Introduction
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a promising anticancer agent. TRAIL selectively
induces apoptosis in malignant tumor cells in vitro and
in vivo and has little to no toxicity in normal cells
(1–4). There are two main pathways that
initiate apoptosis, the extrinsic (death receptor) and the
intrinsic (mitochondrial) pathways. TRAIL binds 5 known
cell-surface receptors, death receptor 4 (DR4), DR5, decoy receptor
1 (DcR1), DcR2 and osteoprotegerin (5–7). Two
of these, DR4 and DR5, possess death domains which recruit
Fas-associated death domain (FADD) upon TRAIL ligation. These
interactions result in the formation of a death-inducing signaling
complex (DISC), leading to stimulation of the extrinsic pathway
through caspase-8 and -10 activation. Importantly, it has been
shown that DR5 is more abundantly expressed in cancer cells than in
normal cells, and that DR5 may contribute more than DR4 to
TRAIL-induced apoptosis in cancer cells which express both DRs
(8–10). Thus, the induction of DR5 can at
least partially contribute to the tumor-selective induction of
apoptosis mediated by TRAIL.
Recombinant human TRAIL (rhTRAIL; dulanermin) is
currently being tested in clinical trials in patients with
refractory malignant tumors. The phase Ia study by Herbst et
al, for the treatment of advanced solid tumors or non-Hodgkin’s
lymphoma, reported that treatment with rhTRAIL as a monotherapy
yielded stable disease in 33 out of 71 (46%) patients and partial
responses in 2 (3%) patients with chondrosarcoma (11). These data indicate that despite its
promising anticancer efficacy, a considerable proportion of
patients with advanced malignant tumors do not respond well to
TRAIL alone.
Peroxisome proliferator-activated receptor γ (PPARγ)
is a member of the ligand-dependent nuclear transcriptional
factors. It is well known to be highly expressed in adipose tissue
and to play important roles in adipocyte differentiation, lipid
metabolism and insulin sensitization (12–14).
There are several synthetic ligands for PPARγ, including those of
the thiazolidinedione class such as troglitazone (TGZ), ciglitazone
and pioglitazone (15,16). TGZ was previously prescribed for
type 2 diabetes mellitus but liver toxicity led to its withdrawal
from clinical use in 2003. In addition to its effect on insulin
sensitization, TGZ exerts antitumor activity against malignant
tumor cells in vitro and in vivo (17,18).
Two phase II studies using TGZ were initiated in patients with
metastatic colorectal and refractory metastatic breast cancer.
However, these clinical trials reported that no objective responses
were observed (19,20).
The endoplasmic reticulum (ER) serves several
important functions in the maintenance of cellular homeostasis.
Perturbations of ER homeostasis affect protein folding and cause ER
stress (21). When ER stress is
persistent or excessive, it triggers apoptosis. One of the
components of the ER stress-mediated apoptotic pathway is the
CCAAT/enhancer-binding protein homologous protein (CHOP) (22). It has been reported that TGZ causes
apoptosis through induction of ER stress in hepatoma cell lines and
through CHOP in a non-small cell lung carcinoma cell line (23,24).
In the present study, we demonstrated that combined
treatment of the synthetic PPARγ ligand TGZ and TRAIL causes
synergistic apoptosis through DR5 induction in several types of
cancer cell lines. The elevation of DR5 expression by TGZ is
mediated through CHOP induction via ER stress. These results
suggest that co-treatment with the DR5 inducer TGZ and TRAIL may be
a promising strategy for cancer therapeutics.
Materials and methods
Cell culture and reagents
Human colon cancer DLD-1 cells were maintained in
RPMI-1640. Human osteosarcoma Saos2, human colon cancer HCT116 and
human lung cancer A549 cells were maintained in Dulbecco’s Modified
Eagle’s Medium (DMEM). Culture media were supplemented with 10%
fetal bovine serum, L-glutamine (2 mmol/l for RPMI-1640 and 4
mmol/l for DMEM), 100 U/ml penicillin and 100 μg/ml streptomycin.
Cell cultures were incubated at 37°C in a humidified atmosphere of
5% CO2. TGZ and ciglitazone were purchased from
Calbiochem. Pioglitazone was kindly provided by Takeda Chemical
Industries. Recombinant human DR5/Fc chimera, zVAD-fmk (pan-caspase
inhibitor), zIETD-fmk (caspase-8 inhibitor) and zAEVD-fmk
(caspase-10 inhibitor) were purchased from R&D Systems. Soluble
recombinant human TRAIL and the PPARγ inhibitor GW9662 were
purchased from PeproTech and Alexis Biochemicals, respectively.
Detection of apoptosis
DNA fragmentation was quantified as the percentage
of cells with hypodiploid DNA (sub-G1). For flow cytometric
analysis, cells were exposed to the agents for the indicated times.
The cells were then treated with Triton X-100 and RNase A, and
their nuclei were stained with propidium iodide. DNA content was
then measured using a FACSCalibur flow cytometer and CellQuest
software (Becton-Dickinson). For all assays, 10,000 cells were
counted.
Western blot analysis
Western blot analysis was performed as previously
described (25). Anti-DR5 (ProSci),
anti-XIAP, anti-cIAP-1, anti-survivin (R&D Systems),
anti-Bcl-2, anti-PARP, anti-Bak, anti-Bax, anti-Bcl-XL,
anti-CHOP, anti-GRP78/Bip (Santa Cruz Biotechnology, Inc.),
anti-Bid, anti-caspase-8, anti-caspase-10 (MBL), anti-caspase-3
(Cell Signaling Technology, Inc.) and anti-β-actin (Sigma)
antibodies were used. The signal was then developed with
Chemilumi-One (Nacalai Tesque) or Immobilon Western (EMD
Millipore).
RNA isolation and real-time reverse
transcription-polymerase chain reaction (RT-PCR) analysis
RNA isolation and RT-PCR were performed as
previously described (26). The
GeneAmp 5700 (Applied Biosystems) was used to quantify the
expression level of CHOP and DR5 mRNAs normalized to 18S rRNA.
Real-time RT-PCR primer probes were purchased from Applied
Biosystems.
Transfection and luciferase assay
A series of CHOP or DR5 reporter plasmids, the ER
stress response element (ERSE) mutant plasmid pCHOP/mt ERSE and the
CHOP mutant plasmid pDR5/mtCHOP were described previously (26). DLD-1 cells were seeded at
1×105 cells/well in 6-well plates. One day later, cells
were transfected with these plasmids or pGVB2 (a vacant control;
1.0 μg) using the DEAE-dextran method and a CellPhect transfection
kit (Amersham Pharmacia Biotech). After 24 h, transfected cells
were treated with 60 μM TGZ for a further 24 h and then harvested.
Luciferase assays were then performed using luciferase assay
reagents (Promega Corporation) and a luminometer.
Small interfering RNA (siRNA)
The DR5 siRNA was purchased from Sigma. The CHOP and
the negative control siRNAs were purchased from Invitrogen Life
Technologies. One day before transfection, DLD-1 cells were seeded
at 5×104 cells/well in 6-well plates without
antibiotics. The CHOP and/or DR5 siRNAs (25 nmol/l) were
transfected into cells using Lipofectamine RNAiMAX (Invitrogen Life
Technologies) according to the manufacturer’s instructions. Twenty
hours after the transfection, cells were treated with or without 60
μM TGZ for 48 h and then harvested.
Statistical analysis
Statistical evaluation of the data was performed
using the Student’s t-test for simple comparison between treatments
and controls. P<0.05 was considered to indicate a statistically
significant difference. Characterization of synergistic
interactions was performed using median dose effect analysis in
conjunction with a commercially available software program
(CalcuSyn; Biosoft).
Results
TGZ and TRAIL synergistically induce
apoptosis in DLD-1 cells
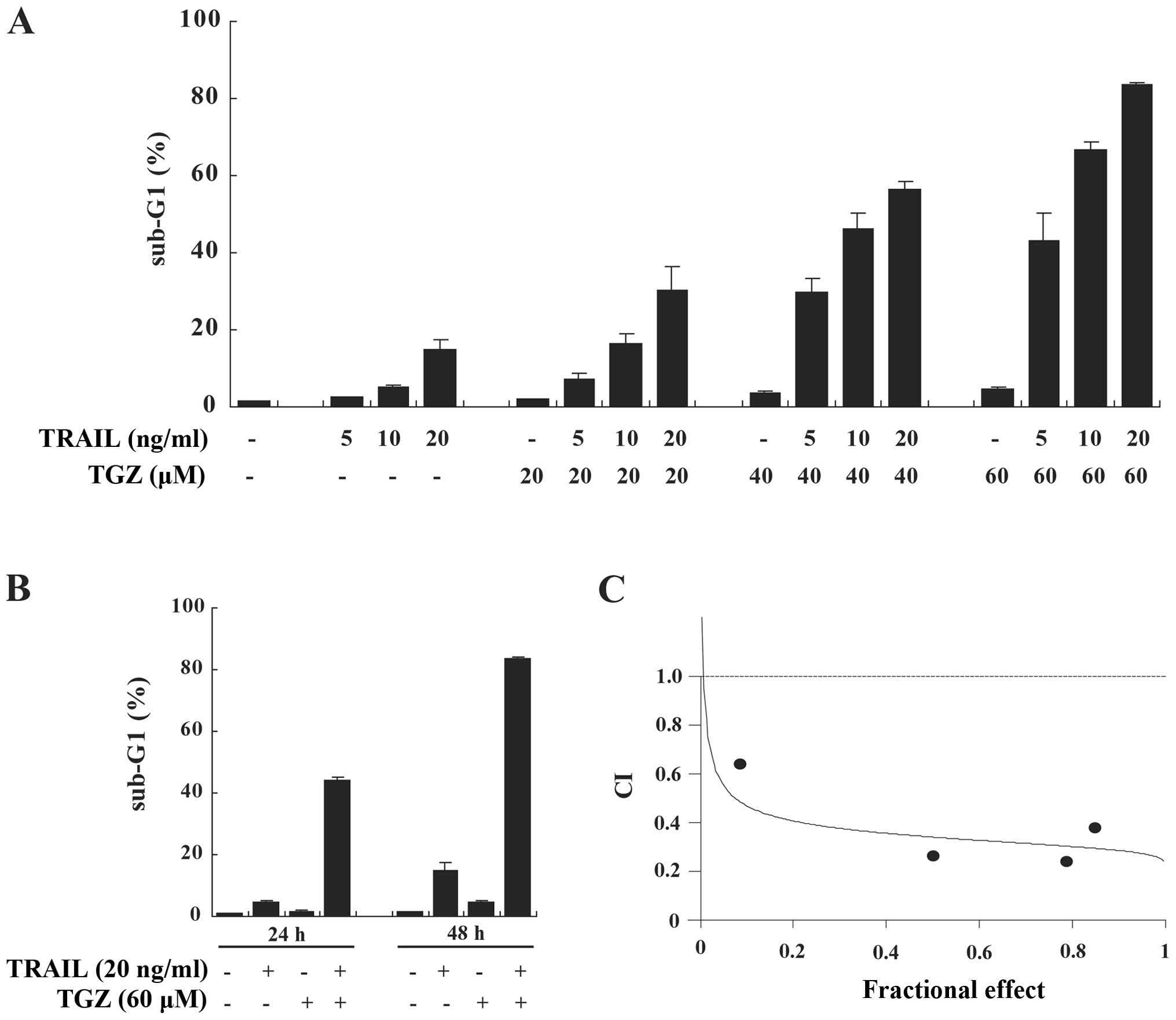
We investigated the effect of combined treatment
with TGZ and TRAIL on apoptosis by measuring the sub-G1 population
in colorectal cancer DLD-1 cells. While TGZ or TRAIL used as a
single agent caused only slight increases in apoptosis, the
combination of TGZ and TRAIL markedly promoted apoptosis in dose-
and time-dependent manners. This suggested that TGZ functions as a
sensitizer for TRAIL-induced apoptosis (Fig. 1A and B). To characterize the nature
of the synergistic apoptosis-inducing effects of co-treatment with
TGZ and TRAIL, DLD-1 cells were incubated with various
concentrations of TGZ and TRAIL at a fixed ratio. The combination
index (CI) value for TGZ and TRAIL was <1.0, indicating
synergistic apoptosis-inducing efficacy (Fig. 1C).
TGZ and TRAIL synergistically induce
caspase-dependent and PPARγ-independent apoptosis in DLD-1
cells
We next investigated the mechanism of
TGZ/TRAIL-induced apoptosis in DLD-1 cells. TGZ and TRAIL
cooperated in the activation of caspase-10, -8 and -3, and in the
cleavages of Bid and PARP (Fig.
2A). In support of these findings, TGZ/TRAIL-induced apoptosis
was blocked by the pan-caspase inhibitor and the caspase-8 and -10
inhibitors (Fig. 2B). To elucidate
whether the sensitization to TRAIL-induced apoptosis by TGZ
occurred via a specific interaction between TRAIL and its
receptors, we used a recombinant human DR5/Fc chimeric protein,
which has a dominant-negative effect by competing with endogenous
DR5 for binding to TRAIL. The DR5/Fc chimera efficiently inhibited
apoptosis induced by TGZ/TRAIL (Fig.
2B). Additionally, to determine whether TGZ/TRAIL-induced
apoptosis was independent of PPARγ activation, we used the PPARγ
irreversible antagonist, GW9662. Pretreatment with GW9662 did not
attenuate TGZ/TRAIL-induced apoptosis (Fig. 2B). Moreover, unlike TGZ,
co-treatment of cells with TRAIL and 2 other PPARγ ligands,
pioglitazone or ciglitazone, did not cause synergistic apoptosis
(Fig. 2C). These results suggest
that TGZ/TRAIL-induced apoptosis is mediated through the death
receptor pathway and is independent of PPARγ activation.
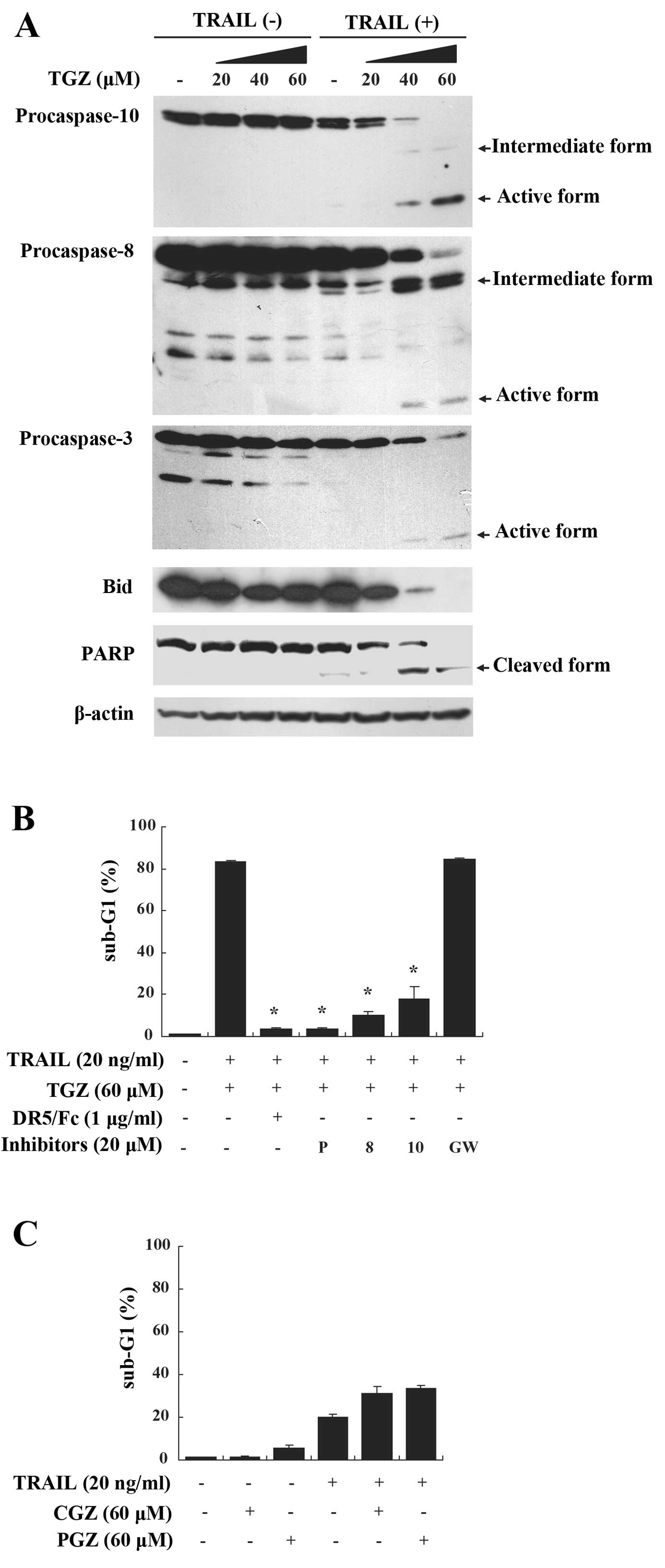 | Figure 2Apoptosis induced by TGZ and TRAIL is
mediated through caspase activation and not PPARγ. (A) DLD-1 cells
were exposed to TGZ at the indicated concentrations with or without
20 ng/ml TRAIL for 48 h. Activation of caspase-10, -8, -3 and Bid,
as well as cleavage of PARP were assessed by western blotting.
β-actin was used as a loading control. (B) DLD-1 cells were
pretreated with or without various caspase inhibitors, DR5/Fc or
GW9662 for 1 h followed by the addition of 60 μM TGZ and 20 ng/ml
TRAIL for 48 h. Apoptosis was determined as above. P, pan-caspase
inhibitor; 10, caspase-10 inhibitor; 8, caspase-8 inhibitor; GW,
GW9662. Columns, means; bars, SD (n=3). *P<0.05 when
compared with cells treated with TGZ and TRAIL. (C) DLD-1 cells
were exposed to 60 μM CGZ or 60 μM PGZ with or without 20 ng/ml
TRAIL for 48 h and apoptosis was determined as above. Columns,
means; bars, SD (n=3). TGZ, troglitazone; TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand. |
TGZ upregulates CHOP and DR5
expression
CHOP is known to be induced under conditions of ER
stress and to play important roles in ER stressor-mediated
apoptosis (22). To elucidate
whether the mechanism of TGZ-mediated enhancement of TRAIL-induced
apoptosis involved this pathway, we examined the expression of
CHOP, the ER stress marker GRP78/Bip and apoptosis-related
molecules using western blot analysis. We found that TGZ induced
the protein expression levels of GRP78/Bip, CHOP and DR5 in a
dose-dependent manner (Fig. 3A).
Expression of GRP78/Bip was upregulated 1 h after treatment with
TGZ, while that of CHOP or DR5 was enhanced at 24 h, raising the
possibility that ER stress caused by TGZ occurs upstream of
increased CHOP and DR5 expression levels (Fig. 3B). Additionally, consistent with
previous reports, TGZ decreased anti-apoptotic molecules such as
bcl-2 and survivin (27,28) (Fig.
3C). Neither pioglitazone nor ciglitazone increased DR5 and
CHOP expression levels (data not shown). These results suggest that
synergistic apoptosis induced by co-treatment with TGZ and TRAIL is
dependent on ER stress and independent of PPARγ activation.
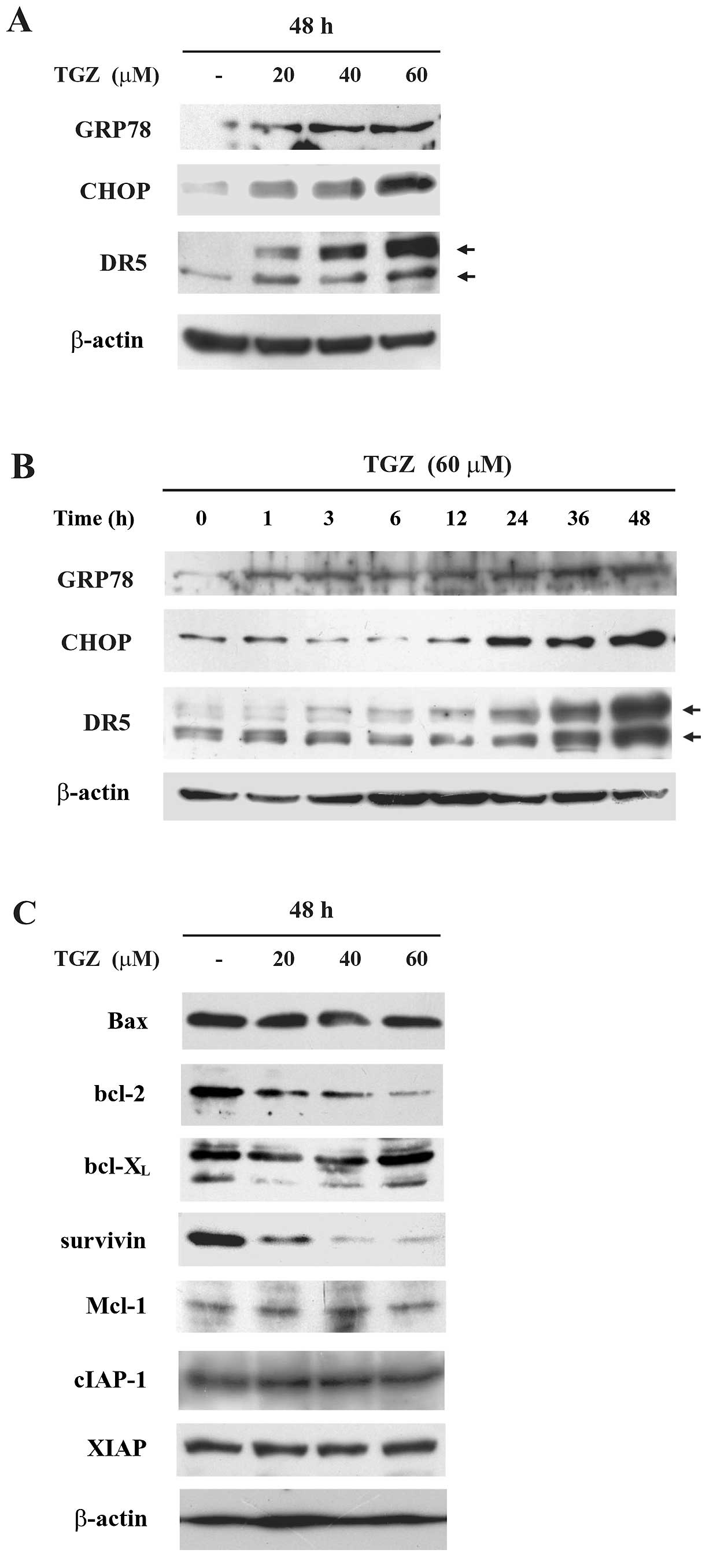 | Figure 3TGZ alters the protein expression
levels of ER stress- or apoptosis-related molecules. (A) DLD-1
cells were treated with TGZ at the indicated concentrations for 48
h. The expression of GRP78, CHOP, DR5 and β-actin (a loading
control) were assessed by western blotting. (B) DLD-1 cells were
treated with 60 μM TGZ for the indicated periods. The expression of
GRP78, CHOP, DR5 and β-actin (a loading control) were assessed by
western blotting. (C) DLD-1 cells were treated with TGZ at the
indicated concentrations for 48 h. The expression of Bax, bcl-2,
bcl-XL, survivin, Mcl-1, cIAP-1, XIAP and β-actin (a
loading control) were assessed by western blotting. TGZ,
troglitazone; CHOP, CCAAT/enhancer-binding protein homologous
protein. |
TGZ increases CHOP and DR5 expression at
their promoter activity levels
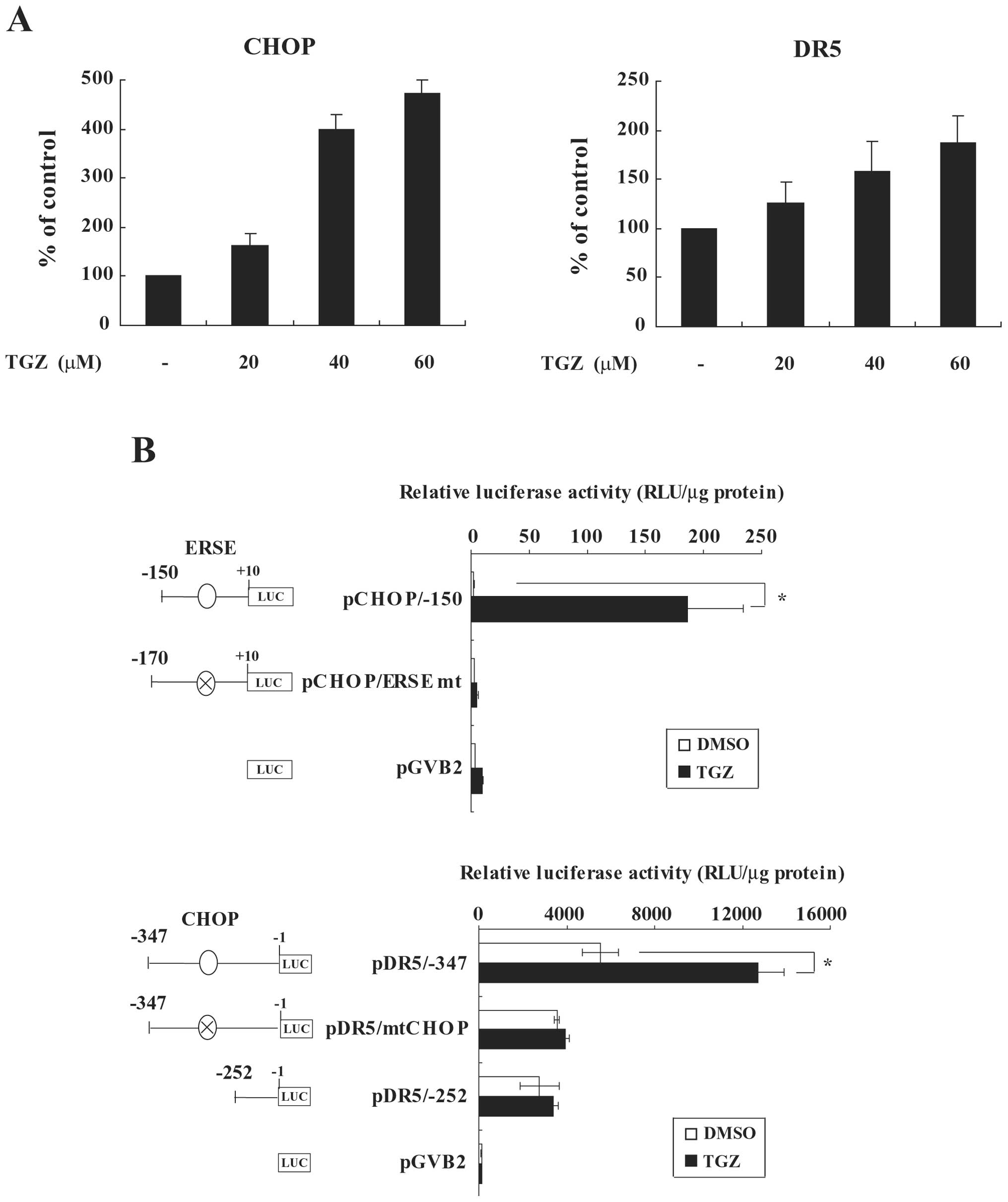
Next, to elucidate the mechanism of CHOP or DR5
upregulation by TGZ, we investigated the mRNA levels of these genes
using quantitative real-time RT-PCR analysis. TGZ increased the
mRNA levels of CHOP and DR5 in a dose-dependent manner (Fig. 4A). To further clarify the molecular
mechanism of TGZ-induced upregulation of CHOP and DR5, we analyzed
the effect of TGZ on their promoter activities using CHOP or DR5
promoter-luciferase fusion plasmids in a transient assay. TGZ
stimulated the promoter activity of pCHOP/−150 (Fig. 4B). It has been shown that the CHOP
promoter harbors an ERSE between −93 and −75 bp from the
transcription start site, which is activated by an ER stress
inducer, tunicamycin (29). Indeed,
mutation of the ERSE abolished the activation of the CHOP promoter
by TGZ (Fig. 4B upper). On the
other hand, the luciferase activity of pDR5/−347 was significantly
increased by TGZ (Fig. 4B lower).
Additionally, the promoter region from pDR5/−252 or pDR5/−347,
which harbored CHOP mutations, did not alter luciferase activity in
response to TGZ. These results indicate that TGZ transcriptionally
induces CHOP through ER stress, resulting in the upregulation of
DR5 at the promoter level.
Upregulation of DR5 and CHOP by TGZ
contributes to the enhancement of TRAIL-induced apoptosis
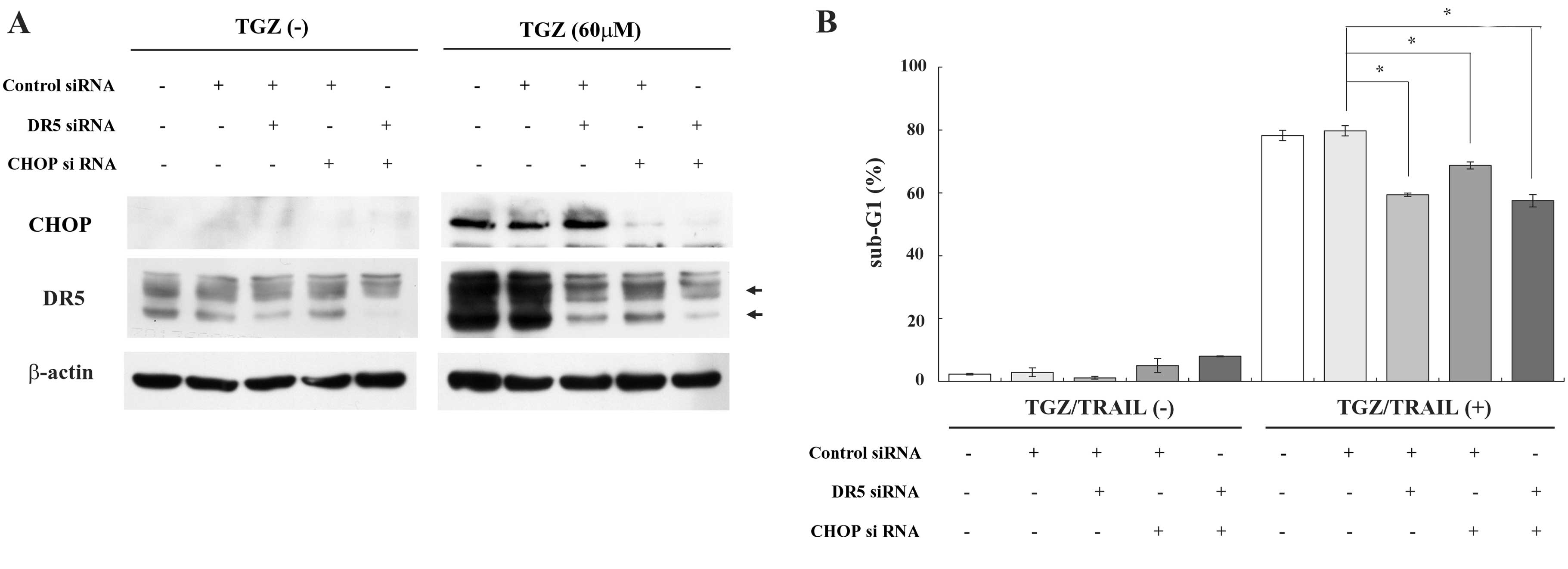
As shown in Fig. 4B,
we observed that CHOP is responsible for the transactivation of the
DR5 promoter by TGZ. Therefore, we next examined whether CHOP
and/or DR5 contributed to TGZ/TRAIL-mediated apoptosis in DLD-1
cells using CHOP and/or DR5 siRNAs. Concomitant with CHOP reduction
by CHOP siRNA, TGZ-induced DR5 upregulation was efficiently
suppressed when compared with control siRNA (Fig. 5A). In addition, transfection of CHOP
and/or DR5 siRNAs, into DLD-1 cells, at least partially impaired
the induction of apoptosis by TGZ. Taken together, these results
suggest that TGZ enhances TRAIL-triggered apoptosis through CHOP
elevation via ER stress and subsequent DR5 induction (Fig. 5B).
TGZ increases both CHOP and DR5
expression and enhances TRAIL-induced apoptosis in other malignant
tumor cells
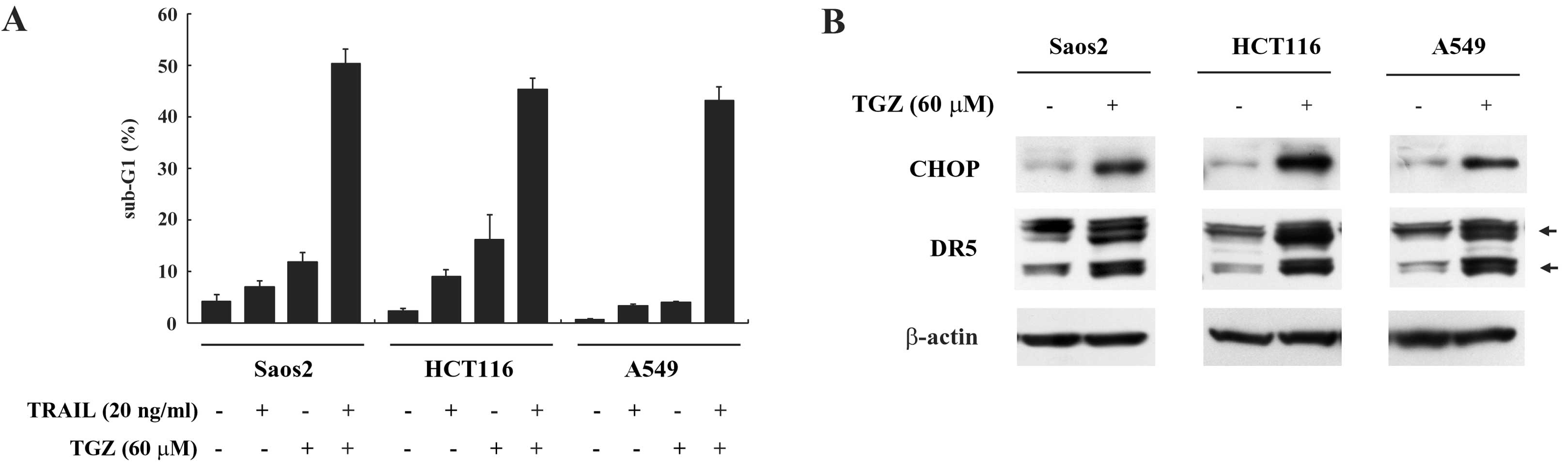
To investigate whether the effects of co-treatment
with TGZ and TRAIL on apoptosis may be observed more generally,
other malignant tumor cell lines such as Saos2, HCT116 and A549
were similarly assayed. We found that TGZ induced DR5 and CHOP
expressions and sensitized TRAIL-induced apoptosis in these cell
lines (Fig. 6A and B). These
results suggest that TGZ sensitizes TRAIL-induced apoptosis through
CHOP and DR5 induction in various malignant tumor cells.
Discussion
In cancer therapeutics, it is essential to induce
apoptosis specifically in malignant tumor cells but not in normal
cells. In this regard, TRAIL has been highlighted as a promising
anticancer agent due to its ability to selectively induce apoptosis
in malignant tumor cells. However, some tumor cells are resistant
to TRAIL-induced apoptosis and the exact mechanisms of resistance
have yet to be fully elucidated. A large number of studies have
clarified some of the molecular mechanisms of sensitivity or
resistance to TRAIL. DcR1, DcR2 and osteoprotegerin inhibit TRAIL
binding by competing with DR4 or DR5, suggesting that resistance to
TRAIL occurs at its receptor level (30–32).
Indeed, transient overexpression of DR5 in TRAIL-resistant cancer
cells restores TRAIL sensitivity (33). DR5 expression in a number of human
T-cell acute lymphoblastic leukemia Jurkat sub-clones has also been
highly correlated with sensitivity to TRAIL (34). In the present study, we demonstrated
that TGZ enhanced TRAIL-induced apoptosis through DR5 elevation,
suggesting that this combined treatment is a rational strategy that
targets DR5 in combination with TRAIL-based therapy.
The antitumor efficacy of TRAIL has been shown to be
enhanced by a variety of anticancer agents, including conventional
chemotherapeutic and molecular-targeted drugs (35). On the basis of these preclinical
data, a clinical phase Ib study of rhTRAIL with rituximab by Yee
et al was initiated in patients with relapsed low-grade
non-Hodgkin’s lymphoma. The co-treatment was active in this
disease, yielding 2 (25%) complete responses, 1 (13%) partial
response and 5 (63%) stable diseases (36). In a clinical phase Ib study by Soria
et al, patients with non-small cell lung cancer (NSCLC) were
treated with rhTRAIL, combined with the antitumor drugs,
paclitaxel, carboplatin and bevacizumab. The combination was well
tolerated and 1 (4%) complete response, 13 (54%) partial responses
and 9 (38%) stable diseases were observed (37). The results of this phase Ib trial
led the researchers to conduct a phase II study using this
combination therapy in patients with NSCLC. However, they reported
that the combined treatment did not improve clinical outcome
(38). Therefore, to enhance the
antitumor activity of TRAIL, more rational therapeutic strategies
based on molecular mechanisms underlying sensitivity and resistance
to TRAIL should be developed.
We previously showed that the proteasome inhibitor
MG132 or the antibiotic tunicamycin increased DR5 expression at the
transcriptional level via CHOP induction in association with
significant sensitization of malignant tumor cells to TRAIL-induced
apoptosis (39,40). The present study described that TGZ,
at least in part, enhances TRAIL-induced apoptosis through CHOP
induction via ER stress and subsequent DR5 elevation at their
transcriptional levels. Collectively, these findings indicate that
a variety of agents that upregulate CHOP possess the ability to
increase DR5 expression and thus enhance TRAIL-induced apoptosis.
Additionally, these agents may reduce the minimal effective dose of
TRAIL required for apoptosis induction in tumor cells and therefore
reduced the side-effects caused by high doses of TRAIL. However,
for clinical application, it remains to be elucidated whether MG132
or tunicamycin can be given safely without toxicity to normal
tissues. The present results suggest that TGZ remains promising as
a DR5 inducer when combined with TRAIL and the fact that TGZ was
clinically used for type 2 diabetes mellitus for several years may
lend a further advantage for safety, although hepatic toxicity
caused by TGZ led to its withdrawal from clinical use (41,42).
In conclusion, we have shown that TGZ induces ER
stress, resulting in CHOP and DR5 expression and subsequent
TGZ-mediated synergism of TRAIL-induced apoptosis in a variety of
human malignant tumor cells. These results suggest that the
combination of TGZ and TRAIL may be promising for treating a broad
spectrum of malignant tumors in a clinical environment.
Acknowledgements
This study was supported by a Grant-in-Aid from the
Japanese Ministry of Education, Culture, Sports, Science and
Technology.
Abbreviations:
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
TGZ
|
troglitazone
|
|
DR5
|
death receptor 5
|
|
CHOP
|
CCAAT/enhancer-binding protein
homologous protein
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keane MM, Ettenberg SA, Nau MM, Russell EK
and Lipkowitz S: Chemotherapy augments TRAIL-induced apoptosis in
breast cell lines. Cancer Res. 59:734–741. 1999.PubMed/NCBI
|
|
4
|
Schow P, Hooley J, Sherwood S, et al:
Differential hepatocyte toxicity of recombinant Apo2L/TRAIL
versions. Nat Med. 7:383–385. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan G, O’Rourke K, Chinnaiyan AM, et al:
The receptor for the cytotoxic ligand TRAIL. Science. 276:111–113.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan G, Ni J, Wei YF, Yu G, Gentz R and
Dixit VM: An antagonist decoy receptor and a death
domain-containing receptor for TRAIL. Science. 277:815–818. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelley RF, Totpal K, Lindstrom SH, et al:
Receptor-selective mutants of apoptosis-inducing ligand 2/tumor
necrosis factor-related apoptosis inducing ligand reveal a greater
contribution of death receptor (DR) 5 than DR4 to apoptosis
signaling. J Biol Chem. 280:2205–2212. 2005. View Article : Google Scholar
|
|
9
|
Ichikawa K, Liu W, Zhao L, et al:
Tumoricidal activity of a novel anti-human DR5 monoclonal antibody
without hepatocyte cytotoxicity. Nat Med. 7:954–960. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koornstra JJ, Kleibeuker JH, van Geelen
CM, et al: Expression of TRAIL (TNF-related apoptosis-inducing
ligand) and its receptors in normal colonic mucosa, adenomas, and
carcinomas. J Pathol. 200:327–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herbst RS, Eckhardt SG, Kurzrock R, et al:
Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a
dual proapoptotic receptor agonist, in patients with advanced
cancer. J Clin Oncol. 28:2839–2846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tontonoz P, Hu E, Graves RA, Budavari AI
and Spiegelman BM: mPPAR gamma 2: tissue-specific regulator of an
adipocyte enhancer. Genes Dev. 8:1224–1234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tontonoz P, Hu E and Spiegelman BM:
Stimulation of adipogenesis in fibroblasts by PPARγ2, a
lipid-activated transcription factor. Cell. 79:1147–1156. 1994.
|
|
14
|
Chawla A, Schwarz EJ, Dimaculangan DD and
Lazar MA: Peroxisome proliferator-activated receptor (PPAR) gamma:
adipose-predominant expression and induction early in adipocyte
differentiation. Endocrinology. 135:798–800. 1994.
|
|
15
|
Fujiwara T, Yoshioka S, Yoshioka T,
Ushiyama I and Horikoshi H: Characterization of new oral
antidiabetic agent CS-045. Studies in KK and ob/ob mice and Zucker
fatty rats. Diabetes. 37:1549–1558. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehmann JM, Moore LB, Smith-Oliver TA,
Wilkison WO, Willson TM and Kliewer SA: An antidiabetic
thiazolidinedione is a high affinity ligand for peroxisome
proliferator-activated receptor γ (PPARγ). J Biol Chem.
270:12953–12956. 1995.
|
|
17
|
Kubota T, Koshizuka K, Williamson EA, et
al: Ligand for peroxisome proliferator-activated receptor γ
(troglitazone) has potent antitumor effect against human prostate
cancer both in vitro and in vivo. Cancer Res. 58:3344–3352.
1998.
|
|
18
|
Grommes C, Landreth GE and Heneka MT:
Antineoplastic effects of peroxisome proliferator-activated
receptor γ agonists. Lancet Oncol. 5:419–429. 2004.
|
|
19
|
Kulke MH, Demetri GD, Sharpless NE, et al:
A phase II study of troglitazone, an activator of the PPARγ
receptor, in patients with chemotherapy-resistant metastatic
colorectal cancer. Cancer J. 8:395–399. 2002.PubMed/NCBI
|
|
20
|
Burstein HJ, Demetri GD, Mueller E, Sarraf
P, Spiegelman BM and Winer EP: Use of the peroxisome
proliferator-activated receptor (PPAR) γ ligand troglitazone as
treatment for refractory breast cancer: a phase II study. Breast
Cancer Res Treat. 79:391–397. 2003.
|
|
21
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maniratanachote R, Minami K, Katoh M,
Nakajima M and Yokoi T: Chaperone proteins involved in
troglitazone-induced toxicity in human hepatoma cell lines. Toxicol
Sci. 83:293–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satoh T, Toyoda M, Hoshino H, et al:
Activation of peroxisome proliferator-activated receptor-γ
stimulates the growth arrest and DNA-damage inducible 153 gene in
non-small cell lung carcinoma cells. Oncogene. 21:2171–2180.
2002.
|
|
25
|
Koyama M, Matsuzaki Y, Yogosawa S, Hitomi
T, Kawanaka M and Sakai T: ZD1839 induces p15INK4b and
causes G1 arrest by inhibiting the mitogen-activated
protein kinase/extracellular signal-regulated kinase pathway. Mol
Cancer Ther. 6:1579–1587. 2007.PubMed/NCBI
|
|
26
|
Koyama M, Izutani Y, Goda AE, et al:
Histone deacetylase inhibitors and
15-deoxy-Δ12,14-prostaglandin J2
synergistically induce apoptosis. Clin Cancer Res. 16:2320–2332.
2010.
|
|
27
|
Elstner E, Müller C, Koshizuka K, et al:
Ligands for peroxisome proliferator-activated receptor γ and
retinoic acid receptor inhibit growth and induce apoptosis of human
breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci
USA. 95:8806–8811. 1998.
|
|
28
|
Lu M, Kwan T, Yu C, et al: Peroxisome
proliferator-activated receptor γ agonists promote TRAIL-induced
apoptosis by reducing survivin levels via cyclin D3 repression and
cell cycle arrest. J Biol Chem. 280:6742–6751. 2005.
|
|
29
|
Ubeda M and Habener JF: CHOP gene
expression in response to endoplasmic-reticular stress requires NFY
interaction with different domains of a conserved DNA-binding
element. Nucleic Acids Res. 28:4987–4997. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walczak H, Degli-Esposti MA, Johnson RS,
et al: Cloning and characterization of TRAIL-R3, a novel member of
the emerging TRAIL receptor family. J Exp Med. 186:1165–1170. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Degli-Esposti MA, Dougall WC, Smolak PJ,
Waugh JY, Smith CA and Goodwin RG: The novel receptor TRAIL-R4
induces NF-κB and protects against TRAIL-mediated apoptosis, yet
retains an incomplete death domain. Immunity. 7:813–820. 1997.
|
|
32
|
Emery JG, McDonnell P, Burke MB, et al:
Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J
Biol Chem. 273:14363–14367. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mitsiades N, Poulaki V, Mitsiades C and
Tsokos M: Ewing’s sarcoma family tumors are sensitive to tumor
necrosis factor-related apoptosis-inducing ligand and express death
receptor 4 and death receptor 5. Cancer Res. 61:2704–2712.
2001.
|
|
34
|
Jang YJ, Park KS, Chung HY and Kim HI:
Analysis of the phenotypes of Jurkat clones with different
TRAIL-sensitivities. Cancer Lett. 194:107–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hellwig CT and Rehm M: TRAIL signaling and
synergy mechanisms used in TRAIL-based combination therapies. Mol
Cancer Ther. 11:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yee L, Fanale M, Dimick K, et al: A Phase
IB safety and pharmacokinetic (PK) study of recombinant human
Apo2L/TRAIL in combination with rituximab in patients with
low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 25:S4602007.
|
|
37
|
Soria JC, Smit E, Khayat D, et al: Phase
1b study of dulanermin (recombinant human Apo2L/TRAIL) in
combination with paclitaxel, carboplatin, and bevacizumab in
patients with advanced non-squamous non-small-cell lung cancer. J
Clin Oncol. 28:1527–1533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soria JC, Márk Z, Zatloukal P, et al:
Randomized phase II study of dulanermin in combination with
paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell
lung cancer. J Clin Oncol. 29:4442–4451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida T, Shiraishi T, Nakata S, et al:
Proteasome inhibitor MG132 induces death receptor 5 through
CCAAT/enhancer-binding protein homologous protein. Cancer Res.
65:5662–5667. 2005. View Article : Google Scholar
|
|
40
|
Shiraishi T, Yoshida T, Nakata S, et al:
Tunicamycin enhances tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis in human prostate
cancer cells. Cancer Res. 65:6364–6370. 2005. View Article : Google Scholar
|
|
41
|
Gitlin N, Julie NL, Spurr CL, Lim KN and
Juarbe HM: Two cases of severe clinical and histologic
hepatotoxicity associated with troglitazone. Ann Intern Med.
129:36–38. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scheen AJ: Thiazolidinediones and liver
toxicity. Diabetes Metab. 27:305–313. 2001.PubMed/NCBI
|