Introduction
Positron emission tomography (PET) is a method for
determining biochemical and physiological processes by using
radiopharmaceuticals labeled with positron-emitting radionuclides.
FDG-PET has been widely applied to assess brain function, heart
muscle metabolism and for the diagnosis of various tumors (1,2);
however, the positive predictive value of prostate cancer has been
considered to be low. A previous study reported that the maximum
standardized uptake value (SUVmax) is not essential for
differential diagnostic criteria of prostatic lesions (3). Prostate cancer shows no or mild FDG
uptake because of its low glucose metabolism (4,6). In
addition, FDG-PET is able to detect some prostate cancers due to
urinary excretion of the radiotracer (5,6). FDG
uptake is known to be unspecific to the cancer and may result from
an inflammatory condition such as prostatitis (7). A previous study reported that FDG-PET
shows 4.0% of histopathology-confirmed prostate cancer (8). On the other hand, cancer surveillance
has revealed that the incidence of second primary cancers is
1.53–8.5% in patients with known first malignancies, and this
incidence has risen due to the increase in the number of elderly
patients and the prolonged cancer survival rate (9,10).
Detection of second primary cancers is important, since they have a
significant influence on patient management, particularly early
cancers that require radical treatment (11).
Incidental uptake in the prostate is often
experienced in clinical practice, but it is difficult to determine
whether the uptake indicates a malignancy or a benign state based
on the SUV alone. Only a few reports have described incidental
prostate uptake on FDG-PET/CT (3).
In the present study, we investigated the frequency and clinical
significance of incidental prostate uptake on FDG-PET/CT and
examined the relationship between the location of FDG uptake and
prostate calcification.
Materials and methods
Patients
From May 2008 to August 2012, 3,236 male patients
underwent 18F-fluorodeoxyglucose
(18F-FDG)-PET/CT scans for various types of cancers at
our hospital. Of the 3,236 PET/CT scan cases, 53 cases demonstrated
incidental FDG uptake in the prostate. Of the 53 PET/CT scans, we
analyzed the 49 cases demonstrating incidental FDG uptake with
sufficient follow-up in this retrospective study, while 4 cases
were excluded from the study due to insufficient follow-up.
18F-FDG PET/CT
In preparation for PET/CT, all patients fasted for
at least 4 h, while water intake was encouraged. Delivered
18F-FDG (FDG scan injectable, 185 MBq on assay date;
Nihon Medi-Physics, Co., Ltd., Tokyo, Japan) was injected
intravenously and scanning was initiated 60 min later. During the
60-min uptake period, the patients drank a sufficient amount of
water. A PET/CT system (Discovery ST Elite 16; GE Healthcare,
Buckinghamshire, UK) was used to acquire all data in 7–8 bed
positions, with an acquisition time of 2.5–3.0 min per bed
position. CT was performed first (30–80 mA, 120 kV, 3.75–3.27 mm
slice thickness). CT data were used for attenuation correction of
PET data, as well as for co-registration with attenuation-corrected
PET images. Then, PET data were acquired immediately from the same
body region. PET, CT and fused PET/CT images were available for
review and were displayed in axial, coronal and sagittal planes on
a viewer system.
18F-FDG PET analysis
FDG uptake in the prostate was visually defined as
positive or negative. In the present study, physiological uptake in
the prostate urethra on coronal, sagittal and axial views was
excluded. The SUVmax for the prostate was obtained from transaxial
views, and analyzed by the Mann-Whitney test (P<0.05,
statistical significance). Prostate sites of FDG uptake (inner or
peripheral zone) and patterns of FDG uptake (focal or diffuse) were
evaluated on the axial view. The CT portion of the FDG-PET/CT was
used to recognize the inner (prostate central gland: central +
transition zones) and peripheral zones according to the contrast
difference (Fig. 1).
Prostate calcification analysis
When prostate calcification and the FDG uptake area
overlapped in 40% or more of the areas, the cases were defined as
‘uptake coexisting with calcification’. The other cases, i.e.
<40% of overlapped areas, were defined as ‘uptake not coexisting
with calcification’ (Fig. 2). In
the present study, the number and position of the prostate
calcifications were not evaluated.
Clinicopathological examinations
Final clinical diagnoses of the patients were
determined based on the summarized results of the biopsy, serum
prostate-specific antigen (PSA) levels, imaging studies (CT, MRI,
follow-up PET/CT) and urological examinations. Urologists performed
a biopsy for suspicious cases, and 12 patients underwent biopsy. In
28 cases, PSA levels were calculated after the PET/CT scan.
Results
Of the 3,236 PET/CT images, incidental FDG uptake of
the prostate was observed in 53 cases (2%), while 4 cases were
excluded. These 49 cases were analyzed in the present study. Of the
49 cases, 8 (16%) had prostate cancer, and 41 (84%) were benign,
i.e., the prostatic cancer discovery rate was 0.25% for all PET/CT
scans. The SUVmax was not significantly different between the two
groups.
Malignant lesions
Of the 8 prostate cancer cases, 7 were ordinary
adenocarcinomas and the other case was signet-ring cell carcinoma.
All 7 cases of adenocarcinoma showed increased serum PSA levels,
but the case of signet-ring cell carcinoma exhibited serum PSA
levels within the normal range. The mean SUVmax of the 8 malignant
lesions was 7.2±2.0. Prostate calcifications were present near the
FDG uptake in 2 cases. All 8 cancer cases showed FDG uptake not
coexisting with calcification, while the cancer lesions were
located in the peripheral zone in 7 cases, and in both inner and
peripheral zones in one case (Table
I).
 | Table IMalignant cases (n=8). |
Table I
Malignant cases (n=8).
| Patients | Age (years) | Indication for
PET/CT | Gleason score | PSA (ng/ml) | SUVmax | Site | Calcification |
|---|
| Patient 1 | 57 | Lung cancer | 3+4 | 30.55 | 6.7 | Peripheral | Absent |
| Patient 2 | 67 | Bone tumor | 4+5 | 85.20 | 9.0 | Peripheral | Absent |
| Patient 3 | 73 |
Cholangiocarcinoma | 4+4 | 8.23 | 5.7 | Peripheral | Absent |
| Patient 4 | 76 | Head and neck
cancer | 4+5 | 49.10 | 7.9 | Peripheral | Presenta |
| Patient 5 | 80 | Head and neck
cancer | 4+5 | 31.00 | 7.3 | Peripheral | Presenta |
| Patient 6 | 61 | Cancer screening | 5+4 | 0.19 | 7.0 | Peripheral | Absent |
| Patient 7 | 78 | Lung cancer | 4+5 | 19.71 | 3.7 | Peripheral | Absent |
| Patient 8 | 66 | Cancer screening | 4+5 | 79.60 | 10.9 | Peripheral-inner | Absent |
Benign lesions
Of the 41 benign cases, 8 showed increased serum PSA
levels, and 12 exhibited serum PSA levels within the normal range.
PSA levels of the other 21 cases were not evaluated after PET/CT,
but they were diagnosed as benign lesions by follow-up imaging
studies or medical examination by urologists. The mean SUVmax of
the 41 cases was 6.0±1.8. Eighteen cases (44%) exhibited FDG uptake
coexisting with prostate calcifications, i.e. 13 of the 18 cases
showed uptake in the inner zone and 5 in the peripheral zone. Of
the 41 benign cases, 23 did not coexist with prostate
calcification; there was FDG uptake in the inner zone in 6 cases,
peripheral zone in 12 cases, and the inner to peripheral zone in 5
cases (Table II).
 | Table IIBenign cases: Uptake with or without
calcification. |
Table II
Benign cases: Uptake with or without
calcification.
| A, Uptake with
calcification (n=18) |
|---|
|
|---|
| Site | PSA unmeasured | PSA normal | PSA high |
|---|
| Inner zone
(n=13) | 10 | 2 | 1 |
| Peripheral zone
(n=5) | 3 | 1 | 1 |
|
| B, Uptake without
calcification (n=23) |
|
| Site | PSA unmeasured | PSA normal | PSA high |
|
| Inner zone (n=6) | 4 | 1 | 1 |
| Peripheral zone
(n=12) | 1 | 8 | 3 |
| Both lobes (n=4) | 3 | 0 | 1 |
| Right lobes
(n=1) | 0 | 0 | 1 |
Discussion
Our results demonstrated that abnormal
hypermetabolic lesions in the prostate glands are not common (2%),
and most were benign lesions (84%). The results are similar to
those of previous reports (Table
III); however, the incidence of prostate cancer in the present
study was much different from previous studies. Cho et al
(3) described incidental prostate
uptake in 148 of 14,854 scans (1.0%), and prostate cancer in 9 of
67 subjects (13.4%) with further evaluations. Han et al
(12) observed incidental prostate
uptake in 63 of 5,119 scans (1.2%), and prostate cancer in 3 of 55
subjects (5.4%) with further evaluation. Hwang et al
(13) observed incidental prostate
uptake in 184 of 1,2037 scans (1.5%), and prostate cancer was found
in 23 of 120 subjects (19.2%) with further evaluation. The
different frequencies of incidental prostate cancer resulted from
not only the large number of enrolled patients, but also the
different confirmation methods. They also speculated that the
incidence of prostate cancer detected by PET/CT might depend on the
age and characteristics of the population, such as healthy people
or patients with other cancers.
 | Table IIISummary of incidental FDG uptake in
previously reported cases. |
Table III
Summary of incidental FDG uptake in
previously reported cases.
| Authors/(ref.) | Subjects | Cases of incidental
FDG uptake n (%) | Number of
malignancies |
|---|
| Han et al
(12) | 5,119 | 63 (1.2) | 3 |
| Cho et al
(3) | 14,854 | 148 (1.0) | 9 |
| Hwang et al
(13) | 12,037 | 184 (1.5) | 23 |
The incidence of prostatic cancer has recently
increased in elderly people aged 60 and over. Tumor growth is
gradual and it occurs frequently as multiple lesions. More than 95%
of prostate cancer consists of adenocarcinoma histologically, and
most prostate cancers arise from the peripheral zone (14). All of our carcinoma cases existed in
the peripheral zone, whereas benign lesions existed in the inner
zone.
In elderly male patients, corpora amylacea, dense
accumulations of calcified proteinaceous material, are frequently
found in the prostatic ducts of elderly men (Fig. 3) and gradually form prostatic
calcification, often without related clinical symptoms. Prostatic
calcifications are associated with benign hyperplasia, chronic
granulomatous prostatitis, and a history of chronic obstruction or
stasis, such as in patients with urethral strictures and secondary
intraprostatic reflux of urine (15–18).
Bock et al (19) reported
that prostatic calcifications were noted in 47.2% of men under 50
years of age and in 86% of men over 50 years of age.
Histopathologically, corpora amylacea are frequently observed in
benign glands but are rarely noted in carcinomas (20). On the other hand, prostatic
crystalloid (intraluminal eosinophilic structures), intraluminal
acidic mucin and intraluminal amorphous eosinophilic materials are
less frequently found in both benign glands and adenocarcinomas
(21–23). It is extremely important to
determine corpora amylacea as criteria of benign lesions. Prostatic
crystalloid (intraluminal eosinophilic structures), intraluminal
acidic mucin, or intraluminal amorphous eosinophilic materials are
invisible to CT because of their low density of calcification,
while corpora amylacea are visualized by CT (20); therefore, FDG uptake coexisting with
calcification visualized on CT is almost always consistent with
benign findings (Fig. 4). On the
other hand, FDG uptake without calcification in the peripheral zone
is thought to possibly indicate a malignant lesion, but the
differential diagnosis of benign/malignant prostatic lesions is not
easy; and further clinical examinations such as serum PSA, MRI and
biopsy are necessary to verify the diagnosis (24,25).
FDG uptake is not only specific to cancer, but is
also positive in inflammatory conditions such as prostatitis.
Previous studies have reported that inflammation is associated with
prostate cancer (26,27); however, reproducible FDG uptake in
the same area is thought to be a potential malignant lesion, and
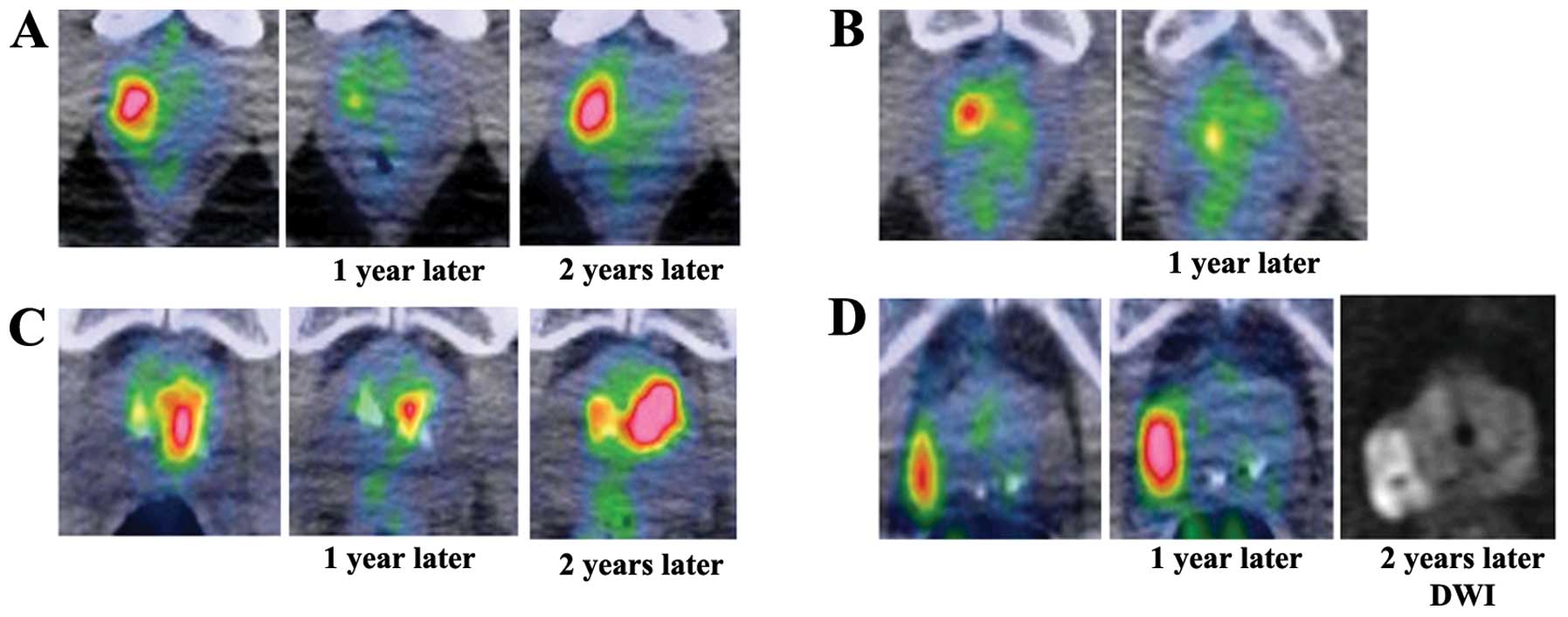
careful observation is recommended (Fig. 5). In the present study, 4 patients
showed reproducible FDG uptake in the same area and 1 patient had
adenocarcinoma. In addition, distribution of the FDG uptake is
important (15,28); FDG uptake in the inner area probably
indicates benign uptake, since prostatic cancer frequently occurs
in the peripheral zone, but not in the inner glands.
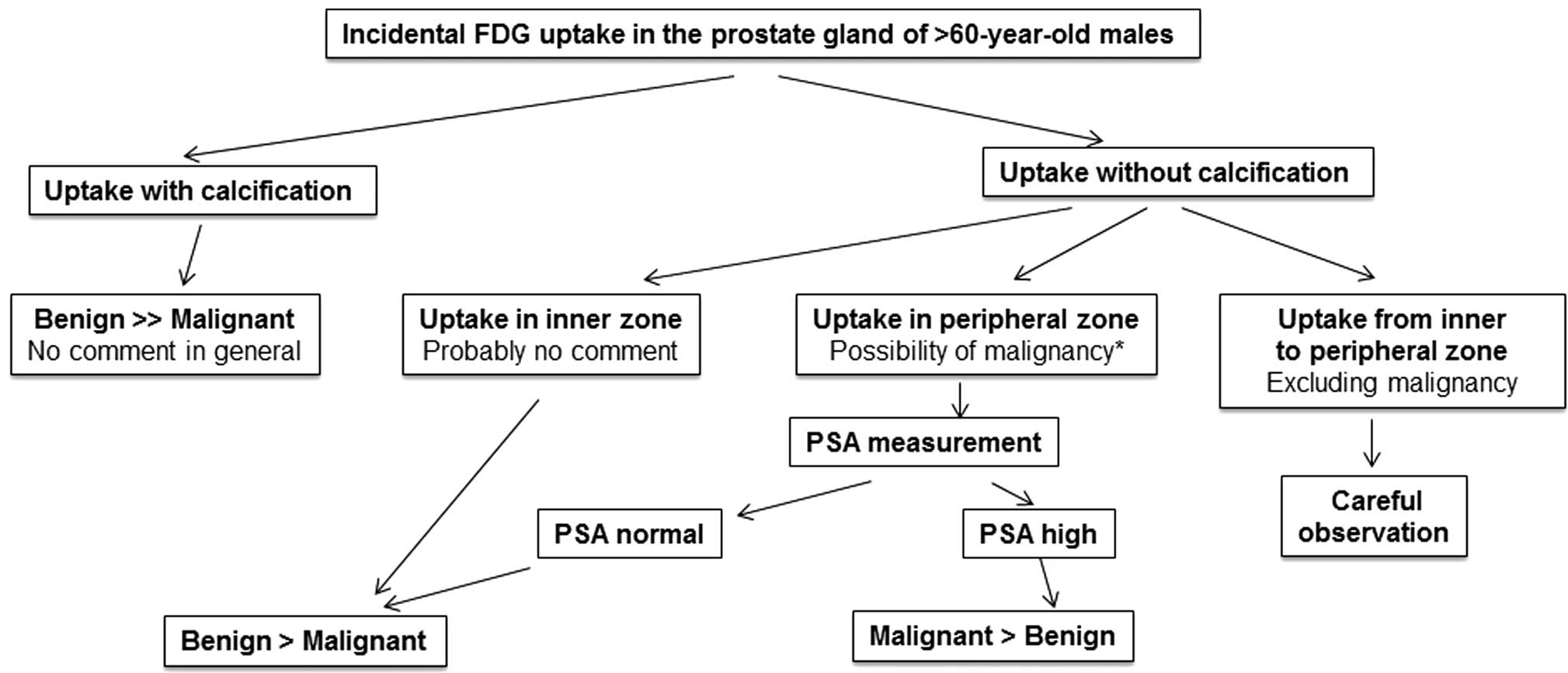
Fig. 6 is a
diagnostic flow chart of incidental FDG uptake in the prostate.
In conclusion, incidental FDG uptake in the prostate
is an extremely rare finding in patients who undergo FDG-PET/CT.
FDG uptake coexisting with calcification is indicative of a benign
lesion; however, FDG uptake without calcification in the peripheral
zone can indicate prostate cancer, and further examinations such as
PSA, MRI and biopsy are necessary to exclude malignancy.
Reproducible FDG uptake should be observed carefully since it can
indicate malignancy or probable malignant potential.
References
|
1
|
Phelps ME: PET: the merging of biology and
imaging into molecular imaging. J Nucl Med. 41:661–681.
2000.PubMed/NCBI
|
|
2
|
Gambhir SS: Molecular imaging of cancer
with position emission tomography. Nat Rev Cancer. 2:683–693. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho SK, Choi JY, Yoo J, Cheon M, Lee JY,
Hyun SH, Lee EJ, Lee KH and Kim BT: Incidental focal
18F-FDG uptake in the prostate: clinical significance
and differential diagnostic criteria. Nucl Med Mol Imaging.
45:192–196. 2011.
|
|
4
|
Effert PJ, Bares H, Handt S, Wolff JM,
Büll U and Janse G: Metabolic imaging of untreated prostate cancer
by PET with 18fluorine labeled deoxyglucose. J Urol.
155:994–998. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofer C, Laubenbacher C, Block T, Breul J,
Hartung R and Schwaiger M: Fluorine-18-fluorodeoxyglucose positron
emission tomography is useless for the detection of local
recurrence after radical prostatectomy. Eur Urol. 36:31–35. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi N, Inoue T, Lee J, Yamaguchi T
and Shizukuishi K: The roles of PET and PET/CT in the diagnosis and
management of prostate cancer. Oncology. 72:226–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kao PF, Chou YH and Lai CW: Diffuse FDG
uptake in acute prostatitis. Clin Nucl Med. 33:308–310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu IJ, Zafar MB, Lai YH, Segall GM and
Terris MK: Fluorodeoxyglucose positron emission tomography studies
in diagnosis and staging of clinically organ-confined prostate
cancer. Urology. 57:108–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong C and Hemminki K: Second primary
neoplasms among 53 159 haemotolymphoproliferative malignancy
patients in Sweden, 1958–1996 a search for common mechanisms. Br J
Cancer. 85:997–1005. 2001.PubMed/NCBI
|
|
10
|
Ueno M, Muto T, Oya M, Ota H, Azekura K
and Yamaguchi T: Multiple primary cancer: an experience at the
Cancer Institute Hospital with special reference to colorectal
cancer. Int J Clin Oncol. 8:162–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leön X, Ferlito A, Myer CM III, Saffiotti
U, Shaha AR, Bradley PJ, Brandwein MS, Anniko M, Elluru RG and
Rinaldo A: Second primary tumors in head and neck cancer patients.
Acta Otolaryngol. 122:765–778. 2002.
|
|
12
|
Han EJ, HOJ, Choi WH, Yoo IR and Chung SK:
Significance of incidental focal uptake in prostate on
18-fluro-2-deoxyglucose positron emission tomography CT images. Br
J Radiol. 83:915–920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang I, Chong A, Jung SI, Hwang EC, Kim
SO, Kang TW, Kwon DD, Park K and Ryu SB: Is further evaluation
needed for incidental focal uptake in the prostate in
18-fluori-2-deoxyglucose positron emission tomography-computed
tomography images? Ann Nucl Med. 27:140–145. 2013. View Article : Google Scholar
|
|
14
|
McNeal JE: The zonal anatomy of the
prostate. Prostate. 2:35–49. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kilmas R, Bennett B and Gardner WA Jr:
Prostate calculi: a review. Prostate. 7:91–96. 1985. View Article : Google Scholar
|
|
16
|
Eykyn S, Bultitude MI, Mayo ME and
Lloyd-Davies RW: Prostatic calculi as a source of recurrent
bacteriuria in the male. Br J Urol. 46:527–532. 1974.PubMed/NCBI
|
|
17
|
Meares EM: Infection stones of prostate
gland. Laboratory diagnosis and clinical management. Urology.
4:560–566. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cross PA, Bartley CJ and McClure J:
Amyloid in prostatic corpora amylacea. J Clin Pathol. 45:894–897.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bock E, Calugi V, Stolfi V, Rossi P,
D’Ascenzo R and Solivetti FM: Calcifications of the prostate: a
transrectal echographic study. Radiol Med. 77:501–503. 1989.(In
Italian).
|
|
20
|
Cohen RJ, McNeal JE, Redmond SL, Meehan K,
Thomas R, Wilce M and Dawkins HJ: Luminal contents of benign and
malignant prostatic glands: correspondence to altered secretory
mechanisms. Hum Pathol. 31:94–100. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luna More S: Argyrophil crystalloids in
adenocarcinoma of the prostate. Prostate. 24:309–315.
1993.PubMed/NCBI
|
|
22
|
Luna More S, Florez P, Ayala A, Diaz F and
Santos A: Neutral and acid mucins and eosinophilic and argyrophil
crystalloids in carcinoma and atypical adenomatous hyperplasia of
the prostate. Pathol Res Pract. 193:291–298. 1997.PubMed/NCBI
|
|
23
|
Epstein JI: Diagnostic criteria of limited
adenocarcinoma of the prostate on needle biopsy. Hum Pathol.
26:223–229. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee R, Localio AR, Armstrong K, Malkowicz
SB and Schwartz JS: A meta-analysis of the performance
characteristics of the free prostate-specific antigen test.
Urology. 67:762–768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wefer AE, Hricak H, Vigneron DB, Coakley
FV, Lu Y, Wefer J, Meller-Lisse U, Carroll PR and Kurhanewicz J:
Sextant localization of prostate cancer: comparison of sextant
biopsy, magnetic resonance imaging and magnetic resonance
spectroscopic imaging with step section histology. J Urol.
164:400–404. 2000. View Article : Google Scholar
|
|
26
|
De Marzo AM, Platz EA, Sutcliffe S, Xu J,
Grönberg H, Drake CG, Nakai Y, Issacs WB and Nelson WG:
Inflammation in prostate carcinogenesis. Nat Rev Cancer. 7:256–269.
2004.
|
|
27
|
Vral A, Magri V, Montanari E, Gazzano G,
Gourvas V, Marras E and Perletti G: Topographic and quantitative
relationship between prostate inflammation, proliferative
inflammatory atrophy and low-grade prostate intraepithelial
neoplasia: a biopsy study in chronic prostatitis patients. Int J
Oncol. 41:1950–1958. 2012.
|
|
28
|
McNeal JE, Redwine EA, Freiha FS and
Stamey TA: Zonal distribution of prostatic adenocarcinoma.
Correlation with histologic pattern and direction of spread. Am J
Surg Pathol. 12:897–906. 1988. View Article : Google Scholar : PubMed/NCBI
|