Introduction
Gastric cancer is second only to lung cancer as the
leading cause of cancer-related deaths worldwide. Although the
overall incidence of gastric cancer has declined, it remains high
in Asian countries (1). The 5-year
survival rate for patients with gastric cancer is only ~20%. The
high mortality rates of patients with gastric cancer are known to
be associated with metastatic spread of cancer cells from the
stomach to common sites such as the liver and peritoneum (2). Metastasis is the result of several
sequential steps including proliferation, invasion, detachment of
tumor cells, migration into lymph nodes and blood vessels, adhesion
and survival in the circulation, and extravasation into the target
organ, where, again, proliferation occurs. These are also the key
elements influencing clinical treatment and prognosis (3). Therefore, understanding of the
molecular and genetic mechanisms involved in gastric cancer
progression may provide us with novel biomarkers and highlight
potential avenues of investigation for targeted therapies.
The allograft inflammatory factor-1 (AIF-1) is a
17-kDa interferon (IFN)-γ-inducible Ca2+-binding EF-hand
protein that is encoded within the human leukocyte antigen (HLA)
class III genomic region on chromosome 6p21.3, which is known for
clusters of genes involved in the inflammatory response (4). AIF-1 is closely associated with
cardiac allograft vasculopathy (5),
rheumatoid arthritis (6),
inflammatory skin disorders (7) and
systemic sclerosis (8). It has been
reported that AIF-1 may promote breast cancer proliferation through
activation of the NF-κB/cyclin D1 pathway (9) and breast cancer cell migration by
upregulation of TNF-α-mediated activation of the p38 MAPK signaling
pathway (10). In addition, AIF-1
may play a significant role in the pathophysiology and progression
of hemangiomas (11). However, it
has not yet been reported whether AIF-1 is also involved in the
development of gastric cancer.
In the present study, to elucidate the potential
role of AIF-1 expression in gastric cancer, we evaluated AIF-1
staining in 78 primary gastric cancer biopsies and matched
non-cancerous gastric tissues using tissue microarray (TMA)
technology and immunohistochemistry. In addition, we investigated
the effects of AIF-1 on the proliferation and migration of the
gastric cancer cell lines BGC-823 and SGC-7901 in vitro.
Materials and methods
Patients and specimens
The study cohort included 103 patients who underwent
radical gastrectomy at Nantong Cancer Hospital from May 1, 1990 to
June 1, 1995. A TMA including whole gastric cancer samples and
matched non-cancerous gastric mucosa was constructed. Due to
missing data in processing, the cohort included 103 patients but 20
samples were omitted, and 5 samples were lost during antigen
retrieval or without tumor cells in the core. Finally 78 paired
tissues were evaluated for AIF-1 expression. Written informed
consent was obtained from each patient prior to tissue acquisition.
Institutional approval was acquired from the Ethics Review Board of
Nanjing Medical University prior to the present study. Detailed
clinicopathological information was obtained from the medical
records of the hospital. The histological types of gastric cancer
were classified according to Lauren (12) and staged according to the
tumor-node-metastasis (TNM) guidelines (13). Only confirmed intestinal, diffuse
and mixed types were included.
TMA construction and
immunohistochemistry
The gastric cancer TMAs were constructed as
previously described (14).
Duplicate 1.0-mm diameter cores of tissue from each sample were
punched from the paraffin tumor block and corresponding non-tumoral
tissues in the cohort. As a tissue control, the biopsies of normal
gastric epithelium tissues were inserted in the four angles and the
center of each slide.
A standard protocol was used for the immunostaining
of the TMAs, as described in our previous study (14). The polyclonal rabbit anti-AIF-1
antibody (1:200 dilution; Abgent Technology, San Diego, CA, USA)
was used. The omission of the primary antibody served as the
negative control. The staining scores of the tissue controls in
each microarray slide were pre-evaluated as quality control for the
immunostaining.
TMA slides were de-waxed at 55°C for 20 min followed
by three 5-min washes with xylene. Rehydration of tissues was
performed by 5-min washes in 100, 95 and 80% ethanol and distilled
water, respectively. Antigen retrieval was performed by heating the
samples at 95°C for 30 min in 10 mM sodium citrate (pH 6.0).
Endogenous peroxidase activity of the tissue was blocked by
incubation in 3% hydrogen peroxide for 30 min. After a 30-min
blocking with Universal Blocking serum (Dako Diagnostics,
Carpinteria, CA, USA), the sections were incubated with the
anti-AIF-1 antibody at 4°C overnight. The sections were then
incubated for 30 min each with a biotin-labeled secondary antibody
and then streptavidin-peroxidase (Dako Diagnostics). The samples
were developed using 3,3′-diaminobenzidine substrate and
counterstained with hematoxylin. Dehydration was then performed
following a standard procedure, and the slides were sealed with
coverslips.
Evaluation of immunohistochemistry
By applying a semi-quantitative immunoreactivity
score (IRS) in the cohort, staining of AIF-1 in the tissues was
scored independently by 2 pathologists blinded to the clinical
data, as reported elsewhere (15).
Category A categorized the intensity of immunostaining as 0–3 (0,
negative; 1, weak; 2, moderate and 3, strong). Category B
categorized the percentage of immunoreactive cells as 1 (0–25%), 2
(26–50%), 3 (51–75%) and 4 (76–100%). Multiplication of category A
and B resulted in an IRS ranging from 0 to 12 for each tumor or
non-tumor sample.
The optimum cutoff value of IRS was obtained by
receiver-operator characteristic (ROC) analysis. The area under the
curve (AUC) at different cutoff values of AIF-1 IRS for overall
survival (OS) at 1, 3 and 5 years was calculated. The optimum value
of cutoff points for AIF-1 IRS was 3 since the predictive value of
this cutoff point for death was optimal. Therefore, samples with
IRS 0–3 and IRS 4–12 were classified as having low and high AIF-1
expression, respectively, in the tumors.
Cell culture
For the in vitro experiments, BGC-823 and
SGC-7901 human gastric cancer cell lines were purchased from the
Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
Dulbecco’s modified Eagle’s medium (Gibco-BRL Life Technologies,
Grand Island, NY, USA) supplemented with 100 U/ml penicillin, 100
μg/ml streptomycin and 10% fetal bovine serum (Tianhang Biological
Technology, Hangzhou, China). All cells were maintained in a 5%
CO2 atmosphere at 37°C.
siRNA and cell growth assay
Control siRNA or AIF-1 siRNA (both from Ruibo
Biotechnology, Guangzhou, China) was transfected using
Lipofectamine 2000 (Invitrogen Life Technologies) according to
manufacturer’s instructions. For measurement of cell growth, a
colorimetric water-soluble tetrazolium salt assay (Cell Counting
Kit-8; Dojindo Laboratories, Kumamoto, Japan) was used to assess
the number of viable cells at various time points following
transfection.
Western blotting
Western blotting was carried out as previously
described (14). Polyclonal rabbit
anti-AIF-1 antibody (1:500 dilution; Abgent Technology, San Diego,
CA, USA), monoclonal rabbit anti-β-catenin antibody (1:1,000
dilution; Epitomics, Burlingame, CA, USA), monoclonal mouse
anti-tubulin antibody (1:2,000 dilution) and mouse anti-β-actin
antibody (1:2,000 dilution) (both from Beyotime Biotechnology,
Nantong, China) were used as the primary antibody. Immunoreactive
bands were detected with a Phototope®-HRP Western Blot
Detection kit (Cell Signaling Technology, Inc., Beverly, MA, USA).
AIF-1 protein bands on the blots were measured by ImageJ software
(version 1.44, National Institutes of Health, USA), after
normalization to the corresponding tubulin level.
Transwell migration assay
Transwell migration assay was carried out in a
24-well modified 2-chamber plate (Corning, Tewksbury, MA, USA). The
upper surface consisted of 6.5-mm diameter filters with 8-μm pore
size. The transfectants (2×104 cells/well) were
transferred into the upper chamber. After 12 h of incubation, the
migrated cells on the lower surface of filters were fixed with 95%
methanol and stained with crystal violet staining solution
(Beyotime Biotechnology), and stained cell nuclei were counted in
triplicate. We assessed the migration through the uncoated filters
of test cells over that in the control counterparts.
Scratch migration assay
BGC-823 and SGC-7901 cells were transfected in a
6-well plate. Forty-eight hours post-transfection, the cells were
scraped with the fine end of a 10-μl pipette tip (time 0). Plates
were washed twice with PBS to remove detached cells, and incubated
with the complete growth medium. Cell migration into the wounded
empty space was determined after 24 h and photographed.
Statistical analysis
The association between AIF-1 expression and
clinicopathological parameters was evaluated by Fisher’s exact
test. The significance of correlations between AIF-1 staining in
primary tumors and their corresponding non-tumor tissues was
assessed by the Wilcoxon test (grouped) and Spearman’s rank-order
correlation (raw scores). Probability of differences in OS as a
function of time was ascertained by use of the Kaplan-Meier method,
with a log-rank test probe for significance. All the statistical
analyses were performed using Stata Statistical software (version
10.1; StataCorp, College Station, TX, USA). A difference with a
P-value of <0.05 was deemed statistically significant.
Results
AIF-1 expression is decreased in gastric
cancer when compared to that in the non-cancerous tissues
To test AIF-1 protein expression, 7 pairs of human
primary gastric cancer tissues and matched normal gastric mucosa
were randomly selected for western blot analysis. As a result, all
of the gastric cancer tissues exhibited a significant reduction in
AIF-1 when compared with that in the paired normal tissues
(Fig. 1A). Furthermore,
immunohistochemical staining of the gastric cancer TMA was used to
further confirm AIF-1 expression in 78 gastric cancer patients.
Staining of AIF-1 was mainly localized in the cytoplasm (Fig. 1B). Regarding the distribution of the
differences in IRS, AIF-1 expression was significantly decreased in
53 of the 78 (67.9%) gastric cancer tissues when compared with that
in the matched normal tissues (P<0.001, Wilcoxon test; Fig. 1C). The above data showed that the
AIF-1 protein was reduced in gastric cancer tissues when compared
with that in the gastric non-cancerous normal tissues.
 | Figure 1AIF-1 expression in primary tumors and
corresponding non-tumor tissues in human gastric cancer. (A) AIF-1
protein levels in 7 cancer tissues and paired non-cancerous normal
tissues of the gastric cancer patients were analyzed by western
blotting. The level of protein was normalized against tubulin, and
the protein levels in cancer tissues are indicated as a ratio to
the paired non-cancerous normal tissues. T, tumor tissue; N,
non-cancerous gastric tissue. (B) Immunohistochemical staining for
AIF-1 in TMA. T, tumor tissue; N, non-cancerous gastric tissue.
(Top panel, scale bar, 250 μm; bottom panel, scale bar, 50 μm). (C)
The distribution of the difference in AIF-1 staining (ΔIRS = IRS N
- IRS T). P-values were calculated with the Wilcoxon test. AIF-1,
allograft inflammatory factor-1; TMA, tissue microarray; IRS,
immunoreactivity score. |
AIF-1 expression negatively correlates
with clinicopathological features of the gastric cancer
patients
Immunohistochemical staining of AIF-1 levels was
analyzed to determine their correlation with clinicopathological
features. As shown in Table I,
reduced protein expression of AIF-1 in the cancer tissues was
significantly associated with malignant clinicopathological
features, such as lymph node metastasis (N category), distant
metastasis, TNM stage, tumor diameter and histological type
(P<0.05). In addition, more cases with depth of T3/T4 invasion
(T category) were noted in the group with low AIF-1 expression
although the difference did not reach a statistically significant
level. These observations suggest that deficiency in functional
AIF-1 expression may contribute to clinical gastric cancer
progression.
 | Table ICorrelation between expression levels
of AIF-1 and the clinicopathological features of the gastric cancer
patients (n=78). |
Table I
Correlation between expression levels
of AIF-1 and the clinicopathological features of the gastric cancer
patients (n=78).
| AIF-1 expression | |
|---|
|
| |
|---|
| Variables | Low, n (%) | High, n (%) | P-valuea |
|---|
| Total patients | 25 (32.05) | 53 (67.95) | |
| Age (years) | | | 1.000 |
| ≤65 | 21 (84) | 43 (81.13) | |
| >65 | 4 (16) | 10 (18.87) | |
| Gender | | | 0.419 |
| Males | 16 (64) | 40 (75.47) | |
| Females | 9 (36) | 13 (24.53) | |
| Depth of
invasion | | | 0.176 |
| T1/T2 | 4 (16) | 17 (32.08) | |
| T3/T4 | 21 (84) | 36 (67.92) | |
| Lymph node
metastasis | | | <0.001 |
| N0 | 1 (4) | 22 (41.51) | |
| N1/N2/N3 | 24 (96) | 31(58.49) | |
| Distant
metastasis | | | 0.004 |
| M0 | 14 (56) | 46 (86.79) | |
| M1 | 11 (44) | 7 (13.21) | |
| TNM stage | | | <0.001 |
| I | 1 (4) | 9 (16.98) | |
| II | 1 (4) | 22 (41.51) | |
| III | 12 (48) | 12 (22.64) | |
| IV | 11 (44) | 10 (18.87) | |
| Tumor diameter
(cm) | | | 0.041 |
| ≤5 | 12 (48) | 36 (67.92) | |
| >5 | 13 (52) | 17 (32.08) | |
| Histological
type | | | 0.007 |
| Intestinal | 16 (64) | 16 (30.19) | |
| Diffuse | 9 (36) | 37 (69.81) | |
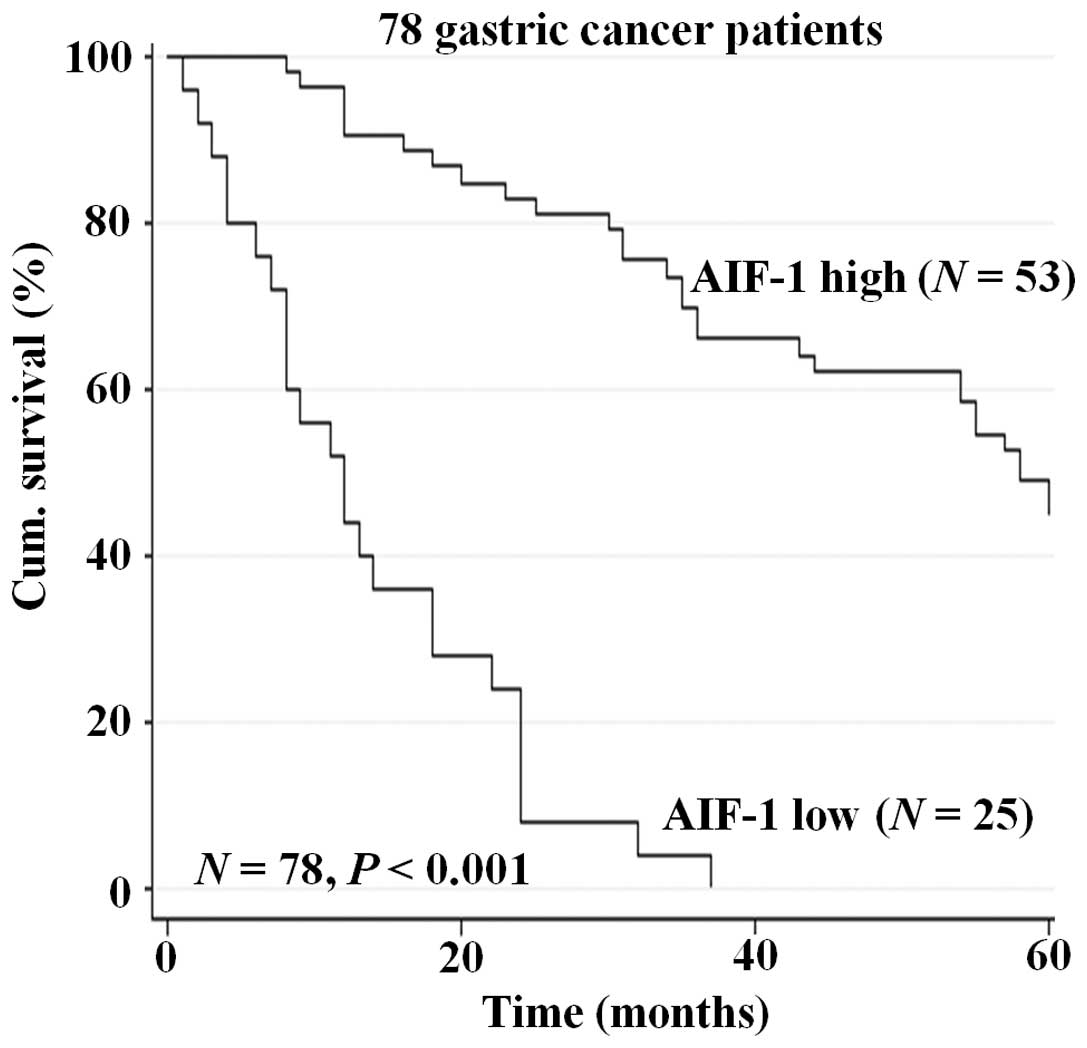
Expression of AIF-1 is an independent
prognostic indicator for overall survival of gastric cancer
patients
To further determine the prognostic value of AIF-1
in gastric cancer, overall survival was analyzed. Kaplan-Meier
survival curves showed that reduced AIF-1 expression in gastric
cancer tissues was significantly correlated with shorten overall
5-year survival in the patients in the cohort (P<0.001, Fig. 2). This suggests that expression of
AIF-1 in gastric cancer tissues may be an independent biomarker of
patient overall survival.
Deficiency of AIF-1 expression
contributes to proliferation and migration in gastric cancer
cells
To determine the potential role of AIF-1 in the
development of gastric cancer, we designed a series of cell culture
models to investigate whether knockdown of AIF-1 affects
proliferation, migration and adhesion of gastric cancer cells. As
shown in Fig. 3, the ability for
cell proliferation was significantly increased after knockdown of
AIF-1 by gene-specific siRNA in both BGC-823 and SGC-7901 cells.
Similarly, the Transwell migration assay revealed that the numbers
of BGC-823 and SGC-7901 cells that migrated through the membrane
into the lower chamber were significantly higher in the si-AIF-1
transfected cells when compared with these numbers in the controls
(Fig. 4). In addition,
AIF-1-deficient cells also demonstrated accelerated wound closure
(Fig. 5). These results indicate
that AIF-1 deficiency contributes to the development of the
malignant phenotype in gastric cancer cells.
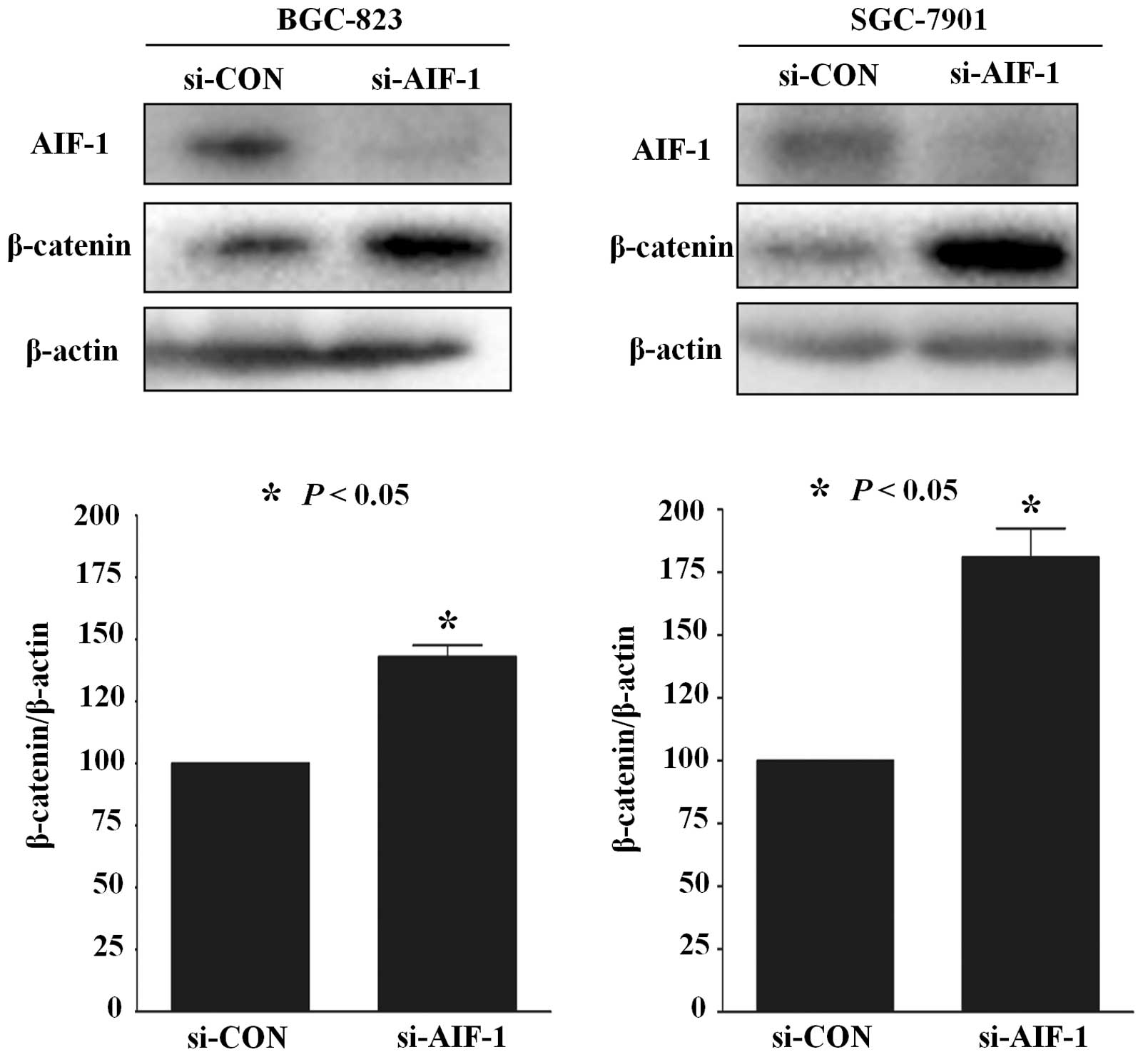
AIF-1 deficiency activates β-catenin
expression in gastric cancer cells
Previous studies have reported that β-catenin
activation plays an important role in gastric cancer (16). To test whether the role of AIF-1 is
realized via β-catenin in gastric cancer cells, AIF-1 and β-catenin
protein in human gastric cancer cell lines was detected by western
blotting. As shown in Fig. 6, the
expression of β-catenin was upregulated in BGC-823 and SGC7901
cells following transfection with si-AIF-1 when compared with the
control (Fig. 6). These data
suggest that AIF-1 deficiency promotes β-catenin expression in
gastric cancer cells.
Discussion
The assessment of biological prognostic factors is
of clinical importance, particularly for diseases with poor outcome
such as gastric cancer. In the present study, we investigated the
role of AIF-1 expression in the prognosis of gastric cancer
patients for the first time. Decreased AIF-1 expression in gastric
cancer when compared to non-cancerous tissues was observed.
Moreover, we showed that AIF-1 expression was associated with
clinical progression and prognosis of the gastric cancer patients.
In the in vitro models, AIF-1 deficiency promoted cell
growth, migration and β-catenin expression in gastric cancer cell
lines BGC-823 and SGC-7901. Thus, the functions of AIF-1 in gastric
cancer cells may be realized through the suppression of β-catenin
expression.
AIF-1 is a cytoplasmic, calcium-binding,
inflammation-responsive scaffold protein that has been implicated
in the regulation of inflammation. The AIF-1 gene is located on
chromosome 6p21.3, which is densely clustered with genes involved
in the inflammatory response, including surface glycoproteins,
complement cascade, TNF-α, TNF-β and NF-κB genes (17). It has been reported that AIF-1 is
closely associated with cardiac allograft vasculopathy, rheumatoid
arthritis, inflammatory skin disorders and systemic sclerosis
(6,7). Since AIF-1 may be involved in the
cytoskeletal signaling network and may contribute to the
progression of EMT (18,19), it is evident that aberrant
regulation of AIF-1 may lead to tumor progression. Previous studies
have revealed that AIF-1 can promote the growth of breast tumors
via the activation of NF-κB signaling, which consequently
upregulates the expression of cyclin D1 (9). Moreover, expression of AIF-1 was found
to be upregulated in cervical cancer tissues (20). These results indicate that AIF-1 may
function as an oncogene. However, in the present study, our results
provided novel evidence of a strong association between AIF-1
upregulation and clinicohistopathological parameters, indicative of
a more favorable outcome in gastric cancer patients. This suggests
that there may be another mechanism by which AIF-1 is involved in
gastric cancer progression.
We noted that the roles of β-catenin in mediating
intercellular adhesion and regulation of cell growth,
differentiation, invasion and metastasis have been well
characterized (21,22). The β-catenin-TCF/LEF complex
regulates and activates its downstream target transcription genes
which are involved in the development and progression of cancer
(23–25). The abnormal activation of β-catenin
frequently occurs in gastric cancer and has been proven to promote
tumor growth, invasion and metastasis (26,27).
Furthermore, previous studies have confirmed that high β-catenin
expression is an independent indicator of poor prognosis for these
carcinomas and is closely correlated with enhanced tumor
progression (28,29).
In the present study, we found that AIF-1 knockdown
promoted β-catenin expression in gastric cancer. Our observations
were consistent with the previous finding that β-catenin activity
is negatively correlated with bacteria-induced inflammation
(30). Further studies are required
to obtain a detailed profile of the exact mechanisms involved in
the regulation of β-catenin by AIF-1 in gastric tumor
development.
This is the first study to report the association
between AIF-1 expression and the clinicopathological features of
gastric cancer. Our data demonstrate that AIF-1 functions as a
tumor suppressor possibly by regulating β-catenin in gastric
cancer. Moreover, loss of AIF-1 expression may represent a novel
indicator for the progression and prognosis of gastric cancer.
Nevertheless, despite highly significant results in our patient
cohort, further studies must be carried out to evaluate whether
AIF-1 may be beneficial as a future preventive and therapeutic
target for gastric cancer.
Acknowledgements
The present study was supported by the Priority
Academic Program Development (PAPD) of Jiangsu Higher Education
Institutions, and the National Natural Science Foundation of China
(nos. 30930080 and 81161120537).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia B, Liu H, Kong Q and Li B: RKIP
expression associated with gastric cancer cell invasion and
metastasis. Tumour Biol. 33:919–925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Utans U, Quist WC, McManus BM, et al:
Allograft inflammatory factory-1. A cytokine-responsive macrophage
molecule expressed in transplanted human hearts. Transplantation.
61:1387–1392. 1996.
|
|
5
|
Autieri MV, Kelemen S, Thomas BA, Feller
ED, Goldman BI and Eisen HJ: Allograft inflammatory factor-1
expression correlates with cardiac rejection and development of
cardiac allograft vasculopathy. Circulation. 106:2218–2223. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura M, Kawahito Y, Obayashi H, et al: A
critical role for allograft inflammatory factor-1 in the
pathogenesis of rheumatoid arthritis. J Immunol. 178:3316–3322.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orsmark C, Skoog T, Jeskanen L, Kere J and
Saarialho-Kere U: Expression of allograft inflammatory factor-1 in
inflammatory skin disorders. Acta Derm Venereol. 87:223–227.
2007.PubMed/NCBI
|
|
8
|
Del Galdo F, Maul GG, Jiménez SA and
Artlett CM: Expression of allograft inflammatory factor 1 in
tissues from patients with systemic sclerosis and in vitro
differential expression of its isoforms in response to transforming
growth factor β. Arthritis Rheum. 54:2616–2625. 2006.PubMed/NCBI
|
|
9
|
Liu S, Tan WY, Chen QR, et al:
Daintain/AIF-1 promotes breast cancer proliferation via activation
of the NF-κB/cyclin D1 pathway and facilitates tumor growth. Cancer
Sci. 99:952–957. 2008.PubMed/NCBI
|
|
10
|
Li T, Feng Z, Jia S, et al: Daintain/AIF-1
promotes breast cancer cell migration by up-regulated TNF-α via
activate p38 MAPK signaling pathway. Breast Cancer Res Treat.
131:891–898. 2012.PubMed/NCBI
|
|
11
|
Jia J, Bai Y, Fu K, Sun ZJ, Chen XM and
Zhao YF: Expression of allograft inflammatory factor-1 and CD68 in
haemangioma: implication in the progression of haemangioma. Br J
Dermatol. 159:811–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lauren P: The two histological main types
of gastric carcinoma: diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
13
|
Aiko T and Sasako M: The new Japanese
classification of gastric carcinoma: points to be revised. Gastric
Cancer. 1:25–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Wu X, Chen Y, et al: Prognostic
and predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weichert W, Röske A, Gekeler V, et al:
Association of patterns of class I histone deacetylase expression
with patient prognosis in gastric cancer: a retrospective analysis.
Lancet Oncol. 9:139–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takasu S, Tsukamoto T, Cao XY, et al:
Roles of cyclooxygenase-2 and microsomal prostaglandin E synthase-1
expression and β-catenin activation in gastric carcinogenesis in
N-methyl-N-nitrosourea-treated K19-C2mE transgenic
mice. Cancer Sci. 99:2356–2364. 2008.
|
|
17
|
Iris FJ, Bougueleret L, Prieur S, et al:
Dense Alu clustering and a potential new member of the
NFκB family within a 90 kilobase HLA class III segment. Nat
Genet. 3:137–145. 1993.
|
|
18
|
Autieri MV, Kelemen SE and Wendt KW: AIF-1
is an actin-polymerizing and Rac1-activating protein that promotes
vascular smooth muscle cell migration. Circ Res. 92:1107–1114.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hugo HJ, Wafai R, Blick T, Thompson EW and
Newgreen DF: Staurosporine augments EGF-mediated EMT in PMC42-LA
cells through actin depolymerisation, focal contact size reduction
and Snail1 induction - a model for cross-modulation. BMC Cancer.
9:2352009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song JY, Bae HS, Koo do H, et al:
Candidates for tumor markers of cervical cancer discovered by
proteomic analysis. J Korean Med Sci. 27:1479–1485. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozawa M, Ringwald M and Kemler R:
Uvomorulin-catenin complex formation is regulated by a specific
domain in the cytoplasmic region of the cell adhesion molecule.
Proc Natl Acad Sci USA. 87:4246–4250. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, Blechman J, Savagner P and Ben-Ze’ev A: Autoregulation of
E-cadherin expression by cadherin-cadherin interactions: the roles
of β-catenin signaling, Slug, and MAPK. J Cell Biol. 163:847–857.
2003.PubMed/NCBI
|
|
23
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/β-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010.
|
|
25
|
Hervieu V, Lepinasse F, Gouysse G, et al:
Expression of β-catenin in gastroenteropancreatic endocrine
tumours: a study of 229 cases. J Clin Pathol. 59:1300–1304.
2006.
|
|
26
|
Bianchi F, Hu J, Pelosi G, et al: Lung
cancers detected by screening with spiral computed tomography have
a malignant phenotype when analyzed by cDNA microarray. Clin Cancer
Res. 10:6023–6028. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang N, Zhang J, Shuai L, et al:
Krüppel-like factor 4 negatively regulates β-catenin expression and
inhibits the proliferation, invasion and metastasis of gastric
cancer. Int J Oncol. 40:2038–2048. 2012.
|
|
28
|
Lin SY, Xia W, Wang JC, et al: β-Catenin,
a novel prognostic marker for breast cancer: its roles in cyclin D1
expression and cancer progression. Proc Natl Acad Sci USA.
97:4262–4266. 2000.
|
|
29
|
Wong SC, Lo ES, Lee KC, Chan JK and Hsiao
WL: Prognostic and diagnostic significance of β-catenin nuclear
immunostaining in colorectal cancer. Clin Cancer Res. 10:1401–1408.
2004.
|
|
30
|
Duan Y, Liao AP, Kuppireddi S, Ye Z,
Ciancio MJ and Sun J: β-Catenin activity negatively regulates
bacteria-induced inflammation. Lab Invest. 87:613–624. 2007.
|