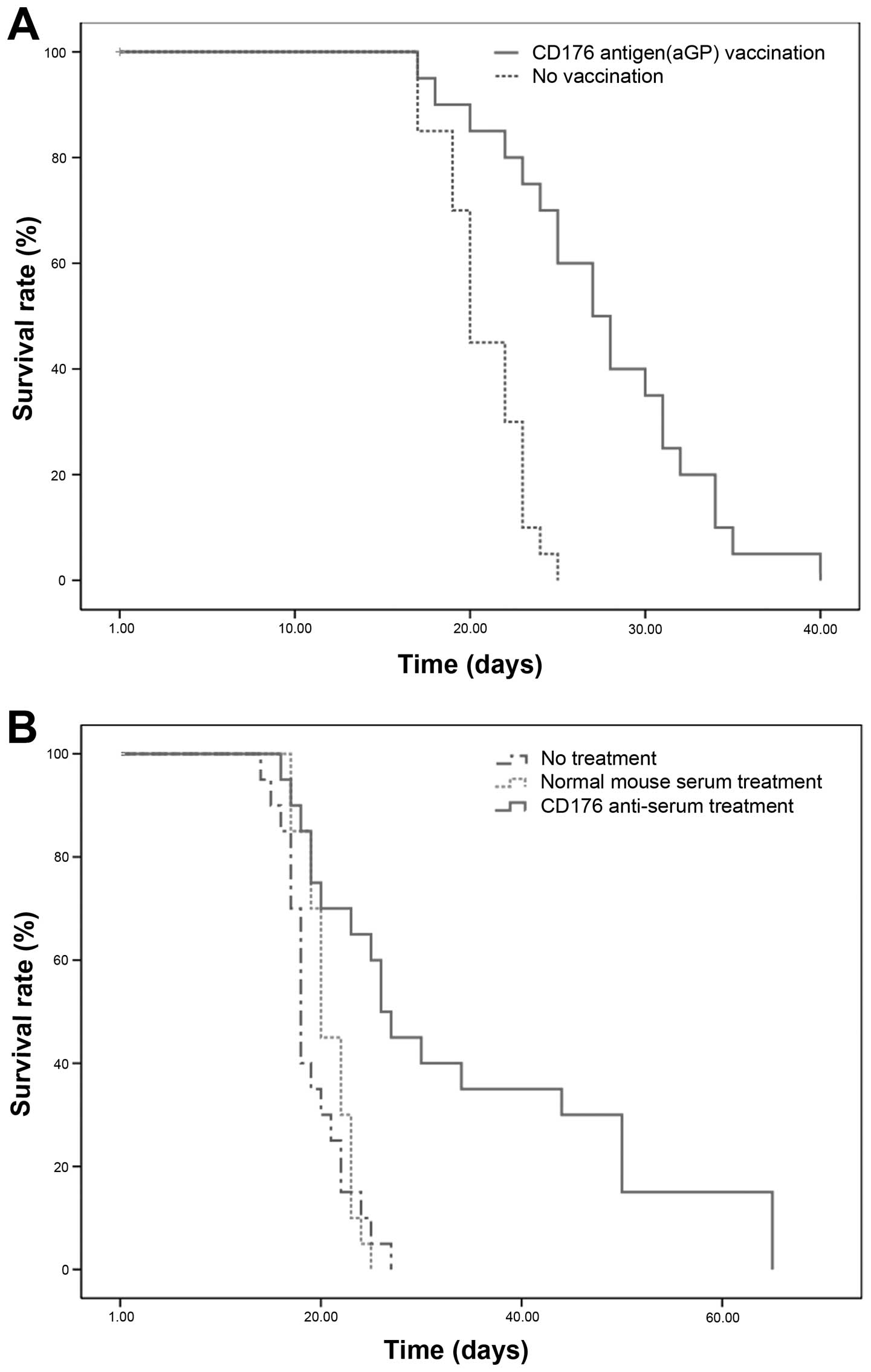
In initiative immunotherapy experiments, the
leukemia mice pre-immunized with aGP (n=20) had a significantly
enhanced survival time in comparison to the non-immunized mice
(n=20) as determined by the Kaplan-Meier analysis (P<0.001;
Fig. 2A). Compared with the control
group, the median survival time of the immunized group was
prolonged by 7 days and the survival time was increased by
32.8%.
The survival time of the leukemic mice is
prolonged by the CD176 antiserum treatment alone
In the first experiments of antibody treatment, the
CD176+ leukemia mice that were treated with the CD176
antiserum alone after inoculation of CD176+ WEHI-3
(n=20), showed a significant survival advantage in comparison to
the mice treated with normal mouse serum (n=20) and the mice
without the treatment (n=20) through the Kaplan-Meier analysis
(P<0.001; Fig. 2B). The median
survival time of the CD176 antiserum treatment group was prolonged
by 6 and 8 days compared with the two control groups,
respectively.
The growth and spreading of
CD176+ cancer cells is inhibited by the CD176 antiserum
treatment alone
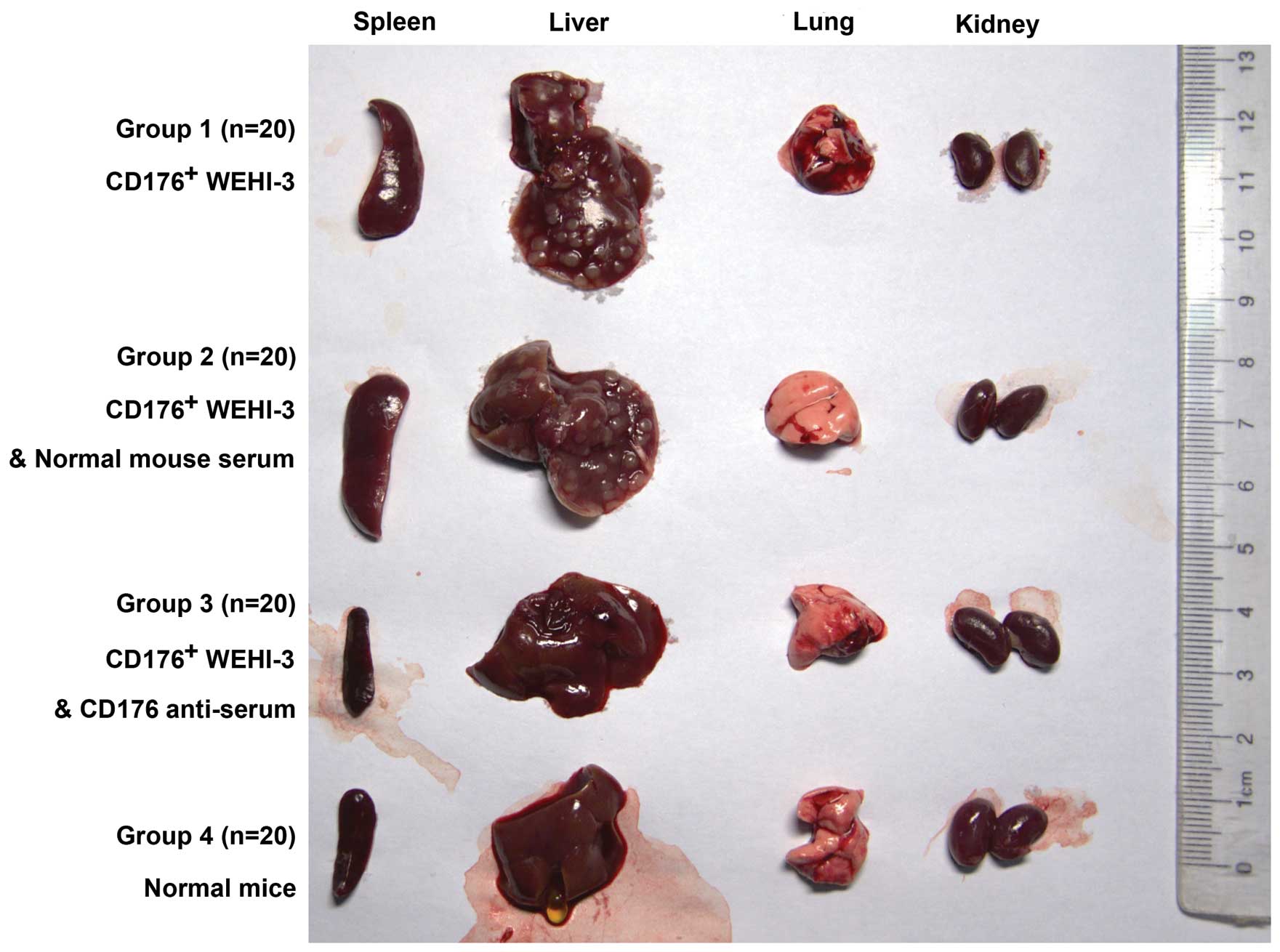
In the second set of antibody treatment experiments,
several changes were observed in the leukemia mice treated with the
CD176 antiserum (n=20) as compared to the leukemia mice treated
with normal mouse serum (n=20) and without the treatment (n=20): i)
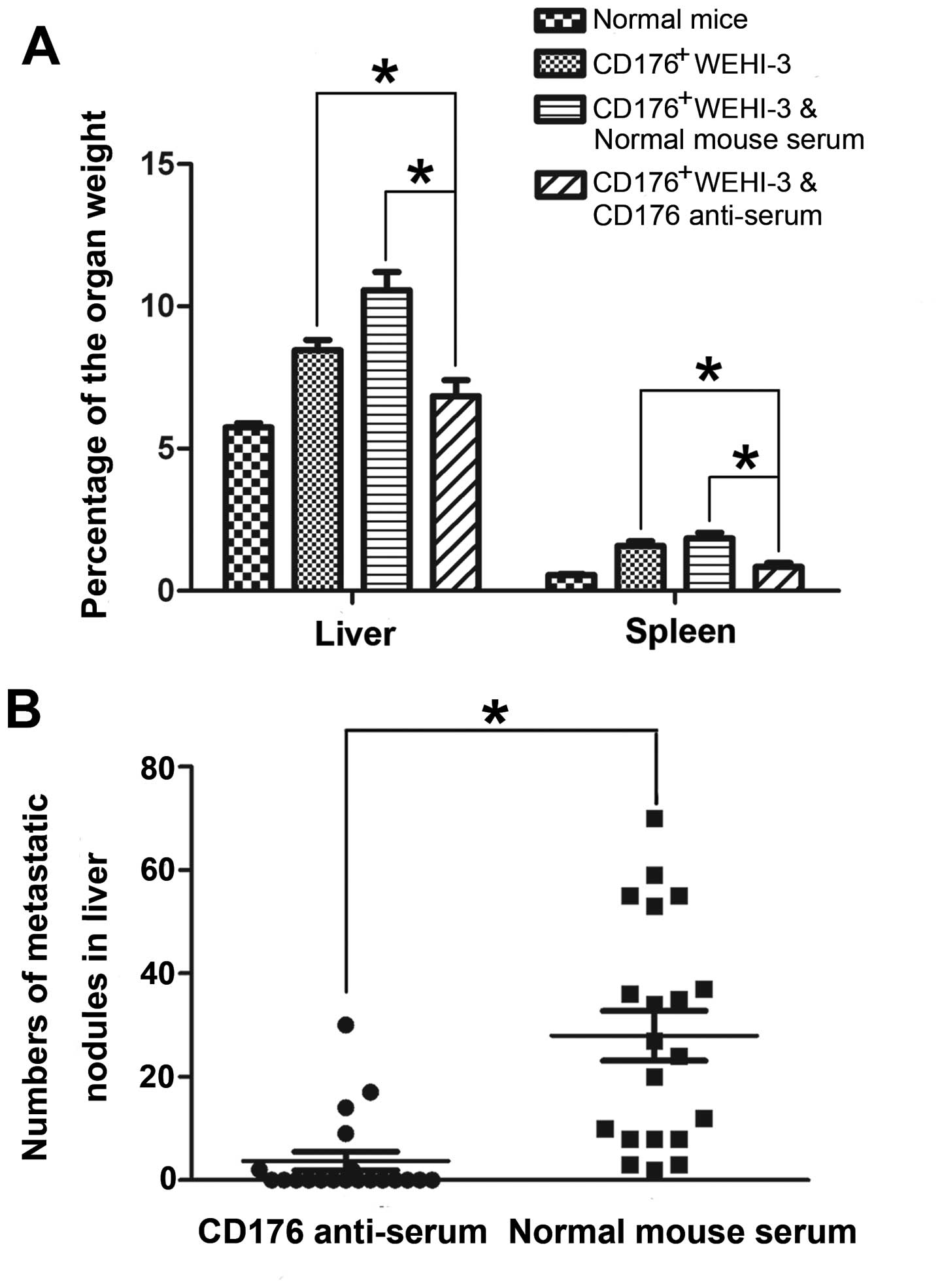
swelling degrees of liver and spleen were significantly reduced
(P<0.001; Figs. 3 and 4A); ii) incidence of the cancer spreading
in the liver and lung was significantly decreased using Fisher’s
exact analysis (Table I); iii)
number of liver cancer spreading nodules was significantly
decreased using paired t-test (P<0.001; Fig. 4B); and iv) there was a marked
decrease in the number of the leukemia cells in lung, liver and
spleen on H&E staining sections (Fig. 5A–D). These data provide strong
evidence that the CD176 antibody treatment effectively inhibits the
growth and spreading of CD176+ leukemia cells.
 | Table IIncidence of liver and lung
metastasis between CD176 antiserum-treated mice and control
mice. |
Table I
Incidence of liver and lung
metastasis between CD176 antiserum-treated mice and control
mice.
| Groups | Incidence of liver
tumor metastasis n (%) | Incidence of lung
tumor metastasis n (%) |
|---|
| CD176+
WEHI-3 (n=20) | 16/20 (80)a | 9/20
(45)b |
| CD176+
WEHI-3 and NMS (n=20) | 20/20 (100)a | 2/20 (10) |
| CD176+
WEHI-3 and CD176 antiserum (n=20) | 6/20 (30)a | 0/20
(0)b |
Number of leukemia cells in bone marrow
is decreased by the CD176 antiserum treatment alone
Through histopathological observation on bone marrow
smears with Giemsa staining and subsequent statistical analysis,
the CD176 antiserum treatment alone significantly reduced the
percentage of leukemia cells in total bone marrow cells (Fig. 5E–H, Table II). The CD176 antibody treatment
also inhibited the growth of CD176+ leukemia cells in
bone marrow.
 | Table IIPercentage of leukemia cells in total
bone marrow cells between CD176 antiserum-treated mice and control
mice. |
Table II
Percentage of leukemia cells in total
bone marrow cells between CD176 antiserum-treated mice and control
mice.
| Percentage of
leukemia cells in total bone marrow cells |
|---|
|
|
|---|
| Groups | +a | ++ | +++ |
|---|
| CD176+
WEHI-3 (n=6) | 0/6b | 0/6 | 6/6 |
| CD176+
WEHI-3 and NMS (n=6) | 0/6 | 0/6 | 6/6 |
| CD176+
WEHI-3 and CD176 antiserum (n=6) | 5/6 | 1/6 | 0/6 |
Discussion
Immunotherapy is of considerable benefit to cancer
patients (20). Anticancer vaccines
targeting tumor-associated carbohydrates, such as CD176, provide an
attractive approach for the prevention and treatment of cancer.
Several studies suggested that the vaccination with a CD176 antigen
could effectively improve breast cancer patient survival (5,14,15).
In patients immunized with CD176 antigen, aside from the activation
of CD176-specific antibody responses, a CD176-specific DTH
reflecting the activation of specific T cells was also observed
(5). An animal study also showed
that mice immunized with synthetic CD176 glycopeptide vaccines
generated a cytotoxic T cell response to CD176+ tumor
cells (21). These results
indicated that tumor vaccines addressing CD176 (TF antigen) could
activate a specific humoral and cellular antitumor response. In the
present study, we used CD176+ leukemia mice as
experimental models and titers of the CD176 IgM and IgG antibody as
immune response index. We found that the mice pre-immunized with
CD176 antigen had a significant survival advantage compared to the
control mice without the immunization. These observations indicated
that high anti-CD176 immune responses and high titers of CD176
antibodies may be beneficial for the prevention of tumor
development. Natural antibodies against CD176 occurring in all
adult sera (22) may be part of a
mechanism of humoral immunosurveillance against CD176+
tumor cells (13), but the natural
CD176 antibody is likely to be consumed and is present in lower
titer in cancer patients. Thus, we presented a hypothesis that the
vaccination with CD176 antigen increased and further maintained the
high anti-CD176 immune response and high CD176 antibody titers for
the prevention of tumor recurrence and metastasis. The development
of new tumor vaccines addressing CD176 merits further
consideration.
In clinical medicine, antibody treatment has unique
advantages for cancer patients. For advanced cancer patients and
immunocompromised patients, passive antibody immunotherapy is
better than tumor vaccine immunotherapy. Antibody drugs have been
widely used for cancer treatment including leukemia. For example,
trastuzumab which triggers apoptosis of HER2-positive breast cancer
(16), and rituximab which targets
surface antigen CD20 of B-cell malignancies, have been used for
clinical treatment (23). In
previous studies, we have found that CD176 antibody can induce
apoptosis of CD176+ leukemia cells (13,17).
Several studies have shown that CD176+ (TF+)
T-cell acute lymphoblastic leukemia (ALL) has a better prognosis
than CD176− ALL (7,8).
Apoptotic destruction induced by CD176 antibody of the natural
antibody repertoire may provide an explanation for this phenomenon
(13). Tumor-specific apoptosis
mediated by natural carbohydrate-reactive IgM antibodies in humans
has been observed and may lead to new strategies for anticancer
immunotherapy (24). Additionally,
CD176 antibody treatment can prevent tumor metastasis (18). Based on these findings, the
application of the CD176 antibody as an anticancer drug may be a
promising approach for current cancer immunotherapy. In order to
investigate whether the CD176 antibody treatment alone has a
therapeutic effect on animals with CD176+ cancer cells
in vivo, we performed passive transfer of the CD176
antiserum in CD176+ leukemia mice. Several inhibitory
experiments with TF (CD176) glycan demonstrated that the CD176
antiserum used in the study reacted only with the tumor-associated
CD176 in cancer cells. The present study clearly demonstrated that
passive immunotherapy using the CD176 antiserum alone provided
CD176+ leukemia mice with a significant advantage for
survival. Furthermore, we observed that the CD176 antiserum
treatment alone could inhibit the growth and spreading of
CD176+ cancer cells in bone marrow, spleen, liver and
lung as determined by histopathological examination. Although the
mechanisms of the CD176 antibody treatment were not fully
understood, CD176 antibody treatment may be involved in the
following process: i) CD176 antibody could inhibit
CD176+ cancer cell metastasis to bone marrow, spleen,
lung and liver through blocking the adhesion of cancer cells to the
endothelium (11,25) and hepatocytes (10); ii) CD176 antibody could mediate the
elimination of CD176+ cancer cells through
complement-dependent cytotoxicity (CDC) and/or antibody-dependent
cellular cytotoxicity (ADCC) performed by natural killer cells,
neutrophils, and macrophages; and finally iii) CD176 antibody could
induce apoptosis of CD176+ leukemia cells (13,17).
In conclusion, the present study provided strong
evidence that both CD176 antigen-based active-immunotherapy and
CD176 antibody-based passive-immunotherapy lead to a therapeutic
response in CD176+ leukemia mice in vivo. Since
CD176 is expressed on the surface of human leukemic cells but is
almost absent in normal and benign adult human tissues (3), CD176 may be an ideal target for
anti-leukemia immunotherapy. Therefore, both CD176 vaccine and
CD176 antibody drug may be beneficial to CD176+ leukemia
patients. The modes of application could be selected according to
the clinical situations of the respective leukemia.
Acknowledgements
The present study was financially supported by
grants from the National Natural Science Foundation of China (no.
81072563), the Yunnan Province Science and Technology Department
(2008CCO15, 2011CI139) and the Chinese Academy of Sciences
(KSCX2-YW-R-196).
Abbreviations:
|
TF
|
Thomsen-Friedenreich antigen
|
|
DTH
|
delayed type hypersensitivity
|
|
aGP
|
asialoglycophorin
|
|
mAb
|
monoclonal antibody
|
|
PAA
|
polyacrylamide
|
|
PBS
|
phosphate-buffered saline
|
|
VCN
|
vibrio cholerae neuraminidase
(sialidase)
|
|
ELISA
|
solid-phase enzyme linked
immunosorbent assay
|
|
BSA
|
bovine serum albumin
|
|
PI
|
propidium iodide
|
|
H&E
|
hematoxylin and eosin
|
|
ssDNA
|
single stranded DNA
|
|
ALL
|
acute lymphoblastic leukemia
|
|
CDC
|
complement-dependent cytotoxicity
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
References
|
1
|
Goletz S, Cao Y, Danielcyk A, Ravn P,
Schoeber U and Karsten U: Thomsen-Friedenreich antigen: the
‘hidden’ tumour antigen. Adv Exp Med Biol. 535:147–162. 2003.
|
|
2
|
Cao Y, Merling A, Karsten U and
Schwartz-Albiez R: Expression of Thomsen-Friedenreich-related
carbohydrate antigens on human leukemia cells. Leucocyte Typing
VII. Mason D, et al: Oxford University Press; Oxford: pp. 204–205.
2002
|
|
3
|
Cao Y, Stosiek P, Springer GF and Karsten
U: Thomsen-Friedenreich-related carbohydrate antigens in normal
adult human tissues: a systematic and comparative study. Histochem
Cell Biol. 106:197–207. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Springer GF: T and Tn, general carcinoma
autoantigens. Science. 224:1198–1206. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Springer GF: Immunoreactive T and Tn
epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol
Med. 75:594–602. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aller CT, Kucuk O, Springer GF and
Gilman-Sachs A: Flow cytometric analysis of T and Tn epitopes on
chronic lymphocytic leukemia cells. Am J Hematol. 52:29–38. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaspers GJ, Veerman AJ, Van Wering ER, et
al: Prognostic significance of peanut agglutinin binding in
childhood acute lymphoblastic leukemia. Leukemia. 10:675–681.
1996.PubMed/NCBI
|
|
8
|
Veerman AJ, Hogeman PH, Huismans DR, Van
Zantwijk CH and Bezemer PD: Peanut agglutinin, a marker for T-cell
acute lymphoblastic leukemia with a good prognosis. Cancer Res.
45:1890–1893. 1985.PubMed/NCBI
|
|
9
|
Lin WM, Karsten U, Goletz S, Cheng RC and
Cao Y: Expression of CD176 (Thomsen-Friedenreich antigen) on lung,
breast and liver cancer-initiating cells. Int J Exp Pathol.
92:97–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y, Karsten UR, Liebrich W, Haensch W,
Springer GF and Schlag PM: Expression of
Thomsen-Friedenreich-related antigens in primary and metastatic
colorectal carcinomas. A reevaluation. Cancer. 76:1700–1708. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glinsky VV, Glinsky GV, Rittenhouse-Olson
K, et al: The role of Thomsen-Friedenreich antigen in adhesion of
human breast and prostate cancer cells to the endothelium. Cancer
Res. 61:4851–4857. 2001.PubMed/NCBI
|
|
12
|
Heimburg J, Yan J, Morey S, et al:
Inhibition of spontaneous breast cancer metastasis by
anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11.
Neoplasia. 8:939–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Y, Merling A, Karsten U, et al:
Expression of CD175 (Tn), CD175s (sialosyl-Tn) and CD176
(Thomsen-Friedenreich antigen) on malignant human hematopoietic
cells. Int J Cancer. 123:89–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
MacLean GD, Bowen-Yacyshyn MB, Samuel J,
et al: Active immunization of human ovarian cancer patients against
a common carcinoma (Thomsen-Friedenreich) determinant using a
synthetic carbohydrate antigen. J Immunother. 11:292–305. 1992.
View Article : Google Scholar
|
|
15
|
Slovin SF, Ragupathi G, Musselli C, et al:
Thomsen-Friedenreich (TF) antigen as a target for prostate cancer
vaccine: clinical trial results with TF cluster (c)-KLH plus QS21
conjugate vaccine in patients with biochemically relapsed prostate
cancer. Cancer Immunol Immunother. 54:694–702. 2005. View Article : Google Scholar
|
|
16
|
Dougan M and Dranoff G: Immune therapy for
cancer. Annu Rev Immunol. 27:83–117. 2009. View Article : Google Scholar
|
|
17
|
Yi B, Zhang M, Schwartz-Albiez R and Cao
Y: Mechanisms of the apoptosis induced by CD176 antibody in human
leukemic cells. Int J Oncol. 38:1565–1573. 2011.PubMed/NCBI
|
|
18
|
Shigeoka H, Karsten U, Okuno K and
Yasutomi M: Inhibition of liver metastases from
neuraminidase-treated colon 26 cells by an
anti-Thomsen-Friedenreich-specific monoclonal antibody. Tumor Biol.
20:139–146. 1999. View Article : Google Scholar
|
|
19
|
Karsten U, Butschak G, Cao Y, Goletz S and
Hanisch FG: A new monoclonal antibody (A78-G/A7) to the
Thomsen-Friedenreich pan-tumor antigen. Hybridoma. 14:37–44. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Topalian SL, Weiner GJ and Pardoll DM:
Cancer immunotherapy comes of age. J Clin Oncol. 29:4828–4836.
2012. View Article : Google Scholar
|
|
21
|
Xu Y, Gendler SJ and Franco A: Designer
glycopeptides for cytotoxic T cell-based elimination of carcinomas.
J Exp Med. 199:707–716. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Butschak G and Karsten U: Isolation and
characterization of thomsen-friedenreich-specific antibodies from
human serum. Tumor Biol. 23:113–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maloney DG: Anti-CD20 antibody therapy for
B-cell lymphomas. N Engl J Med. 366:2008–2016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartz-Albiez R: Naturally occurring
antibodies directed against carbohydrate tumor antigens. Adv Exp
Med Biol. 750:27–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Glinskii OV, Sud S, Mossine VV, et al:
Inhibition of prostate cancer bone metastasis by synthetic TF
antigen mimic/galectin-3 inhibitor lactulose-L-leucine. Neoplasia.
14:65–73. 2012.PubMed/NCBI
|