Introduction
Recent advancements in imaging techniques include
positron emission tomography (PET) with [18F]
2-fluoro-2-deoxy-D-glucose (FDG), which has been widely used for
clinical molecular imaging (1,2).
Several studies have described its usefulness in detecting primary
tumors and metastases for several types of cancer (3,4). In
particular, previous studies reported the increased FDG uptake in
hepatocellular carcinoma (HCC) lesions (5). However, the sensitivity of HCC
detection with FDG-PET is only 50–55% (6–8), which
is significantly lower than the 98% detection rate for liver
metastasis from colorectal cancer (9). FDG-PET is therefore less useful for
detecting primary HCC lesions than conventional ultrasonography or
computed tomography.
Nevertheless, FDG-PET has received considerable
attention as a new modality with the ability to estimate the
malignant potential of tumors. Several studies have demonstrated
that FDG accumulation reflects tumor aggressiveness and predicts
poor survival (10–12). Therefore, FDG-PET has been used to
evaluate the malignant potential of tumors after chemotherapy
(13). New molecular-targeted
cancer therapies [such as vascular endothelial growth factor
inhibitor (bevacizumab) and epidermal growth factor receptor
inhibitor (cetuximab)] require new methods for monitoring treatment
progress (14), as they exert
cytostatic instead of the cytoreductive effects of traditional
chemotherapy. Compared with computed tomography, FDG-PET provides
information on tumor response to bevacizumab therapy earlier
(15). The multiple kinase
inhibitor sorafenib is a new molecular-targeted therapy approved
for the treatment of HCC. Given the strong FDG uptake observed in
HCC tumors, FDG-PET may be useful for evaluating the cytostatic
effects of this type of therapy (16).
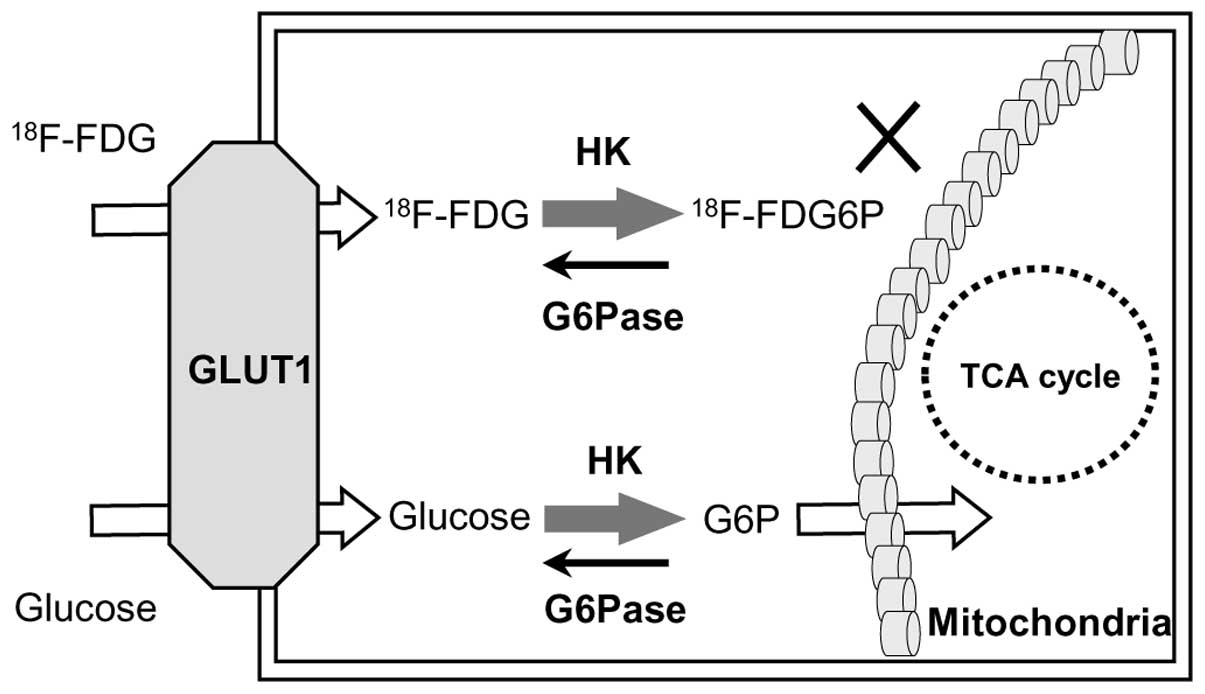
The molecular mechanisms involved in FDG imaging
relate to its uptake by glucose transporter 1 (GLUT1) and
metabolism by hexokinase (HK) and glucose-6-phosphatase (G6Pase).
FDG accumulates in malignant cells via GLUT1 transport and HK
phosphorylation, as shown in Fig. 1
(17). G6Pase, a gluconeogenesis
enzyme strongly expressed in the liver (17,18),
counteracts HK phosphorylation by converting glucose-6-phosphate to
glucose. High G6Pase levels therefore reduce FDG accumulation by
accelerating the conversion of FDG-6-phosphate to FDG, leading to
its release from cells.
Although previous studies have evaluated the
molecular mechanisms underlying FDG-PET, most relied on
non-quantitative immunohistochemistry analysis such as positive or
negative staining. Since interpretation of these results is
subjective, it is difficult to determine the precise relationship
between the standardized uptake value (SUV) and glucose
metabolism-related protein levels. To determine the mechanisms
responsible for low FDG-PET efficacy in HCC, we evaluated glucose
metabolism-related protein expression in HCC with that of another
type of liver cancer (liver metastasis from colorectal cancer)
using quantitative reverse-transcription polymerase chain reaction
(qRT-PCR). We then evaluated the relationship between protein
expression and SUV in each type of liver cancer.
Materials and methods
Patients
The present study included 34 patients with liver
cancer (21 male and 13 female; mean age ± SEM, 67.6±1.7 years) who
underwent FDG-PET prior to surgery in our hospital. Of these
patients, 20 had HCC (HCC group) and 14 had liver metastasis from
colorectal cancer (Meta group). Specimens consisting of the tumors
and surrounding normal liver tissue were immediately snap-frozen in
liquid nitrogen after surgical resection and stored at −80ºC. These
specimens were later thawed for isolation and analysis of mRNA
levels. Of the 20 HCC cases, 16 were moderately differentiated
(M/D) HCC, and four were poorly differentiated (P/D) HCC. The
protocol was approved by the institutional review board and all
patients provided written informed consent.
PET imaging
FDG-PET images were acquired with a PET scanner
(ECAT EXACT HR+, Siemens/CTI, Knoxville, TN, USA).
Patients fasted at least 5 h before FDG injection. Images were
reviewed on a Sun Microsystems workstation (Siemens/CTI) along
transverse, coronal, and sagittal planes with maximum intensity
projection images. The images were interpreted independently by two
experienced nuclear medicine physicians blinded to the clinical
data. Tumor lesions were identified as areas of focally increased
FDG uptake exceeding that of the surrounding normal tissue. A
region of interest was placed over each lesion to include the
highest levels of radioactivity. The maximum SUV was calculated
with the following formula: SUV = cdc/(di/w), where cdc is the
decay-corrected tracer tissue concentration (Bq/g), di is the
injected dose (Bq), and w is the body weight of the patient
(g).
Immunohistochemical staining
Immunohistochemical staining was performed to
determine levels of GLUT1 and GLUT2 in the liver cancer specimens.
Briefly, resected specimens were fixed in 10% buffered formalin
solution, embedded in paraffin, and sectioned (4 μm). Slides were
incubated overnight at room temperature with primary rabbit
polyclonal antibodies against GLUT1 or GLUT2 (1:200 dilution).
Avidin-biotin-peroxidase complex staining was performed according
to the manufacturer’s instructions (Santa Cruz Biotechnology, Santa
Cruz, CA, USA). Finally, the nuclei were counterstained with
hematoxylin.
qRT-PCR
Total RNA was isolated from the specimens by
guanidinium isothiocyanate-acid phenol extraction and quantified by
absorbance at 260 nm. Reverse transcription was carried out with 1
μg RNA, and the resulting cDNA was analyzed by real-time PCR with
Power SYBR Green PCR Master Mix and the ABI Prism 7000 (Applied
Biosystems, Foster, CA, USA). Target-specific oligonucleotide
primers and probes were previously described (19,20).
As endogenous control, the amplification of 18S rRNA was used. The
relative value of mRNA expression indicates the ratio of the mRNA
expression to mean mRNA levels in normal liver after 18S rRNA
normalization. Primers and probes for 18S rRNA were obtained in a
Pre-Developed TaqMan Assay Reagent kit (Applied Biosystems,
Stockholm, Sweden).
Statistical analysis
Results are expressed as mean ± SEM. SUV values were
compared by Student’s t-test. Multiple one-way analysis of variance
(ANOVA) was used to assess differences in mRNA levels. Correlation
analyses were performed with the Spearman’s rank correlation test.
P-values <0.05 were considered to indicate statistically
significant differences.
Results
Tumor detection and SUV with FDG-PET
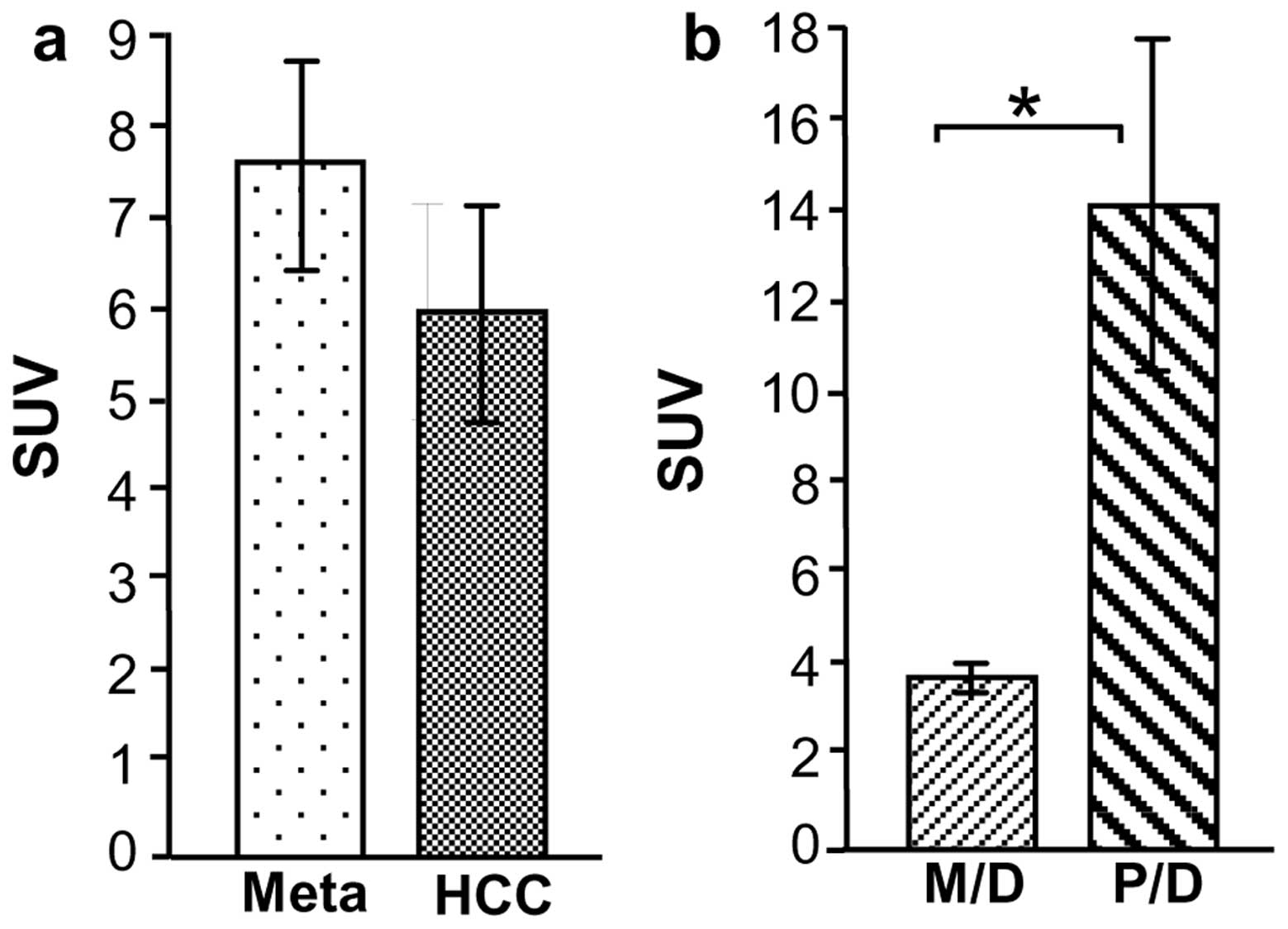
FDG-PET detected 15/20 HCC lesions (SUV, 6.1±1.2)
and 13/14 Meta lesions (SUV, 7.7±1.1) (Fig. 2a). Of the HCC cases, all four P/D
HCC lesions were detected by FDG-PET with a high mean SUV
(14.4±3.7). By contrast, 11/16 M/D HCC lesions were detected by
FDG-PET, and the mean SUV (4.0±0.3) was significantly lower than
that of P/D HCC (P<0.0001) (Fig.
2b).
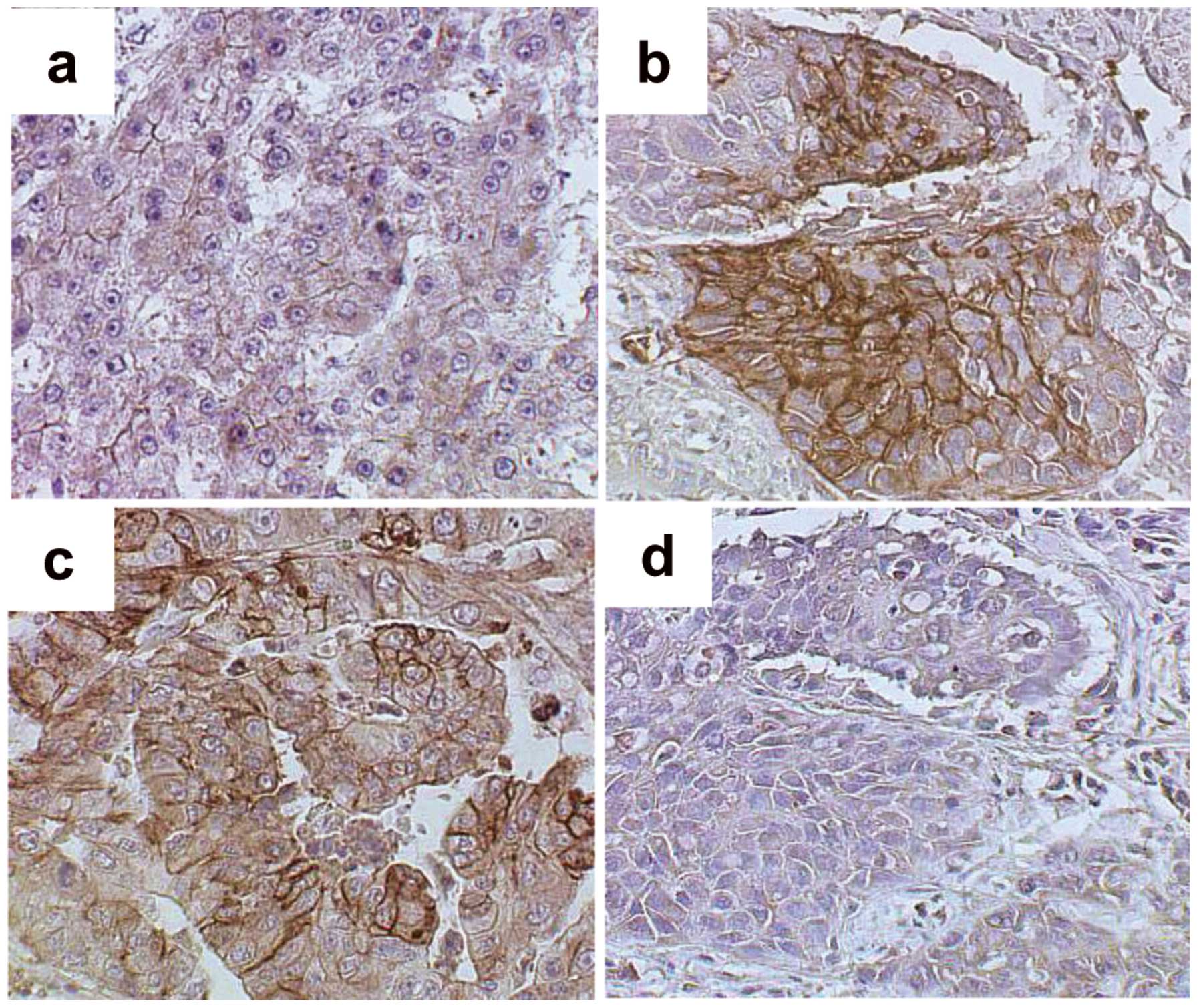
Immunohistochemical analysis of GLUT1 and
GLUT2
To elucidate the molecular mechanisms underlying FDG
accumulation in liver cancer, tumor levels of GLUT1 and GLUT2 were
evaluated. The M/D HCC cell membranes showed only weak staining
with GLUT1 antibody (Fig. 3a), but
P/D HCC and Meta specimens showed relatively strong GLUT1 membrane
staining (Fig. 3b and c). Staining
with the GLUT2 antibody was not observed in P/D HCC cells (Fig. 3d).
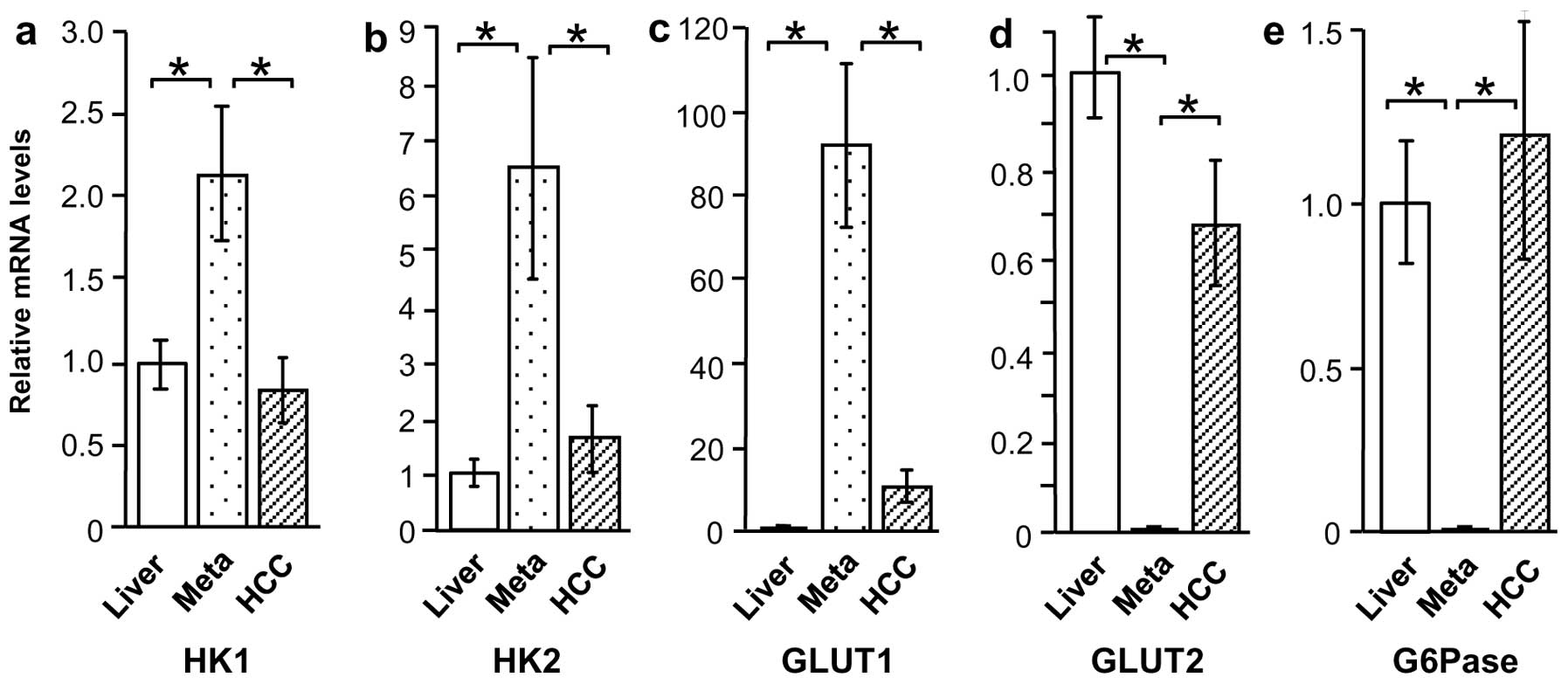
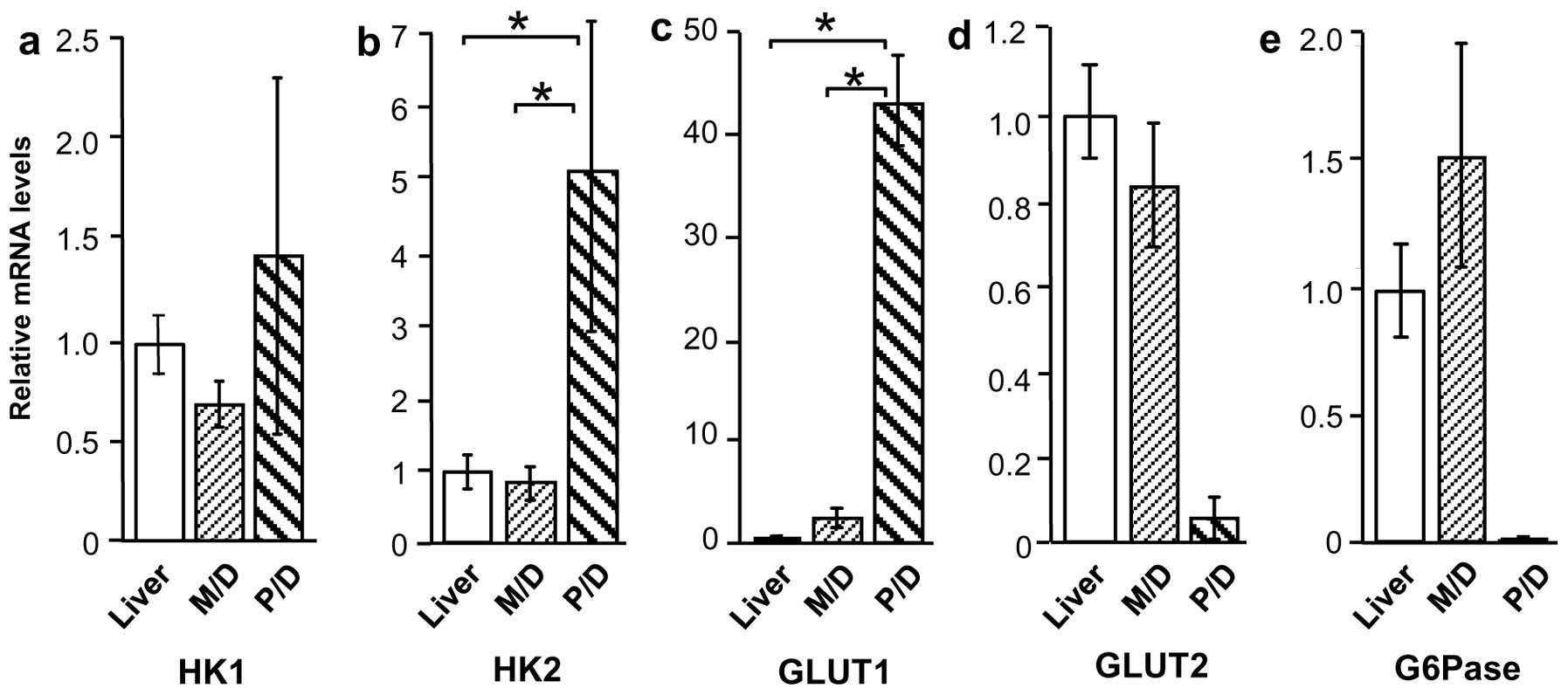
HK1, HK2, GLUT1, GLUT2, and G6Pase mRNA
levels in liver cancer and normal liver tissue
Based on the immunostaining results, the tissue
specimens were evaluated by qRT-PCR to determine expression of
glucose metabolism-related proteins GLUT1, GLUT2, HK1, HK2, and
G6Pase. Compared with normal liver tissue, HK1 and HK2 mRNA levels
were higher in Meta specimens (P<0.01), but were unchanged in
HCC specimens (Fig. 4a and b).
Similarly, GLUT1 expression was higher in Meta specimens than in
normal liver tissue (P<0.01), but was not significantly higher
in HCC specimens (Fig. 4c). By
contrast, GLUT2 and G6Pase mRNA levels were considerably lower in
Meta specimens (P<0.01 and P<0.05, respectively) than in
normal tissue, but were unchanged in HCC specimens (Fig. 4d and e).
HK1, HK2, GLUT1, GLUT2, and G6Pase mRNA
levels according to HCC differentiation
Expression of glucose metabolism-related proteins in
M/D HCC and P/D HCC was also compared, revealing a considerable
difference in expression patterns. Although HK1 mRNA levels were
similar in the two groups, HK2 and GLUT1 mRNA levels were higher in
P/D HCC compared with M/D HCC and normal liver tissue (P<0.01)
(Fig. 5a–c). By contrast, GLUT2 and
G6Pase appeared to be lower in P/D HCC (18- and 142-fold,
respectively), but this increase was not significant (Fig. 5d and e).
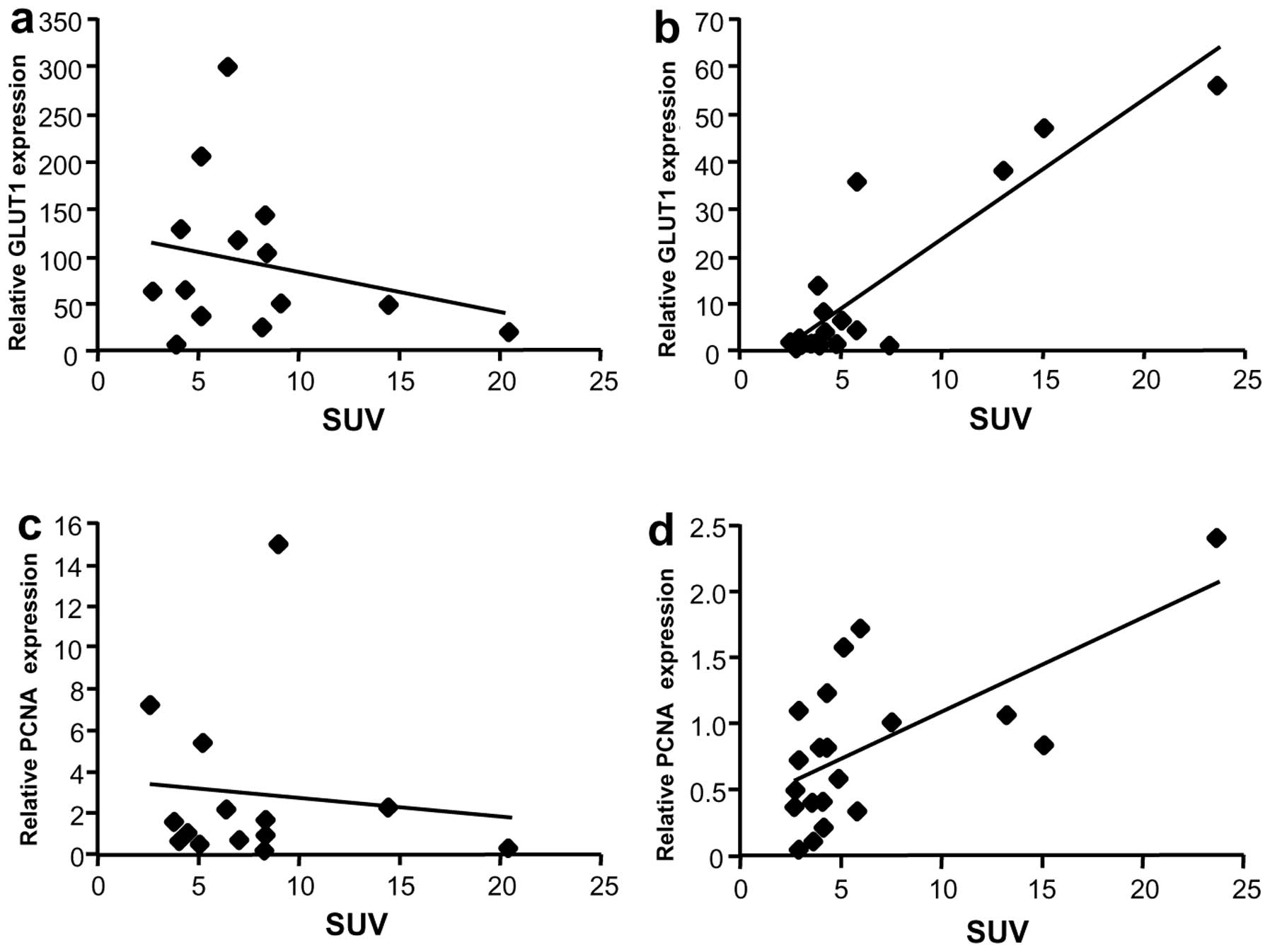
Correlation between SUV and mRNA levels
of GLUT1 or proliferating cell nuclear antigen
Since GLUT1 is overexpressed in liver cancer, the
relationship between GLUT1 mRNA expression and SUV was evaluated.
Although GLUT1 mRNA levels were not associated with SUV in the Meta
group (Fig. 6a), a correlation was
observed in the HCC group (rs=0.69, P=0.002) (Fig. 6b). To further evaluate the
relationship between SUV and tumor growth, mRNA levels of a
proliferation marker, proliferating cell nuclear antigen (PCNA),
were analyzed. No correlation was observed between PCNA mRNA levels
and SUV in Meta specimens (Fig.
6c), but a weak correlation was observed in HCC specimens
(Fig. 6d, rs=0.58, P=0.01).
Discussion
FDG-PET is used for diagnosis and tumor staging
essential for appropriate cancer management. However, FDG
accumulation is low in HCC tumors compared with other types of
cancer, such as lung cancer or malignant lymphoma (21,22).
Therefore, we investigated the mechanisms underlying FDG
accumulation by comparing two major forms of liver cancer, HCC and
colorectal cancer liver metastasis (Meta). We found that SUV
correlated with GLUT1 expression in HCC but not Meta specimens.
This finding may be due to the lower GLUT1 expression in HCC
tumors, particularly M/D HCC cases. Compared with surrounding
normal liver tissue, GLUT1 levels were 11-fold higher in HCC
specimens and 92-fold higher in Meta specimens. Thus, the number of
GLUT1 receptors in Meta tumors likely exceeds the requirements for
maximum FDG transport, accounting for the lack of association
between SUV and GLUT1 expression. The lower GLUT1 levels in HCC
suggest that this transporter may still be a rate-limiting factor
for FDG uptake, with the SUV dependent on GLUT1 increase.
SUV also correlated with tumor growth (assessed by
PCNA expression) in HCC specimens but not Meta specimens. The
increased energy provided by GLUT1 overexpression likely plays a
role in both HCC and Meta tumor growth; therefore, the reason for
the contrasting PCNA results is unclear. However, the PCNA
expression in Meta specimens was consistent with a previous study
(20). These results indicate that
SUV cannot always estimate tumor growth or prognosis in the
patients with liver metastasis of colorectal cancer.
GLUT1 is responsible for the cell basal glucose
requirements. The role of GLUT1 as the primary transporter in FDG
uptake for HCC is controversial as there are >10 GLUT family
isoforms, which are expressed in a tissue-specific manner and have
specific roles (23). Paudyal et
al (24) reported GLUT2
expression in 71% of HCCs and GLUT1 expression in only 16% of HCCs.
Roh et al (25) reported
that GLUT1 was expressed in 81.3% of cholangiocarcinomas but only
4.5% of HCCs. Taken together, these immunohistochemical studies
indicate that GLUT1 overexpression does not occur in all HCCs
(24). Our immunohistochemical
results showed relatively strong GLUT2 expression in HCC specimens;
however, the qRT-PCR results demonstrated that GLUT2 expression was
similar to that of the surrounding normal liver tissue. By
contrast, GLUT1 was clearly overexpressed in HCC in the present
study, with levels ~11-fold higher than those of the surrounding
normal tissue.
GLUT2 is expressed in hepatocytes, pancreatic β
cells, and the basolateral membranes of intestinal and renal
epithelial cells (26). In the
liver, GLUT2 expressed on hepatocyte membranes allows for the
bidirectional glucose transport, allowing glucose flux in and out
of cells (27). Unlike other GLUT
family members, GLUT2 is important for sensing glucose
concentration in the pancreas, intestinal glucose uptake, glucose
resorption by the kidney, and glucose uptake and release in the
liver (28). In humans, the
physiological glucose concentration is 5.6 mmol/l, and the GLUT1
and GLUT2 Michaelis constants are <20 mmol/l and 40 mmol/l,
respectively (28). Thus, GLUT1 has
a higher affinity for glucose than GLUT2, indicating that GLUT1
overexpression may provide a major advantage regarding glucose
uptake in cancer cells in the liver.
Previous studies reported that HK overexpression
also contributes to strong FDG uptake in HCC (29,30).
In this study, the mRNA levels of HK1 and HK2 were higher in Meta
specimens than in HCC specimens. The difference in HKs was
relatively small compared with the large difference in GLUT1
expression of the Meta or HCC. However, this transport protein may
still play a role in FDG uptake.
A molecular mechanism was also required to explain
differences in FDG uptake between M/D HCC and P/D HCC specimens.
However, it is important to note that the P/D HCC results were
derived from a small number of cases and showed considerable
variability in the results. Torizuka et al (17) reported that G6Pase activity was
lower in P/D HCC than in well-differentiated HCC or M/D HCC. Our
mRNA expression data were consistent with these results. In
addition, GLUT and HK mRNA levels were similar in P/D HCC and Meta
specimens. Of note, the G6Pase mRNA level of M/D HCC specimens was
218-fold greater than that of P/D HCC. Overexpression of G6Pase may
allow the FDG trapped in cells to be released into the bloodstream.
The SUV of HCC tumors may thus reflect how well HCC retains the
nature of normal liver tissue. High FDG accumulation in P/D HCC may
reflect increased GLUT1 expression and decreased G6Pase expression
compared with M/D HCC, which would explain why differentiated HCC
shows lower FDG accumulation compared with other malignancies.
In conclusion, the present study suggests that the
molecular mechanism for FDG-PET molecular imaging depends on GLUT1
and G6Pase expression. Low GLUT1 and high G6Pase expression
contribute to low FDG uptake in HCC tumors, preventing efficient
tumor detection. A pattern of high GLUT1 and low G6Pase expression
in P/D HCC facilitated FDG uptake similar to that of liver
metastasis from colorectal cancer. Finally, GLUT2 expression, while
important in normal hepatocytes, did not contribute to FDG uptake
in either type of liver cancer.
Acknowledgements
The authors are grateful to all the clinical staff
who cared for these patients. We also thank Dr Shoji Kimura for his
reliable experimental suggestions.
References
|
1
|
Fass L: Imaging and cancer. Mol Oncol.
2:115–152. 2008. View Article : Google Scholar
|
|
2
|
Mawlawi O and Townsend DW: Multimodality
imaging: an update on PET/CT technology. Eur J Nucl Med Mol
Imaging. 36(Suppl 1): 15–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schaefer O and Langer M: Detection of
recurrent rectal cancer with CT, MRI and PET/CT. Eur Radiol.
17:2044–2054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugiyama M, Sakahara H, Torizuka T, Kanno
T, Nakamura F, Futatsubashi M and Nakamura S: 18F-FDG PET in the
detection of extrahepatic metastases from hepatocellular carcinoma.
J Gastroenterol. 39:961–968. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwata Y, Shiomi S, Sasaki N, et al:
Clinical usefulness of positron emission tomography with
fluorine-18-fluorodeoxyglucose in the diagnosis of liver tumors.
Ann Nucl Med. 14:121–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan MA, Combs CS, Brunt EM, et al:
Positron emission tomography scanning in the evaluation of
hepatocellular carcinoma. J Hepatol. 32:792–797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trojan J, Schroeder O, Raedle J, et al:
Fluorine-18 FDG positron emission tomography for imaging of
hepatocellular carcinoma. Am J Gastroenterol. 94:3314–3319. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagaoka S, Itano S, Ishibashi M, et al:
Value of fusing PET plus CT images in hepatocellular carcinoma and
combined hepatocellular and cholangiocarcinoma patients with
extrahepatic metastases: preliminary findings. Liver Int.
26:781–788. 2006. View Article : Google Scholar
|
|
9
|
Akiyoshi T, Oya M, Fujimoto Y, et al:
Comparison of preoperative whole-body positron emission tomography
with MDCT in patients with primary colorectal cancer. Colorectal
Dis. 11:464–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo S, Hatano E, Higashi T, et al:
Fluorine-18 fluorodeoxyglucose positron emission tomography
predicts tumor differentiation, P-glycoprotein expression, and
outcome after resection in hepatocellular carcinoma. Clin Cancer
Res. 13:427–433. 2007. View Article : Google Scholar
|
|
11
|
Lee JD, Yun M, Lee JM, et al: Analysis of
gene expression profiles of hepatocellular carcinomas with regard
to 18F-fluorodeoxyglucose uptake pattern on positron emission
tomography. Eur J Nucl Med Mol Imaging. 31:1621–1630. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatano E, Ikai I, Higashi T, et al:
Preoperative positron emission tomography with
fluorine-18-fluorodeoxyglucose is predictive of prognosis in
patients with hepatocellular carcinoma after resection. World J
Surg. 30:1736–1741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Byström P, Berglund A, Garske U, et al:
Early prediction of response to first-line chemotherapy by
sequential [18F]-2-fluoro-2-deoxy-D-glucose positron emission
tomography in patients with advanced colorectal cancer. Ann Oncol.
20:1057–1061. 2009.PubMed/NCBI
|
|
14
|
Chau I and Cunningham D: Treatment in
advanced colorectal cancer: what, when and how? Br J Cancer.
100:1704–1719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Langen AJ, van den Boogaart V,
Lubberink M, et al: Monitoring response to antiangiogenic therapy
in non-small cell lung cancer using imaging markers derived from
PET and dynamic contrast-enhanced MRI. J Nucl Med. 52:48–55.
2011.PubMed/NCBI
|
|
16
|
Lee JH, Park JY, Kim do Y, et al:
Prognostic value of 18F-FDG PET for hepatocellular carcinoma
patients treated with sorafenib. Liver Int. 31:1144–1149. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torizuka T, Tamaki N, Inokuma T, et al: In
vivo assessment of glucose metabolism in hepatocellular carcinoma
with FDG-PET. J Nucl Med. 36:1811–1817. 1995.PubMed/NCBI
|
|
18
|
Caracó C, Aloj L, Chen LY, Chou JY and
Eckelman WC: Cellular release of [18F]2-fluoro-2-deoxyglucose as a
function of the glucose-6-phosphatase enzyme system. J Biol Chem.
275:18489–18494. 2000.
|
|
19
|
Kameyama R, Yamamoto Y, Izuishi K, Sano T
and Nishiyama Y: Correlation of 18F-FLT uptake with equilibrative
nucleoside transporter-1 and thymidine kinase-1 expressions in
gastrointestinal cancer. Nucl Med Commun. 32:460–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Izuishi K, Yamamoto Y, Sano T, et al:
Molecular mechanism underlying the detection of colorectal cancer
by 18F-2-fluoro-2-deoxy-d-glucose-positron emission tomography. J
Gastrointest Surg. 16:394–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murakami K: FDG-PET for hepatobiliary and
pancreatic cancer: Advances and current limitations. World J Clin
Oncol. 2:229–236. 2011.PubMed/NCBI
|
|
22
|
Lin WY, Tsai SC and Hung GU: Value of
delayed 18F-FDG-PET imaging in the detection of hepatocellular
carcinoma. Nucl Med Commun. 26:315–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao FQ and Keating AF: Functional
properties and genomics of glucose transporters. Curr Genomics.
8:113–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paudyal B, Paudyal P, Oriuchi N, Tsushima
Y, Nakajima T and Endo K: Clinical implication of glucose transport
and metabolism evaluated by 18F-FDG PET in hepatocellular
carcinoma. Int J Oncol. 33:1047–1054. 2008.PubMed/NCBI
|
|
25
|
Roh MS, Jeong JS, Kim YH, Kim MC and Hong
SH: Diagnostic utility of GLUT1 in the differential diagnosis of
liver carcinomas. Hepatogastroenterology. 51:1315–1318.
2004.PubMed/NCBI
|
|
26
|
Wood IS and Trayhurn P: Glucose
transporters (GLUT and SGLT): expanded families of sugar transport
proteins. Br J Nutr. 89:3–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown GK: Glucose transporters: structure,
function and consequences of deficiency. J Inherit Metab Dis.
23:237–246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leturque A, Brot-Laroche E, Le Gall M,
Stolarczyk E and Tobin V: The role of GLUT2 in dietary sugar
handling. J Physiol Biochem. 61:529–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ong LC, Jin Y, Song IC, Yu S, Zhang K and
Chow PK: 2-[18F]-2-deoxy-D-glucose (FDG) uptake in human tumor
cells is related to the expression of GLUT-1 and hexokinase II.
Acta Radiol. 49:1145–1153. 2008.
|
|
30
|
Paudyal B, Oriuchi N, Paudyal P, et al:
Clinicopathological presentation of varying 18F-FDG uptake and
expression of glucose transporter 1 and hexokinase II in cases of
hepatocellular carcinoma and cholangiocellular carcinoma. Ann Nucl
Med. 22:83–86. 2008. View Article : Google Scholar
|