Introduction
The natural flavonoids quercetin and rutin, and
synthetic flavonoid monoglucosyl rutin (alpha-glucosyl rutin™) are
commercially available and widely utilized in our daily diet.
Quercetin, the representative of the flavonol subclass of
flavonoids, remains a key portion of the human diet occurring in
fruits, vegetables, leaves and grains (1,2).
Quercetin and rutin are also used as a dietary antioxidant
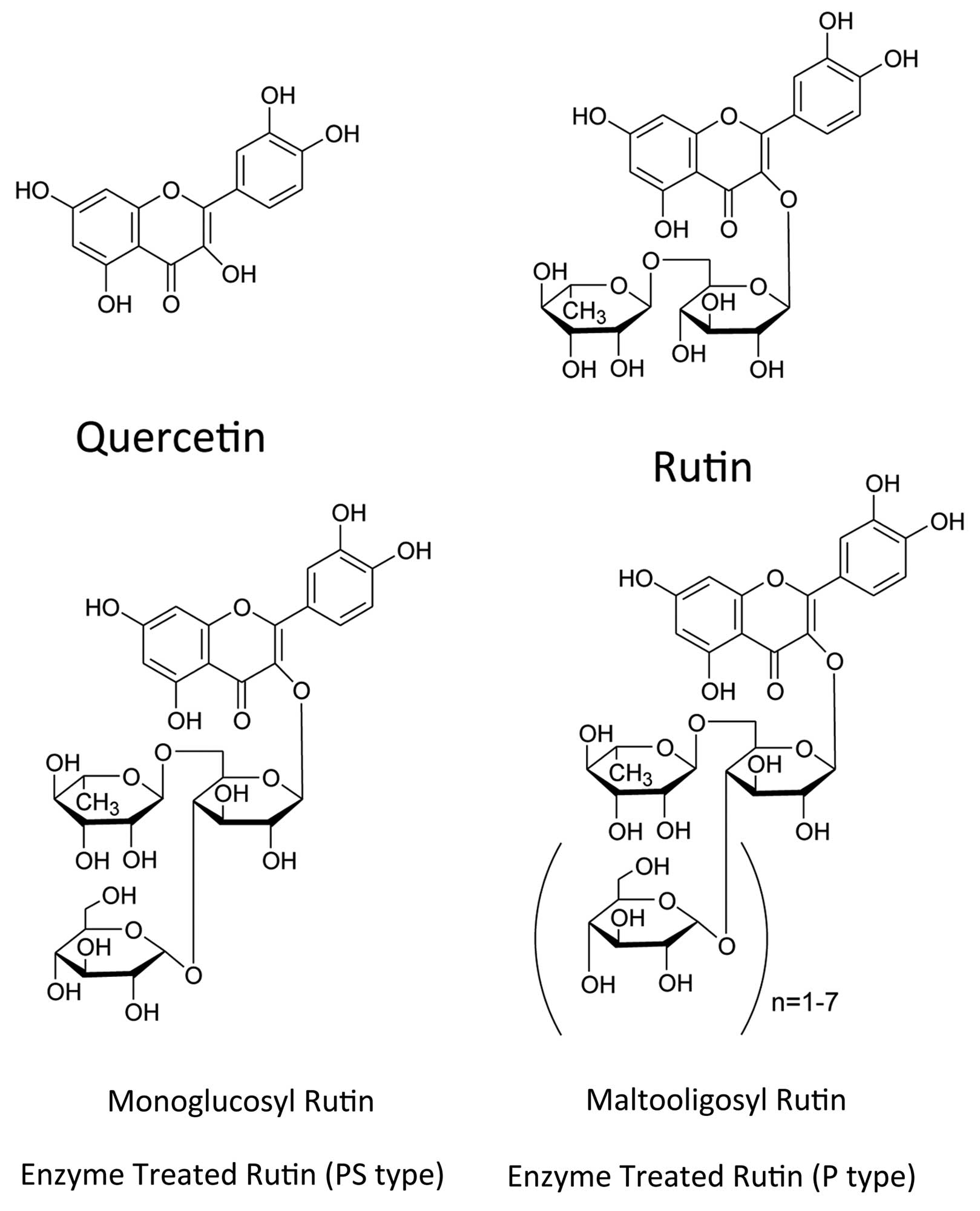
supplement in food and beverages (3). Rutin has two glucosyl residues with
quercetin, monoglucosyl rutin has three glucosyl residues, and
maltooligosyl rutin has four to seven glucosyl residues (Fig. 1) (4). In addition to the antioxidant
activity, quercetin and rutin have been identified to have multiple
inhibitory effects in mammalian cells with quercetin being used for
a variety of beneficial health effects including the treatment of
heart conditions, inflammation and diabetes (5–7).
Moreover, the role of the flavonoid potential in the antitumor
effect and cancer prevention has been studied against various types
of cancers (8). Through
investigating apoptotic effects of quercetin in human myeloid
leukemia cells, this flavonoid proved to induce the
poly(ADP-ribose) polymerase (PARP) inhibitory effect (9).
Synthetic lethality where cell death occurs due to
the combination of a gene and small molecule has been a focus in
cancer therapy (10). Studies have
suggested synthetic lethal interaction between PARP inhibition and
BRCA deficiency (11). In
vitro and in vivo evidence has supported the utilization
of PARP inhibitors as single agents to reduce cancer cells that
possess a defect in DNA repair that additionally have BRCA1 and
BRCA2 mutations (12). PARP is an
enzyme to form large poly(ADP-ribose) polymers in live cells that
have DNA damage (13). PARP plays a
significant role in single strand DNA break repair that leads to no
repair occurring if there is a defect in homologous recombination
repair (HRR) present with synthetic lethality of PARP inhibitors in
HRR-defective cells. PARP inhibitors render HRR-defective cells
unable to maintain their genome during replication and die
(14,15). Currently, more than five PARP
inhibitors are employed in clinical trial development (16).
Heterozygous mutation of BRCA1 or BRCA2 causes
hereditary breast and ovarian cancer syndromes at an early age and
increases the chance of bilateral cancers (17,18).
This gene mutation is transmitted in an autosomal dominant pattern
in families (19). In the general
population this gene mutation is rare; in the US, one in 400–800
individuals have the BRCA1 or BRCA2 heterozygous mutations
(20). However, BRCA1 and BRCA2
mutations are dangerous mutations that produce a hereditary breast
ovarian cancer at an estimation of 40.7% in these mutant carriers
(21,22). The focus of the present study was
the impact of flavonoids on PARP when combined with BRCA mutated
cells. We tested the activities of PARP inhibition with variations
of glucosyl modifications of quercetin by analyzing PARP inhibition
assay in vitro and poly(ADP-ribose) immunostaining. We
investigated the existence of synthetic lethality between BRCA2
mutants and flavonoids through the cytotoxicity assay along with
the γ-H2AX foci experiment.
Materials and methods
Cell culture
Chinese hamster ovary (CHO) cells, Chinese hamster
lung V79 cells wild-type and its BRCA2 mutant (V-C8) (23), and V-C8 hBRCA2, genetically
complimented mutant with human BRCA2 cDNA were cultured in Minimum
Essential Medium (MEM)-α (Gibco, Indianapolis, IN, USA) and
supplemented with 10% fetal bovine serum (FBS; Sigma, St. Louis,
MO, USA) and 1% antibiotics and antimycotics (Gibco). They were
maintained at 37°C in a humidified atmosphere of 5% CO2
in air.
Chemicals
Quercetin, rutin, monoglucosyl rutin and
maltooligosyl rutin were provided from Toyo Sugar Refining Co.,
Ltd. (Tokyo, Japan) (Fig. 1).
AlphaGrutinPS™ and alphaGrutinP™ are trademarks of Toyo Sugar
Refining. Quercetin was prepared in dimetyl sulfoxide (DMSO) for a
1% (w/v) solution. Rutin was prepared in DMSO for 10% (w/v)
solution. Monoglucosyl rutin and maltooligosyl rutin were prepared
in phosphate-buffered saline (PBS) for 10% (w/v) solution. For the
positive control, 3-aminobenzamide (Trevigen, Gaithersburg, MD,
USA) was utilized. The concentrations testing in the present study
were in the range from 0.00001 to 1% (w/v).
PARP inhibitor assay in vitro
PARP inhibitory ELISA kit from Trevigen was used for
the present study (24). PARP was
incubated in a 96-well microplate with a reaction mixture
containing 50 μM β-NAD+ (10% biotinylated
β-NAD+), 90% unlabeled β-NAD+, 1 mM
1,4-dithiothreitol and 1.25 mg/l nicked DNA. The formation of the
poly(ADP-ribose) polymers was detected with peroxidase-labeled
streptavidin (Invitrogen, Grand Island, NY, USA) and
3,3′,5,5′-tetramethylbenzidine. PARP-1 activity was expressed as
absorbance at 450 nm. PARP-1 inhibition of flavonoids was evaluated
by addition of these compounds to the reaction mixture. NanoDrop
spectrophotometer (Thermo Fischer Scientific, Waltham, MA,
USA)measured absorbance.
Poly(ADP-ribose) immunostaining
The suppression of poly(ADP-ribose) formation by
flavonoids was investigated by immunostaining. Cultured CHO cells
were treated with natural and synthetic flavonoids for 30 min to
one day. H2O2 (2 mM) was added for 10 min
before fixation. Cells were fixed in 4% paraformaldehyde for 15 min
and treated in 0.2% Triton X-100 solution for 10 min, following
overnight blocking in PBS with 10% goat serum. Immunostaining was
carried out with anti-poly(ADP-ribose) monoclonal antibody (BD
Biosciences, San Jose, CA, USA) (25) with 1:300 dilution. Poly(ADP-ribose)
formation was analyzed under Zeiss Axioplan fluorescence microscope
(Carl Zeiss, Oberkochen, Germany) with QImaging EXi Aqua digital
camera (QImaging, Surrey, BC, Canada).
Cytotoxicity test by colony formation
assay
Exponentially growing cells were trypsinized and
plated on P-60 dishes to obtain ~100 colonies per dish. Different
concentrations of natural and synthetic flavonoids were added. Ten
days later, cells were fixed in 100% ethanol and stained by 0.1%
Crystal violet solution (Sigma). Colonies containing >50 cells
were counted as survivors.
γ-H2AX foci formation
Following treatment of V79 and mutant cells with
flavonoids, cells were treated as poly(ADP ribose) immunostaining.
The primary antibody utilized was mouse monoclonal γ-H2AX antibody
(Millipore, Billerica, MA, USA) in 1:300 dilution. Secondary
antibodies used were Alexa 488 Fluor-conjugated goat anti-mouse
antibody (Invitrogen). DNA was counterstained by DAPI
(4′,6-diamidino-2-phenylindole) with Prolong Gold Antifade
(Invitrogen). Images were captured and analyzed with Zeiss Axioplan
fluorescence microscope with QImaging EXI Aqua digital camera.
Nuclei containing >100 foci per cell were scored as massive DNA
damage from synthetic lethality.
Statistics
All experiments were carried out at least three
times and error bars indicate standard error of the means. Analysis
of variance was used to determine statistical significance with
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). For
all analyses, P-values of <0.05 were considered to indicate a
statistically significant result.
Results
In vitro ELISA assay for PARP
activity
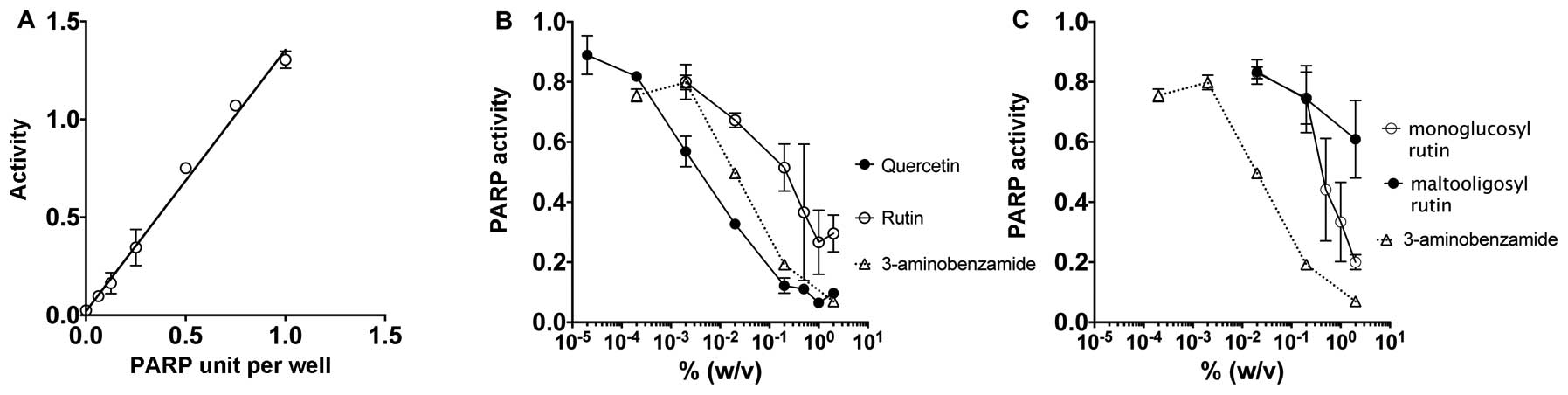
The in vitro assay for PARP activity was
carried out with a screening of PARP inhibiting assay through the
measurement of polyADP-ribosylation of histone proteins (Fig. 2A–C). 3-aminobenzamide was utilized
as the positive control based on its popularity as a PARP
inhibitor. Fig. 2A shows the
standard curve of the PARP activity that demonstrates a linear
relationship with increasing concentration. Fig. 2B and C demonstrate a dose-dependent
relationship where an increase in dosage for all four flavonoid
drugs and the positive control 3-aminobenzamide results in
decreased PARP activity. Quercetin displayed the strongest
inhibitory effects on PARP. The two-glucosyl residues that form
rutin from the quercetin structure dramatically changed PARP
inhibitory effects, however, rutin still carried PARP inhibitory
effect. Rutin displayed a greater PARP inhibitory effect than
monoglucosyl rutin with three glucosyl residues and maltooligosyl
rutin with up to seven glucosyl residues.
Inhibition of PARP in vivo
The PARP activity inhibition by flavonoids was
confirmed by immunostaining to detect poly(ADP-ribose) formation
with CHO cells (Fig. 2D–F). Clear
poly(ADP-ribose) formation in CHO wild-type cells was observed
after 10 min of H2O2 (2 mM) treatment under a
fluorescence microscope (Fig. 2D and
E). On the other hand, pretreatment of quercetin [0.01% (w/v),
0.6 mM] inhibited H2O2 induced
poly(ADP-ribose) formation in the CHO cells (Fig. 2F). Although other flavonoids did not
suppress poly(ADP-ribose) formation as well as quercetin, overnight
pretreatment of rutin, monoglucosyl rutin and maltooligosyl rutin
showed inhibition of the poly(ADP-ribose) formation.
Synthetic lethality in BRCA2 mutant cells
with drug treatment
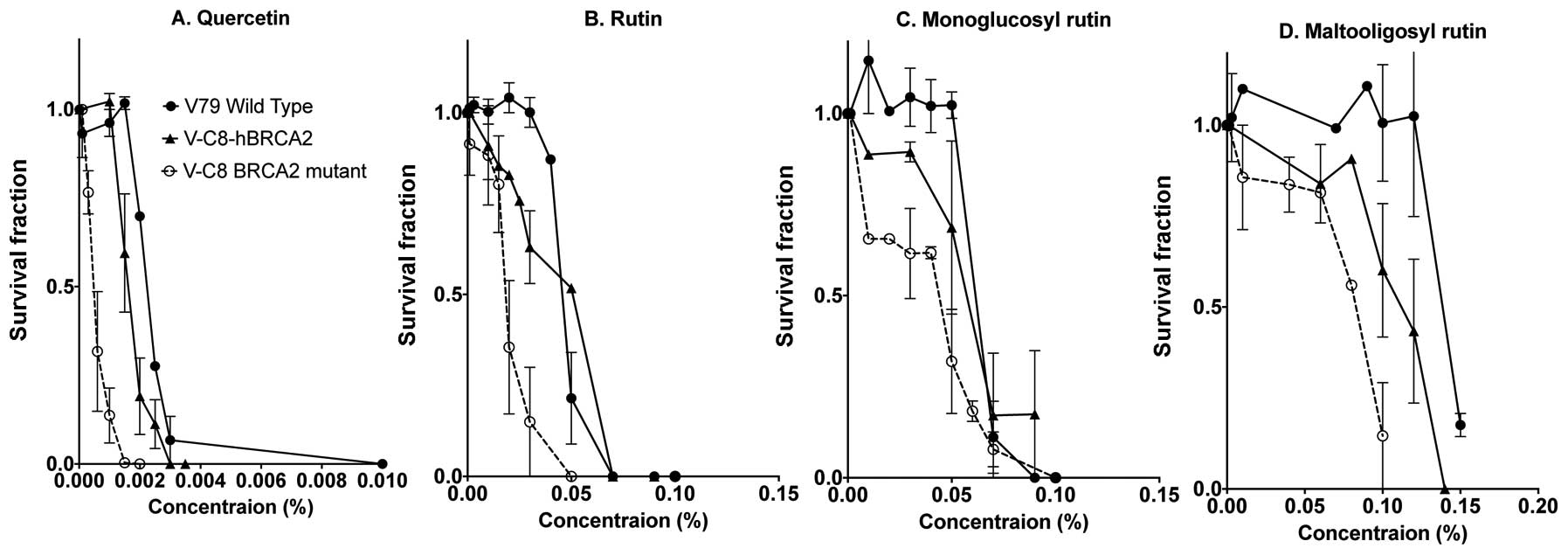
Cytotoxicity studies were carried out with V79
wild-type and its BRCA2 mutant (V-C8) and human BRCA2 gene
complimented V-C8 (V-C8 hBRCA2) cells (Fig. 3). Each quercetin and the three
variations of glucosyl modifications of rutin, monoglucosyl rutin
and maltooligosyl rutin were continuously present in media during
colony formation. Quercetin showed the highest cytotoxicity among
the four flavonoids. V-C8 cells exposed to flavonoids possessed a
higher lethality to the survival fraction with a lower
concentration in comparison with V79 wild-type and genetically
complimented V-C8 hBRCA2 cells. The concentration required for 90%
cell death for quercetin was <0.003% (0.1 mM), rutin was at a
concentration of 0.06%, monoglucosyl rutin increased to ~0.075%,
and maltooligosyl rutin had the highest concentration at 0.15% for
V79 cells. It was also apparent that additional glucosyl residues
reduced cytotoxicity for V79 wild-type, V-C8 and V-C8 hBRCA2
cells.
DNA double strand breaks from synthetic
lethality
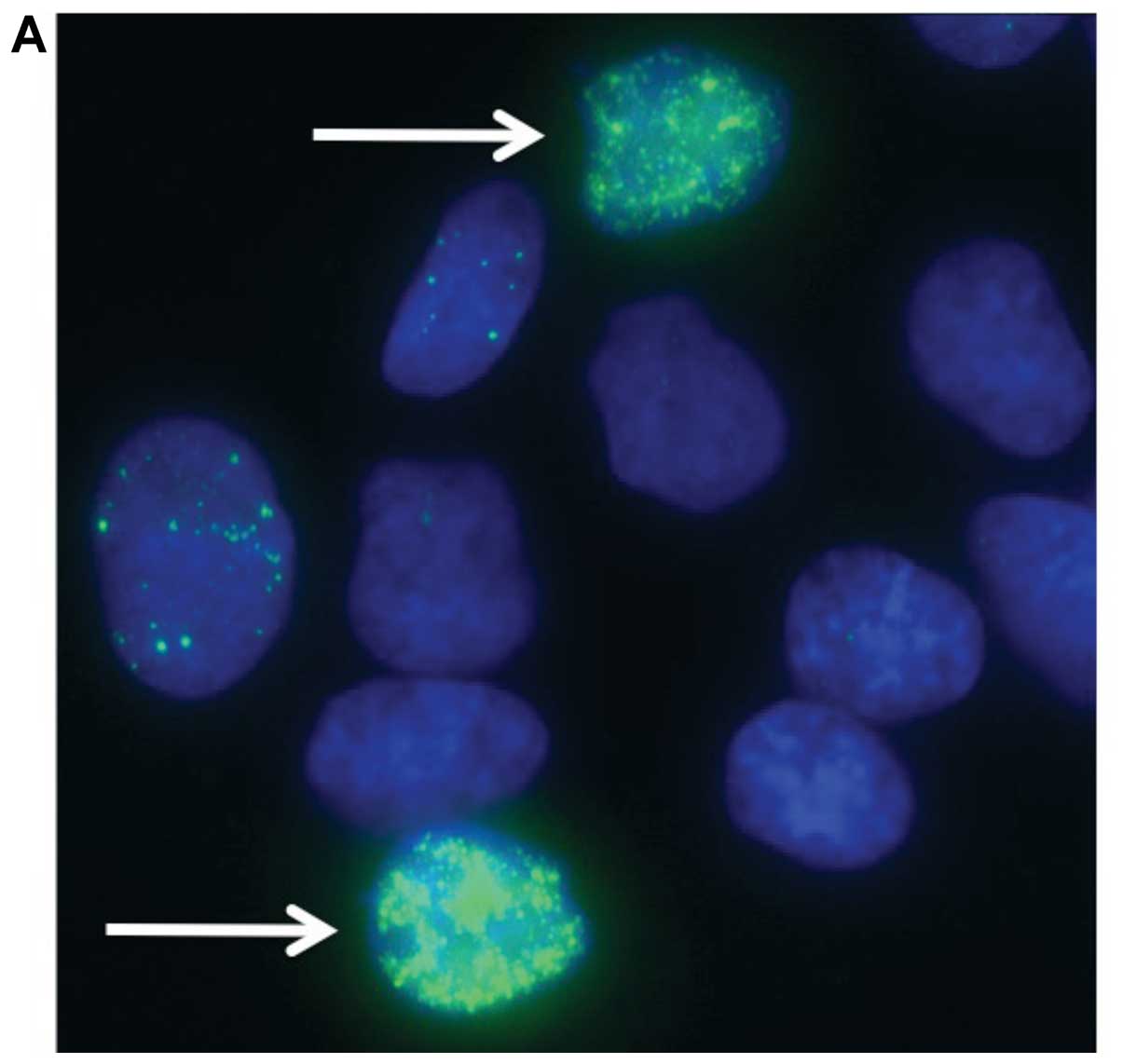
To assess DNA damage from synthetic lethality, we
used γ-H2AX foci, a marker of DNA double strand breaks (Fig. 4) (26). V79 wild-type cells, its BRCA2 mutant
(V-C8) and genetically complimented mutant with human BRCA2 (V-C8
hBRCA2) were exposed each to 0.01% flavonoid for 12 h. Synthetic
lethality induced DNA damage was observed in pan nuclear region and
clearly distinguished from background level focus (Fig. 4A). The fraction of population having
>100 nuclear foci was scored as massive DNA damage from
synthetic lethality (Fig. 4B). The
V-C8 cells displayed the greatest fraction of cells displaying
massive DNA damage compared with V79 in the four drug treatments.
Human BRCA2 complementation to V-C8 cells was visible in the drug
treatments of quercetin and rutin. The wild-type cells possessed
fewer number of massive DNA damage in the four drug exposures.
Quercetin demonstrated the greatest effect regarding DNA damage on
the three cell lines. Rutin had a less significant effect in
comparison with quercetin in all three cell lines. Monoglucosyl
rutin and maltooligosyl rutin did not cause an equivalent amount of
damage to cells in comparison with quercetin and rutin.
Discussion
Our studies showed that the natural and glucosylated
flavonoids quercetin, rutin, monoglucosyl rutin and maltooligosyl
rutin inhibited PARP activity and induced synthetic lethality in
BRCA2 deficient Chinese hamster cells (Figs. 2 and 3). Synthetic lethality was observed
through the greatest production of DNA double strand breaks from
the combination of BRCA2 deficient V-C8 cells with PARP inhibition
through flavonoid exposure in comparison with the other two BRCA2
proficient cell lines (Fig. 4).
Quercetin demonstrated the greatest effect regarding PARP
inhibition in conjunction with V-C8 cells in comparison with
flavonoids that possessed glucosyl modifications, which speaks to
the lethality occurring in a glucosyl-dependent manner.
The observed effect of quercetin and glucosyl
flavonoids in BRCA2 mutant cells as a result of another pathway
than PARP inhibition has to be formally considered. For example, we
can not exclude that previous studies have identified flavonoid
cytotoxicity on cells as a result of increasing intracellular
reactive oxygen species levels or inhibiting DNA topoisomerase
(27,28). However, synthetic lethality effect
is likely because we confirmed the inhibition of PARP both in
vitro and in cells, and the flavonoids selectively targeted
BRCA2 mutated cells, which is consistent with a cytotoxic mechanism
involving the conversion of single strand breaks into double strand
breaks during DNA replication that cannot be repaired efficiently
in cells with an HRR defect. Thus, the activity of flavonoids was
clearly dependent on BRCA2 dysfunction and represents a new
clinical application for BRCA2 mutant in breast cancer.
The modification of physiochemical and biological
properties of flavonoid structures is of great scientific and
industrial interest. Our observations suggest that the efficacy of
the flavonoids as PARP inhibitors appears to result from the number
of glucosyl residues (Figs. 2A–C
and 3). Monoglucosyl rutin and
maltooligosyl rutin act as PARP inhibitors, but are not as
effective as quercetin. These quercetin glycosides showed higher
solubility in water than quercetin due to the hydrophilicity of the
sugar moieties, suggesting that the conjugation with glucose
enhances quercetin absorption in small intestine (4). In contrast, rutin, which has
glycosides, has been found to not incorporate well into the cells
(29). One possibility is that the
higher hydrophilicity of rutin and glucosylated rutin caused them
to be barely incorporated into the cells and, therefore, showed
less effect in the present study. It is also possible that glycosyl
residues might block PARP inhibitory activity. As we have shown in
in vitro assay for PARP activity (Fig. 2A–C), quercetin glucosyl derivatives
demonstrated a lower PARP inhibitory activity with increased number
of glucosyl residues in comparison with quercetin. We assume these
additional glucoses would decrease PARP inhibitory activity,
however, a longer exposure duration would allow cells to uptake and
digest the glucosyl residues.
Although there are many in vitro and in
vivo studies related to flavonoid toxic and mutagenic
properties, it remains a vital part of drug exposure to know the
possible genotoxicity and cytotoxicity for natural or synthetic
flavonoids (1). In earlier studies,
the potential mutagenic effects of flavonoids have been reported,
including sister chromatid exchanges and bacterial system tests
(30,31). Several reports supported the absence
of dietary quercetin-related carcinogenicity and toxicity in
vivo (1). While previous animal
studies have demonstrated that following oral consumption of
quercetin, the plasma levels of total quercetin (free and
conjugated) were quite high (0.05 mM), the free un-conjugated
quercetin circulated in plasma at extremely low concentrations. We
observed considerable cytotoxicity of quercetin in wild-type cells
at relatively high concentrations (0.1 mM) and in a dose-dependent
manner. These results are consistent with previous reports of
observed flavonoid cytotoxicity in wild-type CHO and non-cancerous
human cell lines (27,32). Even better cytotoxicity to BRCA2
mutated cells have been achieved by sufficient active flavonoid
concentrations; exposure of high dose of genotoxic flavonoids may
have unwanted effects to the healthy tissues.
Currently, oral intake of PARP inhibitors has shown
a favorable therapeutic score for breast cancer with acceptably low
toxicity in women with the BRCA1 and BRCA2 mutation (33). Moreover, the role of PARP inhibitors
for chemoprevention for breast and ovarian cancer in BRCA1 and
BRCA2 mutation carriers has been proposed. However, caution should
be exercised for long-term use of these drugs in the light of the
induction of resistance, and especially for the lack of knowledge
of the effects of long-term inhibition of PARP. We propose that the
new natural agents may have the potential of reducing cancer risk
by the elimination of BRCA1 or BRCA2 homozygous mutated cancers.
The present study provides support for the clinical use of dietary
flavonoids, and is the beginning of the potential application
regarding cancer prevention and potentially periodic consumption of
appropriate flavonoids.
Acknowledgements
We would like to thank the Dr Akiko M. Ueno
Radiation Biology Research Fund, the Toyo Sugar Refining Co., Ltd.,
Research Fund and the Technology Fee Stipend Student Experimental
Learning Fund in College of Veterinary Medicine and Biosciences in
Colorado State University for helping support our research and
making this project a possibility.
References
|
1
|
Harwood M, Danielewska-Nikiel B,
Borzelleca JF, Flamm GW, Williams GM and Lines TC: A critical
review of the data related to the safety of quercetin and lack of
evidence of in vivo toxicity, including lack of
genotoxic/carcinogenic properties. Food Chem Toxicol. 45:2179–2205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Day AJ and Williamson G: Biomarkers for
exposure to dietary flavonoids: a review of the current evidence
for identification of quercetin glycosides in plasma. Br J Nutr.
86(Suppl 1): S105–S110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: from antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsukawa N, Matsumoto M, Chiji H and Hara
H: Oligo-saccharide promotes bioavailability of a water-soluble
flavonoid glycoside, αG-rutin, in rats. J Agric Food Chem.
57:1498–1505. 2009.PubMed/NCBI
|
|
5
|
Chen YW, Chou HC, Lin ST, et al:
Cardioprotective effects of quercetin in cardiomyocyte under
ischemia/reperfusion injury. Evid Based Complement Alternat Med.
2013:3645192013.PubMed/NCBI
|
|
6
|
Bakhshaeshi M, Khaki A, Fathiazad F, Khaki
AA and Ghadamkheir E: Anti-oxidative role of quercetin derived from
Allium cepa on aldehyde oxidase (OX-LDL) and hepatocytes
apoptosis in streptozotocin-induced diabetic rat. Asian Pac J Trop
Biomed. 2:528–531. 2012.PubMed/NCBI
|
|
7
|
Lee KH and Yoo CG: Simultaneous
inactivation of GSK-3β suppresses quercetin-induced apoptosis by
inhibiting the JNK pathway. Am J Physiol Lung Cell Mol Physiol.
304:L782–L789. 2013.
|
|
8
|
Romano B, Pagano E, Montanaro V, Fortunato
AL, Milic N and Borrelli F: Novel insights into the pharmacology of
flavonoids. Phytother Res. July.4–2013.(Epub ahead of print).
|
|
9
|
Duraj J, Zazrivcova K, Bodo J, Sulikova M
and Sedlak J: Flavonoid quercetin, but not apigenin or luteolin,
induced apoptosis in human myeloid leukemia cells and their
resistant variants. Neoplasma. 52:273–279. 2005.PubMed/NCBI
|
|
10
|
Nijman SMB: Synthetic lethality: general
principles, utility and detection using genetic screens in human
cells. FEBS Lett. 585:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bryant HE, Schultz N, Thomas HD, et al:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Basu B, Sandhu SK and de Bono JS: PARP
inhibitors: mechanism of action and their potential role in the
prevention and treatment of cancer. Drugs. 72:1579–1590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poitras MF, Koh DW, Yu SW, et al: Spatial
and functional relationship between poly(ADP-ribose) polymerase-1
and poly(ADP-ribose) glycohydrolase in the brain. Neuroscience.
148:198–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polyak K and Garber J: Targeting the
missing links for cancer therapy. Nat Med. 17:283–284. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dedes KJ, Wilkerson PM, Wetterskog D,
Weigelt B, Ashworth A and Reis-Filho JS: Synthetic lethality of
PARP inhibition in cancers lacking BRCA1 and BRCA2 mutations. Cell
Cycle. 10:1192–1199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peralta-Leal A, Rodriguez-Vargas JM,
Aguilar-Quesada R, et al: PARP inhibitors: new partners in the
therapy of cancer and inflammatory diseases. Free Radic Biol Med.
47:13–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brandt A, Lorenzo Bermejo J, Sundquist J
and Hemminki K: Breast cancer risk in women who fulfill high-risk
criteria: at what age should surveillance start? Breast Cancer Res
Treat. 121:133–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iqbal J, Ragone A, Lubinski J, et al: The
incidence of pancreatic cancer in BRCA1 and BRCA2 mutation
carriers. Br J Cancer. 107:2005–2009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Christopoulou A and Spiliotis J: The role
of BRCA1 and BRCA2 in hereditary breast cancer. Gene Ther Mol Biol.
10A:95–99. 2006.
|
|
20
|
Whittemore AS, Gong G, John EM, et al:
Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic
Whites. Cancer Epidemiol Biomarkers Prev. 13:2078–2083.
2004.PubMed/NCBI
|
|
21
|
Mavaddat N, Barrowdale D, Andrulis IL, et
al: Pathology of breast and ovarian cancers among BRCA1 and BRCA2
mutation carriers: results from the Consortium of Investigators of
Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev.
21:134–147. 2012. View Article : Google Scholar
|
|
22
|
Teng LS, Zheng Y and Wang HH: BRCA1/2
associated hereditary breast cancer. J Zhejiang Univ Sci B.
9:85–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verhaegh GW, Jongmans W, Morolli B, et al:
A novel type of X-ray-sensitive Chinese hamster cell mutant with
radioresistant DNA synthesis and hampered DNA double-strand break
repair. Mutat Res. 337:119–129. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geraets L, Moonen HJ, Brauers K, Wouters
EF, Bast A and Hageman GJ: Dietary flavones and flavonoles are
inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial
cells. J Nutr. 137:2190–2195. 2007.PubMed/NCBI
|
|
25
|
Kanai Y, Tanuma S and Sugimura T:
Immunofluorescent staining of poly(ADP-ribose) in situ in HeLa cell
chromosomes in the M phase. Proc Natl Acad Sci USA. 78:2801–2804.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuo LJ and Yang LX: γ-H2AX - a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–309.
2008.
|
|
27
|
Matsuo M, Sasaki N, Saga K and Kaneko T:
Cytotoxicity of flavonoids toward cultured normal human cells. Biol
Pharm Bull. 28:253–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boege F, Straub T, Kehr A, et al: Selected
novel flavones inhibit the DNA binding or the DNA religation step
of eukaryotic topoisomerase I. J Biol Chem. 271:2262–2270. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spencer JP, Abd-el-Mohsen MM and
Rice-Evans C: Cellular uptake and metabolism of flavonoids and
their metabolites: implications for their bioactivity. Arch Biochem
Biophys. 423:148–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishikawa M, Oikawa T, Hosokawa M, Hamada
J, Morikawa K and Kobayashi H: Enhancing effect of quercetin on
3-methylcholanthrene carcinogenesis in C57Bl/6 mice. Neoplasma.
32:435–441. 1985.PubMed/NCBI
|
|
31
|
Venskutonis PR, Dedonyte V, Lazutka J, et
al: A preliminary assessment of singlet oxygen scavenging,
cytotoxic and genotoxic properties of Geranium macrorrhizum
extracts. Acta Biochim Pol. 57:157–163. 2010.PubMed/NCBI
|
|
32
|
Nakayama T, Yamada M, Osawa T and
Kawakishi S: Suppression of active oxygen-induced cytotoxicity by
flavonoids. Biochem Pharmacol. 45:265–267. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tutt A, Robson M, Garber JE, et al: Oral
poly(ADP-ribose) polymerase inhibitor olaparib in patients with
BRCA1 or BRCA2 mutations and advanced breast cancer: a
proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar
|