Introduction
Neuroblastoma (NB) is the most common solid tumor
diagnosed in children, and it originates from immature neuronal
cells of the sympathetic nervous system. NBs are also comprised of
multiple cell phenotypes, including neuroblasts, non-neuronal cells
and melanocytes (1). In NB cell
lines, as well as in primary NB tumors, three distinct cell types
have been identified: Schwannian/melanoblastic precursors (S-type),
neuroblastic/neuroendocrine precursors (N-type), and
morphologically intermediate stem cells (I-type). I-type stem cells
represent precursors of both N- and S-type cells, and also
represent the most immature and highly malignant population of NB
cells (2). Based on significant
similarities in the gene expression profiles of normal neuroblasts
and malignant NB, as well as the morphological and functional
differentiation that can be achieved with NB cells in culture, the
differentiation process of NB can be studied in vitro.
A growing body of evidence has demonstrated that
many types of cancers are driven by a small population of cancer
cells, termed cancer stem cells (CSCs), or cancer-initiation cells
(3). CSCs have the capacity to
undergo differentiation, self-renewal and tumor generation.
Moreover, CSCs have the potential to induce tumor growth,
metastasis and resistance to radiation and chemotherapy (4). Understanding the signaling pathways
that mediate functional characteristics is important in preclinical
models, including animal models or in vitro cell lines.
Drosophila delta-like 1 homologue (DLK1) is a member of the
epidermal growth factor-like homeotic protein family. DLK1 has been
known to modulate differentiation signaling in adipocytes and
hematopoietic stem cells and has been characterized as a bona fide
stem cell gene in neuronal tumors (5,6).
Mitogen activated protein kinase (MAPK) signaling pathways have
been shown to play pivotal roles in growth, differentiation and
stress responses (7,8). It has been reported that MAPK
signaling is affected by the expression of DLK1 (9). Correspondingly, extracellular
signal-regulated kinases (ERK)(p42/44) are phosphorylated during
neuronal differentiation and the activation of MEK/ERK signaling
was found to decrease the self-renewal capacity of embryonal stem
cells (8,10).
During tumor treatment, most cancer cells, including
a majority of differentiated tumor cells and actively replicating
cells, respond to cytotoxic treatments. However, acquired multidrug
resistance to conventional therapies can develop in CSCs.
Therefore, the successful elimination of both actively replicating
tumor cells, and slowly dividing CSCs, may represent a more
effective strategy for the complete treatment of various types of
cancer.
β-carotene (BC) is a provitamin A carotenoid, and a
source of retinoids in dark green and orange fruits and vegetables.
Observational studies have reported that an increase in the intake
of dietary BC with increased BC plasma concentrations is associated
with a decreased risk of several types of cancers (11,12).
Accumulating evidence also suggests that mechanisms involving
antioxidant potential, alterations in intracellular signaling
pathways, and regulation of cell proliferation and apoptosis,
contribute to the anticarcinogenic effects associated with BC
(13,14). Retinoic acid (RA) has also been
shown to promote the differentiation of embryonic stem cells and
CSCs (15), and is used as a
chemotherapeutic drug for various types of cancers (16). Moreover, direct, or indirect effects
of several dietary compounds, including sulforaphane and curcumin
on self-renewal pathways, have been identified (17,18).
Currently, little is known about the role of BC in
the differentiation and self-renewal characteristics of CSCs.
Therefore, in the present study, the chemopreventive effects of BC
on the induction of differentiation and attenuation of cancer cell
stemness were investigated. Moreover, based on the potential for
CSCs to contribute to chemoresistance, the chemotherapeutic effect
of administering BC and cisplatin in combination was investigated,
as well as the hypothesis that the effects of BC are mediated
through the targeting of NB cancer stem cell-like cells.
Materials and methods
Reagents and cell culture
BC, all-trans-retinoic acid (RA),
tetrahydrofuran (THF) and cis-diamminedichloroplatinum(II)
(cisplatin) were purchased from Sigma Chemical Co. (St. Louis, MO,
USA). U0126 and PD98059 were obtained from Cell Signaling
Technology Inc. (Danvers, MA, USA). Two human NB cell lines,
SK-N-BE(2)C [BE(2)C] and SH-SY5Y (SY5Y), were obtained from
the American Type Culture Collection (ATCC, Rockville, MD, USA) and
maintained in a 1:1 mixture of Minimum Essential Medium (MEM) and
Ham’s F-12 medium supplemented with 10% fetal bovine serum (FBS)
and antibiotics. BC was freshly prepared in THF containing 0.01%
butylated hyroxytoluene (BHT), then added to the culture medium to
a final concentration of 5–20 μM. The concentration of THF in
culture medium was adjusted to be the same in all experiments, and
the final concentration did not exceed 0.4% (v/v). The various
concentrations of BC (5, 10 and 20 μM) used in the present study
were not associated with any significant cytotoxicity, and cell
culture involving BC was performed under minimal light to minimize
the breakdown of BC. Cisplatin was dissolved in 0.9% NaCl.
Cell viability assay
Cell viability was evaluated using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma) (19). Briefly, cells were
seeded in 96-well plates and incubated overnight at 37°C with 5%
CO2. Varying concentrations of BC were added to the
wells and incubated for 48–96 h. MTT was then added to each well at
a final concentration of 500 μg/ml. After 4 h at 37°C, the
supernatant from each well was removed. Cells were lysed with DMSO,
and the absorbance values at 560 nm were measured using a
microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Measurement of neurite outgrowth
BE(2)C cells were
plated in triplicate in 6-well plates (4×104 cells/well)
and treated with varying doses of BC for 5 days. A process having
at least twice the diameter of the associated cell body, and
possessing a terminal growth edge, was considered as a neurite. The
percentage of cells with outgrowing neurites in ~50 cells/per well
was counted. The mean and standard error of the mean (SEM) are
reported. Three independent experiments for each treatment were
performed.
Clonogenic assays
To determine the self-renewal characteristics of
cells, clonogenic assays were performed as previously described
(20). Briefly, BE(2)C cells were plated in 6-well plates (500
cells/well) and treated with varying concentrations of BC for 10
days. After fixing the cells with 0.9% NaCl, colonies were stained
with crystal violet (Sigma). Colonies containing at least 50
stained cells were counted. Plating efficiency (PE) was calculated
as follows: PE = (colony number/total cell number) × 100%.
Sphere formation assays
To facilitate sphere formation, poly-(2-hydroxyethyl
methacrylate) (polyHEMA; Sigma-Aldrich)-coated plates were prepared
by applying 500 μl of 10% polyHEMA stock solution [12% polyHEMA
solution in 95% ethanol (w/v)] into each well of a 6-well plate
(21). Sphere medium was prepared
with DMEM-F12 (3:1; Invitrogen, Carlsbad, CA, USA) containing 2%
B27 (Invitrogen), 20 ng/ml recombinant human epidermal growth
factor and 40 ng/ml recombinant human fibroblast growth factor
basic (PeproTech, Rocky Hill, NJ, USA). After 6 days, the number of
tumor spheres containing >50 cells was counted.
Antibodies and western blot assay
Western blot assays were performed as previously
described (22). Briefly, cell
lysates were separated by SDS-PAGE and transferred onto
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA). The membranes were incubated with 5% non-fat dried milk,
followed by antibodies against DLK1, SOX2 (Millipore), Notch1
(Novus Biologicals, Littleton, CO, USA), β-tubulin III (Sigma),
phospho-ERK (p42/p44), total ERK (Cell Signaling), or β-actin
(Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bound antibodies
were detected using enhanced chemiluminescence reagents (Animal
Genetics Inc., Suwon, Kyonggi-do, Korea).
RNA isolation and real-time PCR
Cells were treated with various doses of BC for 4
days and total RNA extraction was performed on each sample using
TRIzol reagent (Invitrogen). cDNA of 1 μg total RNA was synthesized
by reverse transcription with RevertAid First Strand cDNA synthesis
kit (Thermo Fisher Scientific, Waltham, MA, USA), and real-time PCR
was performed with StepOnePlus™ (Applied Biosystems, Foster City,
CA, USA) to examine the relative DLK1 mRNA levels among the samples
according to the manufacturer’s instructions. PCR primers were as
follows: human DLK1, 5′-CTG AAGGTGTCCATGAAAGAG-3′ (forward);
5′-GCTGAAGG TGGTCATGTCGAT-3′ (reverse) and β-actin, 5′-ATTGGCA
ATGAGCGGTTC-3′ (forward); 5′-GGATGCCACAGGACT CCAT-3′ (reverse).
β-actin, a housekeeping gene, was used for equal RNA loading.
RNA interference
Small interfering RNA (siRNA) for human DLK1
(nM_003836) was purchased from Dharmacon (Lafayette, CO, USA), and
the 5′-GCACCUGCGUGGAUGU GAUU-3′ oligonucleotides were used to
target nucleotides 578–596 of the DLK1 gene. Non-targeting siRNA
(5′-UAACA AUGAGAGCACGGCUUU-3′) was used as a transfection control.
siRNA transfection was performed using DharmaFECT (Dharmacon)
according to the manufacturer’s instructions.
Statistical analysis
All results are expressed as the means ± SEM.
Comparisons between groups were performed using one-way analysis of
variance (ANOVA) followed by the Newman-Keuls post-hoc test. All
experiments were repeated at least three times to generate
statistically relevant data. Data were considered significant when
the P-value was <0.05.
Results
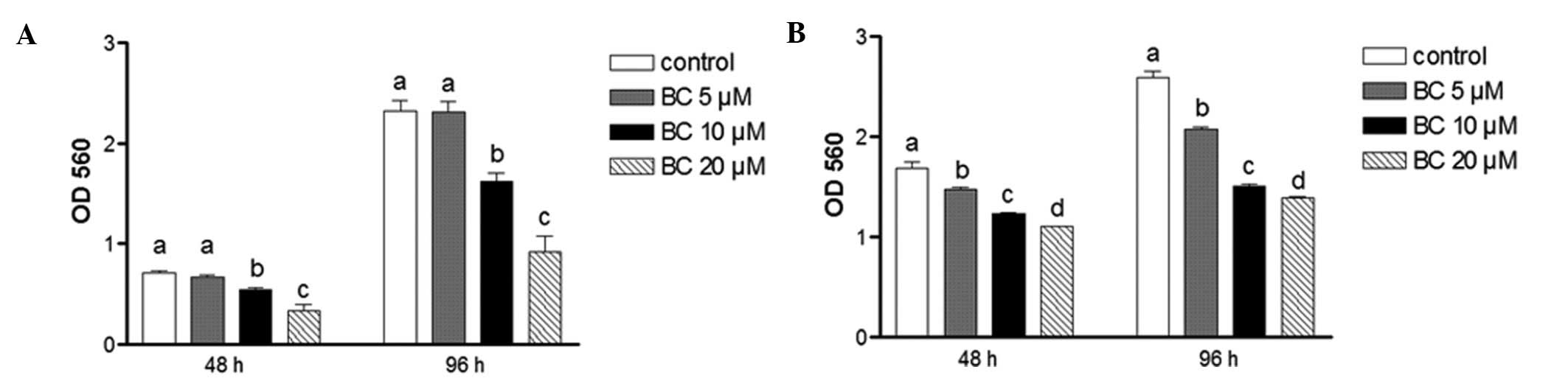
Effects of BC on NB cell growth
Cell growth of BE(2)C and SY5Y cells following 48 or 96 h of
treatment with BC was evaluated using the MTT assay. Treatment with
BC significantly decreased the viability of both cell types when
compared to the controls at both time-points (Fig. 1A and 1B). For example, the viability
of the BE(2)C cells was decreased
by 60% after treatment with 20 μM of BC for 96 h, while the same
treatment decreased the viability of SY5Y cells by 46%.
Furthermore, these inhibitory effects were not due to toxicity
since cell viability for each treatment was found to be >90%
(data not shown). Compared to the N-type cells, SY5Y, the I-type
cells, BE(2)C, are more aggressive
and less differentiated. As a result, BE(2)C cells exhibit more stem cell-like
characteristics than SY5Y cells (2)
and accordingly, were preferentially used in the following
experiments to examine the effect of BC on NB cell stemness.
Effects of BC on cell
differentiation
BE(2)C cells are
I-type NB cells that possess cancer cell stemness and undergo
morphological and functional differentiation in
vitro(20). To examine the
effect of BC on NB cell differentiation, cells were treated with
various concentrations of BC. Following treatment, BE(2)C cells underwent morphological
differentiation in a dose-dependent manner manifested by an
increase in neurite growth, which is a hallmark of neuronal
differentiation (Fig. 2A and B). In
these assays, RA was used as a positive control to induce neuronal
differentiation. The observed increase in neuronal differentiation
following treatment with BC was confirmed with an upregulation of
β-tubulin III that was detected by western blotting (Fig. 2C). Neurite elongation and protein
expression profiles determined following BC treatment were also
very similar to those obtained following RA treatment.
BC-induced neuronal cell differentiation
involves ERK phosphorylation
It has been reported that ERK1/2 (p42/p44) is
phosphorylated during the differentiation of neuronal progenitor
cells (8). Similarly, when
phosphorylation of ERK1/2 was assessed following treatment with BC,
a marked increase was observed, whereas the expression of total ERK
was unchanged (Fig. 3A). To examine
whether MAPK pathways are involved in BC-induced NB cell
differentiation, the MAPK-specific inhibitors, U0126 and PD98059,
were used. In these studies, phosphorylation of ERK1/2 was
completely blocked following treatment with U0126 and 10 or 20 μM
BC, while phosphorylation of ERK1/2 was only partially blocked by
PD98059 at both concentrations of BC (Fig. 3B). Consistent with these results,
BC-induced neurite elongation was also completely blocked by U0126
treatment, and only partially blocked by PD98059 (Fig. 3C). In combination, these data
clearly demonstrate that BC induces neuronal differentiation of NB,
and plays an important role in the MAPK phosphorylation that is
involved in this process.
BC suppresses the self-renewal
characteristics of NB
One of the more notable characteristics of CSCs is
the capacity for self-renewal. Clonogenic assay measures the
ability to form a colony from a single cell and represent the
self-renewal potential of CSCs. Folowing the treatment of NB cells
with 10 or 20 μM BC, clonogenic formation was significantly
suppressed when compared to the controls (Fig. 4A and B). Another widely used assay
to analyze the self-renewal capacity of CSCs is sphere formation
assay, which assesses the ability to grow as non-adherent spheroids
in a specific serum-free medium. The NB cells grown in serum-free
medium were able to form a clonosphere and following treatment with
BC exhibited a marked decrease in clonosphere formation compared to
the controls (P<0.05), and this decrease was dose-dependent
(Fig. 4C and D). To further verify
the effect of BC on neuronal stem cell markers, BE(2)C cells were treated with BC for 4 days
and the protein expression of several well-known stem cell markers,
including Sox2 and Notch1 was assessed. The expression of these
stem cell markers was found to be downregulated (Fig. 4E). Taken together, these results
suggest that BC is highly effective in decreasing the self-renewal
capacity of NB cells, and in eliminating most NB stem cells.
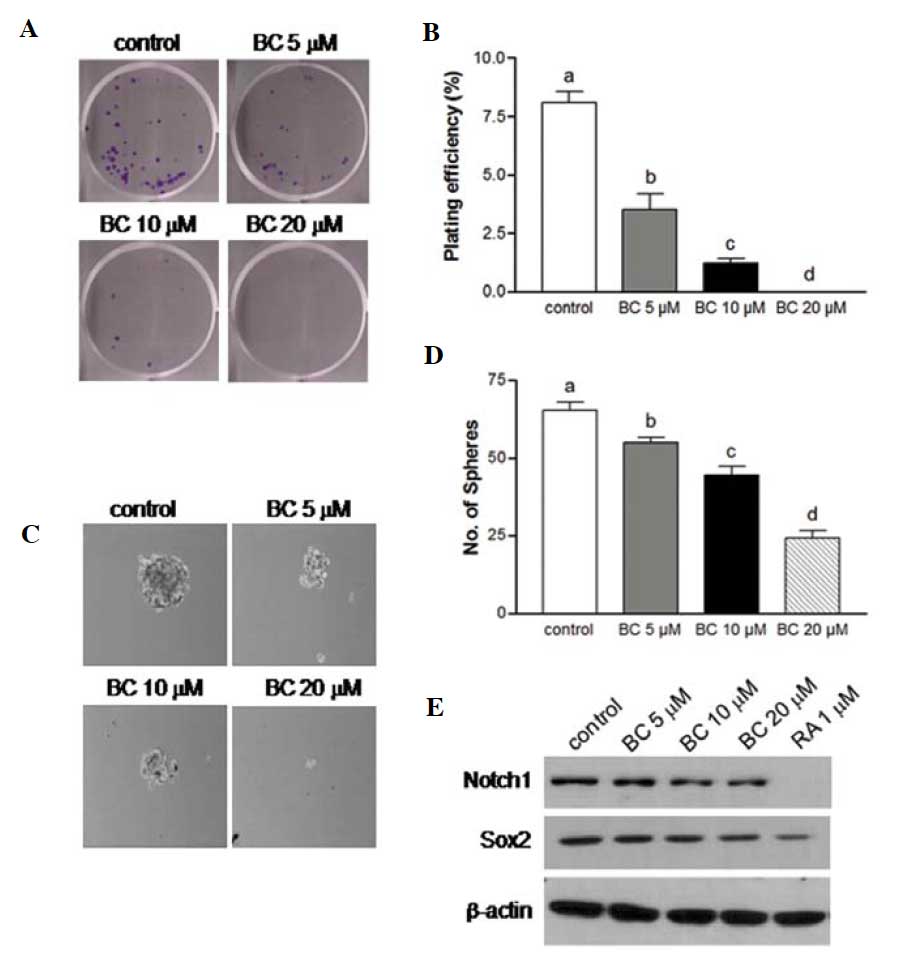 | Figure 4BC attenuates the self-renewal
capacity of NB. (A) BE(2)C cells in 6-well plates were treated with
0, 5, 10 or 20 μM BC. After 7–10 days, colonies were stained and
imaged. (B) The number of colonies with ≥50 cells were counted, and
plating efficiency (PE) was calculated: PE = percentage of colonies
per input ± SEM; (n=6, P<0.05 vs. control). (C and D) BE(2)C
cells were also cultured in polyHEMA-coated tissue culture dishes
and treated with various doses of BC in serum-free sphere media.
After 6 days, sphere formation was imaged and the number of spheres
was counted. Magnification, ×100. Experiments were independently
performed at least three times. Different letters at the top of the
given bars of the histogram indicate that those values are
significantly different from each other (P<0.05). (E)
Representative immunoblot assay of Notch1 and Sox2 expression in
BE(2)C cells following 4 days of treatment with various
concentrations of BC or RA. BC, β-carotene; NB, neuroblastoma; RA,
all-trans-retinoic acid. |
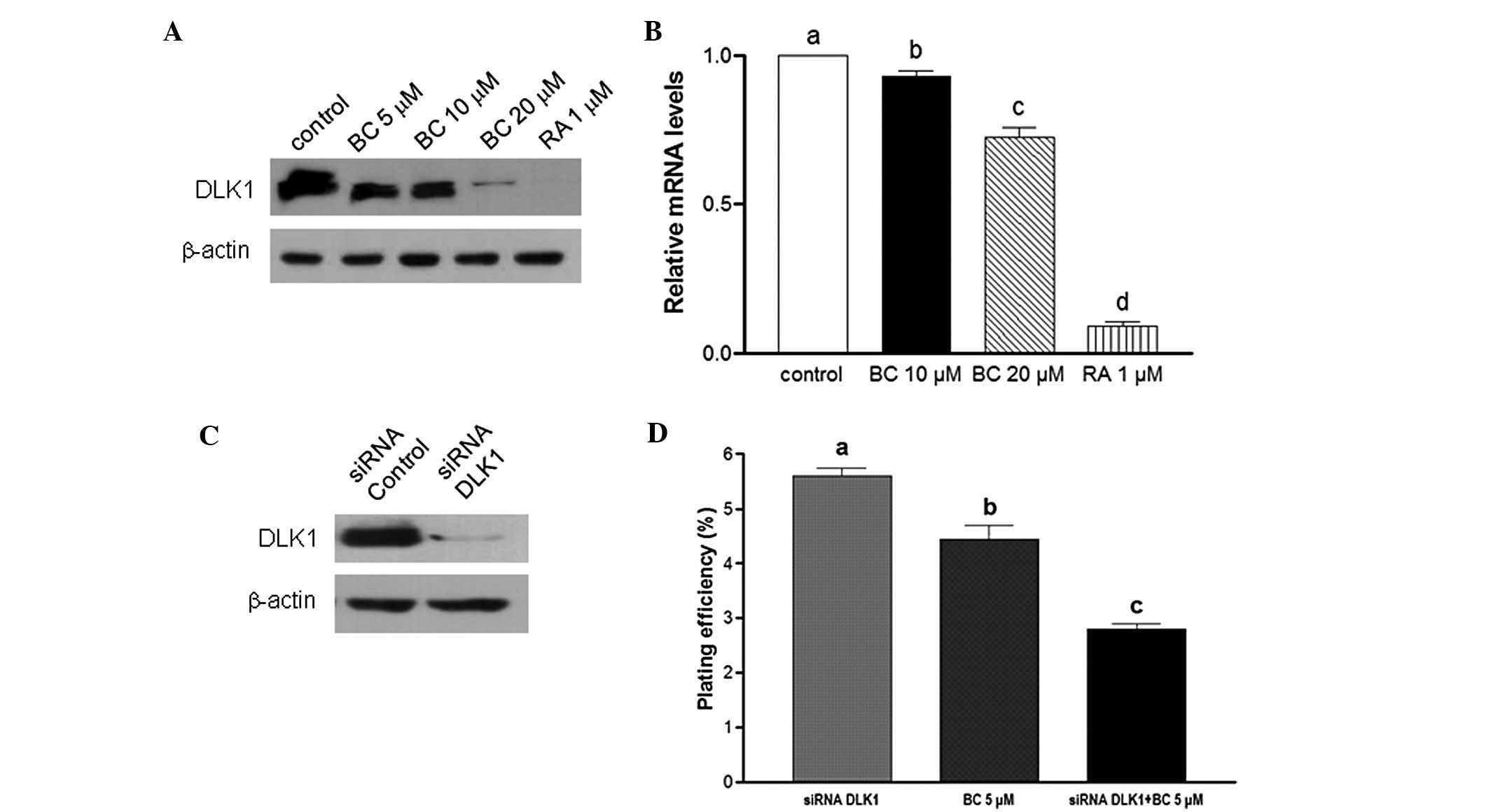
BC downregulates DLK1 expression
DLK1 is recognized as a neuronal stem cell marker
and has been hypothesized to play an important role in the growth
of stem/progenitor cells and cancer cells (20). Here, in cells treated with BC, mRNA
and protein levels of DLK1 were found to be downregulated (Fig. 5A and B). To determine whether a
combination of BC treatment and knockdown of DLK1 inhibits cancer
cell stemness, BE(2)C cells were
transfected with siRNA oligonucleotides designed to target DLK1.
Subsequently, endogenous expression of DLK1 was found to be
completely abrogated (Fig. 5C).
Moreover, in the clonogenicity assay, treatment with BC or
knockdown of DLK1 significantly inhibited colony formation (22 and
49%, respectively, P<0.05). Furthermore, the combination of BC
treatment and DLK1 knockdown resulted in a 65% inhibition in
clonogenicity compared to the control (Fig. 5D). Collectively, inhibition of DLK1
by siRNA was found to potentiate BC-mediated inhibition of the
self-renewal capacity of NB cells. Consequently, BC treatment with
DLK1 knockdown may provide a more effective chemotherapeutic effect
by regulating CSCs.
BC potentiates the anticancer effect of
cisplatin by inhibiting the self-renewal capacity of NB cells
CSCs resist conventional chemotherapeutic drugs and
are likely to play an important role in cancer relapse. Therefore,
it is important to develop cancer therapeutic agents targeting
CSCs. Furthermore, most chemotherapeutic agents have undesirable
side-effects. Therefore, various agents are administered in
combination to increase their therapeutic efficacy while decreasing
associated toxicity. Cell growth was examined using MTT assays to
assess whether BC treatment in the presence of cisplatin has an
additive effect on BE(2)C cells
(Fig. 6A and B). For these assay,
cells were treated with increasing doses of BC and cisplatin over 4
days. IC30 values calculated from the dose-response data
obtained were ~0.59 μM for cisplatin (Fig. 6A) and ~10.8 μM for BC (Fig. 1A). To evaluate the antitumor effects
of BC in combination with cisplatin, NB cells were treated with 0.5
μM cisplatin, 10 μM BC, or both. After 4 days, cell growth was
analyzed using MTT assay. Whereas treatment with BC or cisplatin
alone significantly decreased cell viability, the combination of BC
with cisplatin further suppressed NB cell growth (Fig. 6B). Furthermore, to evaluate the
effects of BC and cisplatin on the self-renewal characteristics of
CSCs, a sphere formation assay was also conducted. The combination
treatment had an additive effect on reducing sphere formation
although either BC or cisplatin showed inhibition of sphere
formation (Fig. 6C and D). These
results suggest that BC has the potential to suppress the growth of
CSCs, and the combination of BC with conventional chemotherapeutic
agents could represent a more effective regimen for the treatment
of this deadly childhood cancer.
Discussion
Neuroblastoma is the most common malignant tumor of
the neural crest and arises within the sympathetic nervous system.
In addition, the non-differentiated stem cell phenotype associated
with NB has been identified as a potential independent risk factor
for NB malignancy. Therefore, NB cells represent a model system
applicable to the study of cancer stem cell-like cells. In the
present study, treatment with BC was observed to induce the
differentiation of NB cells and to decrease the self-renewal
characteristics of CSCs. Therefore, BC may represent a novel
therapeutic strategy for the targeting of CSCs associated with NB,
thereby preventing recurrence and metastasis.
RA is a widely used chemotherapeutic drug that
induces cell differentiation. Similarly, carotenoids have also been
shown to induce cell differentiation (23,24).
For example, in a study by Gross et al(23), both BC and lutein were shown to
induce the differentiation of HL-60 cells. β-cryptoxanthin, one of
the major carotenoids present in blood, has also been shown to
induce the morphological differentiation of mouse Neuro2a cells,
in vitro(24). Prasad et
al(25) also successfully
differentiated the murine NB cell line, NBA2, by treating cells
with BC for 3 days, followed by irradiation with X-rays at a dose
of 20 Gy and higher. However, evidence regarding the
differentiation signaling induced by BC, and the efficacy of BC in
regard to CSCs, remains unclear. In this present study,
phosphorylation of ERK (p42/p44) was observed to markedly increase
following treatment with BC. Moreover, the use of specific ERK
inhibitors, PD98059 and U0126, confirmed the involvement of the
ERK/MEK1/2 pathway in BC-induced neuronal differentiation. It has
previously been proposed that U0126 directly inhibits MEK1/2
activity, whereas PD98059 is an inefficient inhibitor of MEK2
(26). The differential effects of
these inhibitors on osteogenesis and adipogenesis have also been
characterized (27). Similarly, in
a previous study of RA-induced neurite growth, growth was
completely blocked by U0126, yet only partially blocked by PD98059
(28). Collectively, these data
suggest that BC-induced neuronal differentiation requires the
inhibition of both MEK1 and MEK2. Furthermore, BC-induced neuronal
differentiation appears to be partly mediated via a pathway also
used for RA-induced neuronal differentiation. Therefore, the
mechanistic details of the regulation of BC on ERK phosphorylation,
as well as on the phosphorylation of other MAPKs require further
analysis. However, our results suggest that ERK is an important
mediator of BC-mediated neuronal differentiation.
In the present study, BC induced the neuronal
differentiation of human NB cells, and several mechanisms could
account for this observation. Folowing are examples proposed for NB
differentiation induced by BC: i) BC could be converted to RA since
BC is a precursor of RA and then cleaved via a central cleavage
pathway (29); ii) differentiation
may be induced by nuclear receptor-binding activities, subsequently
leading to the induction of several transcription factors and
signaling pathways involved in differentiation; or iii) RA
signaling could be promoted by metabolites of BC. Further studies
are needed to investigate these possibilities, as well as to
determine the contributions of BC to the differentiation of CSCs
and RA precursor function.
The self-renewal capacity of CSCs is associated with
their tumorigenic potential (4).
For example, the ability of CSCs to form colonies as well as
anchorage-independent spheres is directly proportional to the
proportion of self-renewing cells present. Correspondingly, an
evaluation of these two characteristics can determine the effect of
a particular treatment on CSCs. In the present study, NB cells were
observed to form colonies and spheroids in the absence of BC, yet
in the present of BC, the formation of colonies and spheres was
reduced. A decrease in the levels of stem cell markers, DLK1,
Notch1 and Sox2 was also noted in BC-treated cells.
DLK1 is a transmembrane protein that is expressed
extensively in immature cells with regenerative potential (30,31).
It has previously been reported that various tumors express higher
levels of DLK1 (32,33), and exogenous expression of DLK1
increases the self-renewal capacity of NB cells. In contrast,
knockdown of DLK1 has been shown to enhance neuronal
differentiation (20). In the
present study, the combination of BC treatment with knockdown of
DLK1 resulted in a significantly higher inhibition of colony
formation than treatment with either agent alone. Since DLK1 is
involved in MAPK signaling, it is hypothesized that BC and DLK1
also have roles in the same ERK pathway. Taken together, these
results suggest that BC inhibits the self-renewal capacity of CSCs.
Moreover, poorly differentiated tumors have recently been shown to
have similar gene expression profiles as normal embryonic stem
cells or progenitor cells (34,35).
However, studies are ongoing to further elucidate the mechanisms by
which stem cell genes regulate tumor progression and metastasis, as
well as the role of nutrients and tumor microenvironments.
Although initially responsive to cytotoxic
treatment, most tumors acquire a multidrug-resistant (MDR)
phenotype and relapse. CSCs are likely to play a major role in
chemotherapy resistance and cancer relapse. Therefore, it is
important to develop therapies that bypass the drug resistance of
CSCs. Currently, the most promising strategy for the treatment of
cancer is the administration of two or more drugs with different
mechanisms of action to produce synergistic, or at least additive
anticancer effects. Cisplatin was the first platinum-derived drug
produced, and is still used as a first-line therapeutic agent
against several types of cancers, including NB (36). While the chemotherapeutic effects of
cisplatin are based on the formation of an adduct with DNA and
proteins, as well as the disruption of several signaling pathways
(37,38), the administration of cisplatin is
also associated with serious side-effects, including nephrotoxicity
and peripheral neuropathy due to immune suppression (39). Due to these toxicities and
undesirable side-effects, equally effective and safer treatments
are needed. It has been reported that a combination treatment of
minocycline with BC or 13-cis-retinoic acid, along with
cisplatin, can result in decreased cytotoxicity (40). Furthermore, a mixture of three or
four vitamins containing BC showed the potential to enhance the
growth-inhibitory effects of cisplatin on melanoma cells (41). In the present study, we demonstrated
for the first time that BC can potentiate the cytotoxic effects of
cisplatin against NB CSCs. In detail, BC increased the self-renewal
activity of NB cells in vitro and mediated a reduction in
sphere formation. In the latter case, we hypothesize that this role
for BC contributed to the resensitization of CSCs to the
chemotherapeutic agent, cisplatin. Further study of the molecular
mechanisms of BC in relation to the effects of chemotherapeutic
agents are needed to confirm this hypothesis, and are warranted to
develop more potent and less toxic combination therapies. However,
our results are based on in vitro cell culture rather than
in vivo. Therefore, further in vivo studies using
animals should be performed to support and confirm the present
results.
In conclusion, the present study revealed that BC
exhibits excellent anti-CSC qualities, and can induce neuronal cell
differentiation via phosphorylation of MAPK and downregulation of
DLK1. For example, treatment with BC decreased the expression of
stem cell markers and suppressed the self-renewal characteristics
of the NB cells assayed, including their colony and spheroid
formation potential. Furthermore, our results suggest that BC may
represent a potent medical adjunct for the treatment of NB in
combination with cisplatin. However, additional studies are
warranted to elucidate the mechanisms of action of BC in regard to
both neuronal cell differentiation and inhibition of CSCs, thereby
facilitating the application of BC as a therapeutic treatment.
Acknowledgements
This research was supported by the National Research
Foundation of Korea (project no. 2011-0008169). The authors
sincerely appreciate this support.
References
|
1
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciccarone V, Spengler BA, Meyers MB,
Biedler JL and Ross RA: Phenotypic diversification in human
neuroblastoma cells: expression of distinct neural crest lineages.
Cancer Res. 49:219–225. 1989.PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on Cancer Stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
5
|
Smas CM and Sul HS: Pref-1, a protein
containing EGF-like repeats, inhibits adipocyte differentiation.
Cell. 73:725–734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore KA, Pytowski B, Witte L, Hicklin D
and Lemischka IR: Hematopoietic activity of a stromal cell
transmembrane protein containing epidermal growth factor-like
repeat motifs. Proc Natl Acad Sci USA. 94:4011–4016. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hunter T: Protein kinases and
phosphatases: the yin and yang of protein phosphorylation and
signaling. Cell. 80:225–236. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berwick DC, Calissano M, Corness JD, Cook
SJ and Latchman DS: Regulation of Brn-3a N-terminal transcriptional
activity by MEK1/2-ERK1/2 signalling in neural differentiation.
Brain Res. 1256:8–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruiz-Hidalgo MJ, Gubina E, Tull L,
Baladron V and Laborda J: dlk modulates mitogen-activated protein
kinase signaling to allow or prevent differentiation. Exp Cell Res.
274:178–188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burdon T, Smith A and Savatier P:
Signalling, cell cycle and pluripotency in embryonic stem cells.
Trends Cell Biol. 12:432–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayne ST: Beta-carotene, carotenoids, and
disease prevention in humans. FASEB J. 10:690–701. 1996.PubMed/NCBI
|
|
12
|
Comstock GW, Alberg AJ, Huang HY, et al:
The risk of developing lung cancer associated with antioxidants in
the blood: ascorbic acids, carotenoids, α-tocopherol, selenium, and
total peroxyl radical absorbing capacity. Am J Epidemiol.
168:831–840. 2008.PubMed/NCBI
|
|
13
|
Liu C, Russell RM and Wang XD: Low dose
β-carotene supplementation of ferrets attenuates smoke-induced lung
phosphorylation of JNK, p38 MAPK, and p53 proteins. J Nutr.
134:2705–2710. 2004.
|
|
14
|
Burton GW and Ingold KU: beta-Carotene: an
unusual type of lipid antioxidant. Science. 224:569–573. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tighe AP and Gudas LJ: Retinoic acid
inhibits leukemia inhibitory factor signaling pathways in mouse
embryonic stem cells. J Cell Physiol. 198:223–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fenaux P, Chevret S, Guerci A, et al:
Long-term follow-up confirms the benefit of all-trans
retinoic acid in acute promyelocytic leukemia. European APL group.
Leukemia. 14:1371–1377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kakarala M, Brenner DE, Korkaya H, et al:
Targeting breast stem cells with the cancer preventive compounds
curcumin and piperine. Breast Cancer Res Treat. 122:777–785. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Corrao S, Scaglione R, Arnone S and Licata
G: Left ventricular diastolic filling alterations in subjects with
mitral valve prolapse: a Doppler echocardiographic study. Eur Heart
J. 14:369–372. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim Y, Lin Q, Zelterman D and Yun Z:
Hypoxia-regulated delta-like 1 homologue enhances cancer cell
stemness and tumorigenicity. Cancer Res. 69:9271–9280. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Folkman J and Moscona A: Role of cell
shape in growth control. Nature. 273:345–349. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin Q, Lee YJ and Yun Z: Differentiation
arrest by hypoxia. J Biol Chem. 281:30678–30683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gross MD, Bishop TD, Belcher JD and Jacobs
DR Jr: Induction of HL-60 cell differentiation by carotenoids. Nutr
Cancer. 27:169–173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noguchi S, Sumida T, Ogawa H, Tada M and
Takahata K: Effects of oxygenated carotenoid beta-cryptoxanthin on
morphological differentiation and apoptosis in Neuro2a
neuroblastoma cells. Biosci Biotechnol Biochem. 67:2467–2469. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prasad KN, Carvalho E, Kentroti S, et al:
Production of terminally differentiated neuroblastoma cells in
culture. Restor Neurol Neurosci. 7:13–19. 1994.PubMed/NCBI
|
|
26
|
Favata MF, Horiuchi KY, Manos EJ, et al:
Identification of a novel inhibitor of mitogen-activated protein
kinase kinase. J Biol Chem. 273:18623–18632. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dang ZC and Lowik CW: Differential effects
of PD98059 and U0126 on osteogenesis and adipogenesis. J Cell
Biochem. 92:525–533. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim Y: Effect of retinoic acid and
delta-like 1 homologue (DLK1) on differentiation in neuroblastoma.
Nutr Res Pract. 4:276–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang XD and Krinsky NI: The bioconversion
of beta-carotene into retinoids. Subcell Biochem. 30:159–180. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Floridon C, Jensen CH, Thorsen P, et al:
Does fetal antigen 1 (FA1) identify cells with regenerative,
endocrine and neuroendocrine potentials? A study of FA1 in
embryonic, fetal, and placental tissue and in maternal circulation.
Differentiation. 66:49–59. 2000. View Article : Google Scholar
|
|
31
|
Tornehave D, Jensen CH, Teisner B and
Larsson LI: FA1 immunoreactivity in endocrine tumours and during
development of the human fetal pancreas; negative correlation with
glucagon expression. Histochem Cell Biol. 106:535–542. 1996.
View Article : Google Scholar
|
|
32
|
Sakajiri S, O’Kelly J, Yin D, et al: Dlk1
in normal and abnormal hematopoiesis. Leukemia. 19:1404–1410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin D, Xie D, Sakajiri S, et al: DLK1:
increased expression in gliomas and associated with oncogenic
activities. Oncogene. 25:1852–1861. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben-Porath I, Thomson MW, Carey VJ, et al:
An embryonic stem cell-like gene expression signature in poorly
differentiated aggressive human tumors. Nat Genet. 40:499–507.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phillips HS, Kharbanda S, Chen R, et al:
Molecular subclasses of high-grade glioma predict prognosis,
delineate a pattern of disease progression, and resemble stages in
neurogenesis. Cancer Cell. 9:157–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Quasthoff S and Hartung HP:
Chemotherapy-induced peripheral neuropathy. J Neurol. 249:9–17.
2002. View Article : Google Scholar
|
|
37
|
Huang H, Zhu L, Reid BR, Drobny GP and
Hopkins PB: Solution structure of a cisplatin-induced DNA
interstrand cross-link. Science. 270:1842–1845. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Donzelli E, Carfi M, Miloso M, et al:
Neurotoxicity of platinum compounds: comparison of the effects of
cisplatin and oxaliplatin on the human neuroblastoma cell line
SH-SY5Y. J Neurooncol. 67:65–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bruce AC: Cancer: Principles and practice
of oncology. Anticancer Drugs. DeVita VT, Lawrence TS and Rosenberg
SA: JB Lippincot Company; Washington: pp. 325–340. 1993
|
|
40
|
Teicher BA, Schwartz JL, Holden SA, Ara G
and Northey D: In vivo modulation of several anticancer agents by
beta-carotene. Cancer Chemother Pharmacol. 34:235–241. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prasad KN, Hernandez C, Edwards-Prasad J,
Nelson J, Borus T and Robinson WA: Modification of the effect of
tamoxifen, cis-platin, DTIC, and interferon-α 2b on human
melanoma cells in culture by a mixture of vitamins. Nutr Cancer.
22:233–245. 1994.PubMed/NCBI
|