Introduction
It is well known that pancreatic cancer is a lethal
disease that is difficult to treat. Surgical resection remains the
only potential curative approach to a small proportion in early
stage (1). There is a need for the
continual development of multimodality treatments including
immunotherapy to improve patient prognosis (2).
As a member of the transmembrane mucin family
(3,4), MUC4 is considered to have considerable
potential as an immunotherapy target for pancreatic cancer
(5,6). MUC4, in particular, is aberrantly
expressed in pancreatic ductal adenocarcinoma (PDAC) and
precancerous pancreatic intraepithelial neoplasias (PanINs), but
not in benign or normal parts of pancreas (7–9). The
level of MUC4 expression correlates significantly with a poor
prognosis of PDAC (9,10). Previous studies suggested that
cancer cells could use MUC4 for invasion, metastasis and resistance
to chemotherapy (11–14). MUC4 antigen-pulsed vaccines are
promising to eradicate focus in early stage and eliminate the
minimal residual lesion remaining after surgical resection with
minimizing damage to normal cells. Thus, the development in
MUC4-antigen related vaccines is favorable for prevention of tumor
recurrence and the approaches posses safety and tolerability with
less substantial toxicity.
In our previous studies (15–17),
we developed MUC4-antigen related dendritic cell (DC) vaccines
which elicited MUC4 antigen-specific cytotoxic T lymphocyte
(MS-CTL) response against MUC4-expressing tumor cells in
vitro. During examination of the cytotoxic activity of the
vaccine in vitro, we observed the apoptosis of CTLs and
noted that the apoptotic rate of CTLs co-cultured with
MUC4+ tumor cells was significantly increased compared
to those co-cultured with T2 cells of pulsed MUC4-epitope- peptide,
suggesting MUC4+ tumor cells could counterattack MS-CTLs
via some pathway during the cytolytic process.
MUC4-bearing tumor cells lose their polarity,
allowing MUC4 to be uniformly expressed all over the cell surface
(18). With a larger size (ranges
between 1.1 and 2.1 μm, depending on the length of the central
tandem repeat domain) above the cell surface, MUC4 could mask most
cell surface molecules (not exceeding a length of 35 nm) and
interrupt their functions on cell-cell interactions (18). On the other hand, the aberrant
overexpression or effusion of MUC4 mucin, including distinct types
protein (secreted or membrane-associated forms), confers on tumor
cells potential ligands for interaction with other receptors at the
cell surface, and they might inactivate immune effector cells
through receptor-ligand interactions (19). Thus, we postulated that MUC4 might
contribute to the enhanced apoptotic rate of MS-CTLs during the
killing process.
In the present study, we first isolated pure
CD8+ T cells before the induction and amplification of
MS-CTLs to eliminate the killing influence of Tregs. Secondly, we
fixed basic parameter and defined precise gating template for
apoptosis measurements by flow cytometry in order to investigate
the nature of the apoptosis of MS-CTLs in co-incubation system.
Thirdly, we repeated comparative analysis of the apoptosis rate of
MS-CTLs incubated with MUC4+ tumor cells and
pulsed-peptide T2 cells to confirm the previous observations. Then,
we addressed whether high levels of MUC4 by pancreatic cancer cells
would have an effect on the significant increase of apoptosis rate
of MS-CTLs: i) clonal MUC4-knockdown HPAC sublines with different
MUC4 expression were selected for co-incubation system and further
analyses were performed; ii) the total apoptosis-induced effects of
supernatants on MS-CTLs were compared among distinct co-cultured
groups; iii) analyses of the blocking effect of Fas on MS-CTL
surface were applied to further elucidate whether the Fas-FasL
pathway might be involved in MS-CTL apoptosis in co-incubation
system.
Materials and methods
Cell lines, cytokines, culture medium,
epitope peptide
T2 cells (HLA-A2+/MUC4−, TAP
deficient) were a generous gift from Weifeng Chen (Immunity
Department, Peking University). The three human
HLA-A2+/MUC4+ tumor cell lines were obtained
from the American Type Culture Collection (ATCC; Manassas, VA,
USA), including HCT 116 (colorectal carcinoma), CFPAC-1 and HPAC
(pancreatic adenocarcinoma). HCT 116 cells were grown in McCoy’s-5A
medium (Sigma-Aldrich, St. Louis, MO, USA). Other lines were
cultured in DMEM (Gibco, Auckland, New Zealand), supplemented with
10% heat-inactivated fetal calf serum (Gibco, Carlsbad, CA, USA), 2
mM glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin at 37°C
in 5% CO2.
Recombinant cytokines of human GM-CSF, IL-4, TNF-α,
IL-2 were purchased from PeproTech (Rocky Hill, NJ, USA). The
HLA-A2-restricted and MUC4-specific CTL epitope peptides
(LLGVGTFVV) were synthesized by Shanghai Sangon Biological
Engineering Technology (Shanghai, China).
HLA-A2+ volunteers
Three healthy HLA-A2+ donors (male,
average age 24–30 years) from Jiangsu Provincial Blood Center
(Nanjing, China) were screened from 45 volunteers, and the
verification of HLA-A2 subtype was performed as previously
described (16,20). Samples were collected after
obtaining patient informed consent and the present study was
approved by the Ethics Committees of The First Affiliated Hospital
of Nanjing Medical University (reference no: 2011-SRFA-100).
Induction of human HLA-A2-restricted and
MUC4-specific CTL
Peripheral blood mononuclear cells (PBMCs) were
isolated from buffy coats with Ficoll-Hypaque (TBD Science,
Tianjin, China), as previously described (16). CD8+ T lymphocytes in the
PBMCs separated from the blood samples were isolated by CD8
Dynabeads (Daynal Biotech, USA), according to the manufacturer’s
instructions. The isolated CD8+ T cells were analyzed by
flow cytometry with anti-CD8-FITC antibodies (BD Pharmingen, San
Jose, CA, USA).
DCs were induced by IL-4 and GM-CSF cocktail as
previously described (16). On day
5, 1,000 U/ml of tumor necrosis factor-α (TNF-α) was added to
induce final maturation. After 7 days of culture, DCs were
harvested and were confirmed with mature DC-specific phenotype by
FACS. Matured DCs (1×105 cells/well) were pulsed with 20
μg/ml epitope peptide (LLGVGTFVV) for 2 h, washed, and irradiated
by Co60 (2 Gy/min for 15 min). The irradiated, peptide-loaded DCs
were mixed with 1×106 cells/well CD8+ T cells
at a final DC: CD8+ T cell ratio of 1:10. The
co-cultures were seeded in U-bottom 96-well plates and incubated
for 3 days at 37°C, 5% CO2. IL-2 (10 U/ml) was added on
day 4 and the same amount of loaded DCs was added on day 7 and day
10. On day 14, cells were harvested as MS-CTLs and used for
subsequent MS-CTL/tumor cell interaction experiments.
Selection of clonal MUC4-knockdown HPAC
sublines with different MUC4 expression
Construction of clonal MUC4-knockdown
HPAC sublines
The target DNA sequences corresponding to MUC4-shRNA
were previously reported (21,22).
The selected sequences are present in all splice variants of MUC4
characterized thus far, as follows: forward oligo:
5′-ccggAACGCAAGCATCGG ACTTCACctcgagGTGAAGTCCGATGCTTGCGTTtttttg-3′;
reverse oligo: 5′-aattcaaaaaAACGCAAGCATCGGACTTC
ACctcgagGTGAAGTCCGATGCTTGCGTT-3′, with AgeI and EcoRI
restriction enzyme sites (5′ and 3′ ends, respectively). The target
DNA was ligated to the pLKO.1-TRC cloning vector (23) (Addgene ID #10879), which was
supplied by PlusGene Center of Nanjing Medical University (Nanjing,
China) and verified by sequencing. The verified recombinant vector
plasmid (pLKO.1/MUC4-shRNA), the packaging plasmid pΔ8.2
and pVSV-G were co-transfected into 293T cells by Lipofectamine™
2000 reagent (Invitrogen, Carlsbad, CA, USA). The supernatant of
the cultured 293T cells was collected to infect the HPAC cells. The
pLKO.1-scramble shRNA vector (Addgene ID #1864; negative control
vector containing scrambled shRNA insert) was used to package virus
and infect HPACs as control. Stable clones were then selected in a
medium containing puromycin (5 μg/ml; Sigma). MUC4 expression in
the derived sublines was confirmed via real-time RT-PCR, western
blot analysis and flow cytometry.
Identification of clonal MUC4-knockdown
HPAC sublines
Quantitative real-time PCR
Total RNA was extracted from the selected
MUC4-knockdown HPAC sublines with TRIzol reagent (Invitrogen). The
reverse transcription using iScript cDNA synthesis kit (Bio-Rad
Laboratories, Hercules, CA, USA) and quantitative real-time PCR
using TaqMan Gene Expression assays (Applied Biosystems, Foster
City, CA, USA) were performed as previously described (9). Each quantification PCR was performed
in triplicate. The mRNA concentration was defined as the ratio of
target mRNA copies relative to GAPDH mRNA copies.
Western blot analysis
The HPAC-derived clones were processed for protein
extraction and western blotting using standard procedures. Cell
lysates were prepared as previously described (24). Protein concentrations were
determined by using the Bradford assay and proteins (20 μg/lane)
were resolved on 4–20% Mini-Protean® TGX™ precast gels
(#456–1093; Bio-Rad Laboratories). Resolved proteins were
transferred onto the polyvinylidene difluoride (PVDF) membrane and
blocked in 5% non-fat milk in PBS for 2 h and subjected to the
standard immunodetection procedure using specific antibodies. The
following primary antibodies were used for labeling of the
membranes: 1 μg/ml anti-MUC4 mouse monoclonal antibody (ab60720;
Abcam, Cambridge, UK) for MUC4 immunodetection; 1:1,000-diluted
anti-GAPDH mouse monoclonal antibody (AG019; Beyotime Institute of
Biotechnology, Haimen, China) for GAPDH immunodetection. The
membranes were incubated at 4°C overnight followed by six 10-min
washes in TBST [50 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl, and
0.05% Tween-20]. Membranes were then incubated with 1:2,000-diluted
horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (A0216;
Beyotime) for 1 h at room temperature followed by six 10-min washes
with TBST. The blots were developed with an enhanced
chemiluminescence kit (Amersham Biosciences, Freiburg, Germany).
PageRuler™ Plus Prestained Protein Ladder (#26619/SM1811, 10–250
kD; Fermentas) were used as internal molecular weight standards.
Each blot was repeated three times. The value of optical density
(OD) was measured by the software (Quantity One v462; Bio-Rad
Laboratories) and the relative protein expression levels of MUC4
were calculated with the following ratio: (OD value of MUC4 - OD
value of background) / (OD value of GAPDH - OD value of
background).
FACS analysis of surface expression of
MUC4
Cells (1×106) were suspended in 100 μl of
PBS containing 1% BSA and incubated on ice for 30 min with 5 μg/ml
monoclonal anti-MUC4 (clone 1G8) antibodies (mouse IgG1;
Invitrogen). After washing twice with 2 ml of cold PBS, the cells
were incubated on ice for 30 min with 1:1,000 diluted fluorescein
isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody
(eBiosciences), washed twice with PBS, and fixed in 1%
paraformaldehyde and, finally, 10,000 gated events were analyzed
using a FACSCalibur flow cytometer (BD Biosciences). Each
measurement was repeated three times. Geometric mean fluorescence
intensity (GMFI) values of equal quantity cells only labeled
primary antibodies were calculated as background values. GMFI
values of equal quantity cells only labeled second antibodies were
calculated as negative control (NC). Fluorescence index (FI) was
calculated with the following formula: FI = (GMFI sample - GMFI
background) / GMFI background.
MS-CTL/tumor-cell co-incubation
experiments
Fixed basic co-incubation condition
and standardizing measurements
Co-incubated system with basic condition and fixed
parameter: MS-CTLs suspended in 10% FCS RPMI-1640 medium were
seeded into a round-bottomed 96-well plate at 5×105
cells/100 μl/well in triplicate. The prepared target cells were
added into the corresponding wells at 1×105 cells/100
μl/well to make the effector/target ratios equal 5:1, respectively.
At the same time 10% FCS RPMI-1640 medium without target cells was
added into MS-CTLs suspended at a final volume of 200 μl/well as
background control. The plates were spinned at 1,000 rpm for 10 min
and then incubated for 4 h at 37°C, 5% CO2, the same as
the standard procedures of 51Cr release assay
(cytotoxicity assays) (25). After
incubation, the plates were spinned again at 1,000 rpm for 20 min;
100 μl supernatant were harvested for subsequent tests and 100 μl
co-incubated cells were harvested for the following apoptosis
measurements of CTLs.
FACS-apoptosis measurements of CTLs from the
co-incubated system: the co-incubated cells at a volume of 100 μl
were harvested into tube and labeled with 5 μl of anti-CD8-APC
antibodies (Invitrogen) for 20 min at room temperature, protected
from light. Then, cells were resuspended in buffer and were stained
with an Annexin V-FITC kit (Invitrogen Systems). Cell suspensions
were evaluated using a FACSCalibur flow cytometer (BD Biosciences)
within 1 h and the results were analyzed using CellQuest version
3.1 software (BD Biosciences). At least 10,000 events per sample
were collected with debris and aggregates excluded using low
forward and orthogonal light scatter values. Compensations were
established using single color controls. The gates for CD8 positive
cells were set to calculate CTL (CD8+) apoptosis. Cells
that stained positively with Annexin V were considered to be
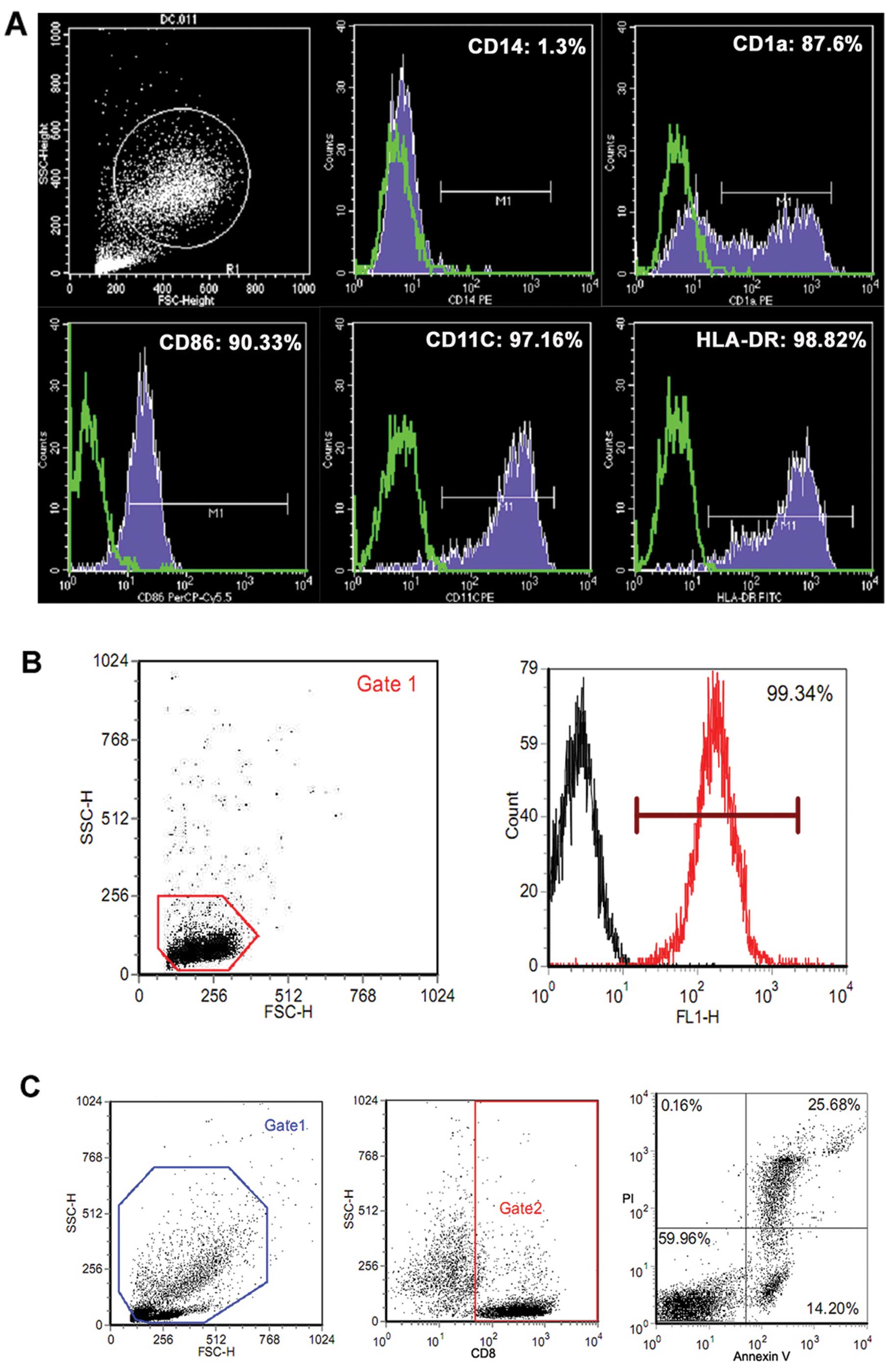
apoptotic. Fig. 1C shows that CTLs
were sorted out easily from co-incubated cells by CD8-APC positive
gate and were calculated accurately.
Co-incubation experiments
A series of experiments was carried out based on
basic condition (see above) as follows: i) the apoptosis rates of
MS-CTLs incubated with MUC4+ tumor cells (HCT 116,
CFPAC-1 and HPAC) and pulsed-peptide T2 cells were compared to
confirm the previous observations; ii) clonal MUC4-knockdown HPAC
sublines with different MUC4 expression were selected as target
cells and further MS-CTL apoptosis was examined; iii) the harvested
supernatant of distinct groups, the same as ii), were used to
incubate MS-CTLs for 4 h and following apoptosis measurement for
the analysis of the influence of supernatant; iv) effect of
blockade of Fas on MS-CTL surface. We hypothesized that the
Fas-FasL pathway might contribute to MS-CTL apoptosis as one of the
effect factors. Before co-incubating with target cells, also the
same as ii), MS-CTLs were cultured for 1 h at 37°C in the presence
of antagonistic mouse anti-human Fas, ZB4 (Beckman Coulter, Brea,
CA, USA; 2 μg/ml). The effective blocking dose was defined
according to lymphocyte counting using tetrazolium bromide (MTT).
After incubation, cells were collected for the detection of
apoptosis.
Statistical analysis
Results are expressed as means ± standard error
(SEM). Data were analyzed using one-way analysis of variance
between groups (ANOVA). Correlations between CTL apoptosis rates
and determined factors were analyzed using linear regression
analysis. The variations in blocking effect of Fas on CTL surface
were analyzed by 2-way ANOVA. Statistical analysis was performed
with SPSS 17.0 software. Differences were considered statistically
significant at P<0.05.
Results
Generation of mature DCs and MS-CTLs
After 7 days of culture in medium containing GM-CSF,
IL-4 and TNF-α, FACS analysis showed that DCs from blood samples of
three HLA-A*0201 volunteers displayed typical mature phenotypic
characteristics. The frequency of expression of the phenotypic
markers CD14, CD86, CD1a, CD11c and HLA-DR was 1.32±0.12,
89.13±3.76, 90.24±8.63, 97.32±1.23 and 96.55±2.59%, respectively
(one representative FACS histogram is depicted in Fig. 1A). CD8+ T cells (purities
>99%, as shown in Fig. 1B) were
induced into human HLA-A2-restricted and MUC4-specific CTLs by
co-culture with epitope peptide-loaded DCs. The specific cytotoxic
activity of MS-CTLs is consistent with our previous reports
(16) (data not shown).
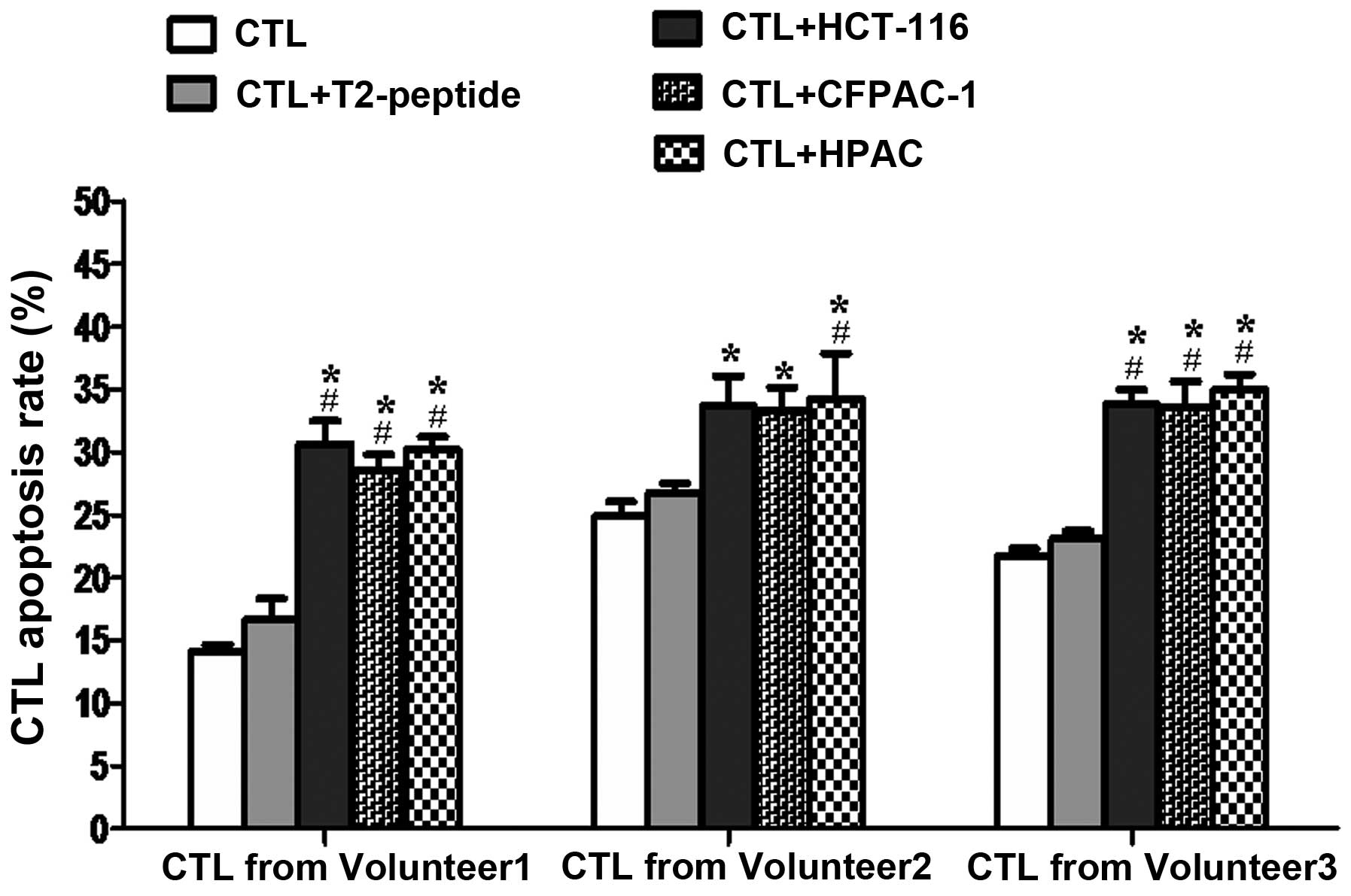
The apoptosis rate of MS-CTLs increases
significantly when co-cultured with MUC4+ tumor cells
compared to the background control
Induction of MS-CTLs using blood sample from
volunteer 1; compared to the background control group (CTLs without
target cells added) or T2 cell group, the apoptosis rate of MS-CTLs
in MUC4+ tumor cell (HCT 116, CFPAC-1 and HPAC) groups
increased significantly, P<0.05, respectively (Fig. 2). Using blood samples from
volunteers 2 and 3, the changing trend of MS-CTL apoptosis rate was
similar, although the changing extent had a slight difference
(Fig. 2).
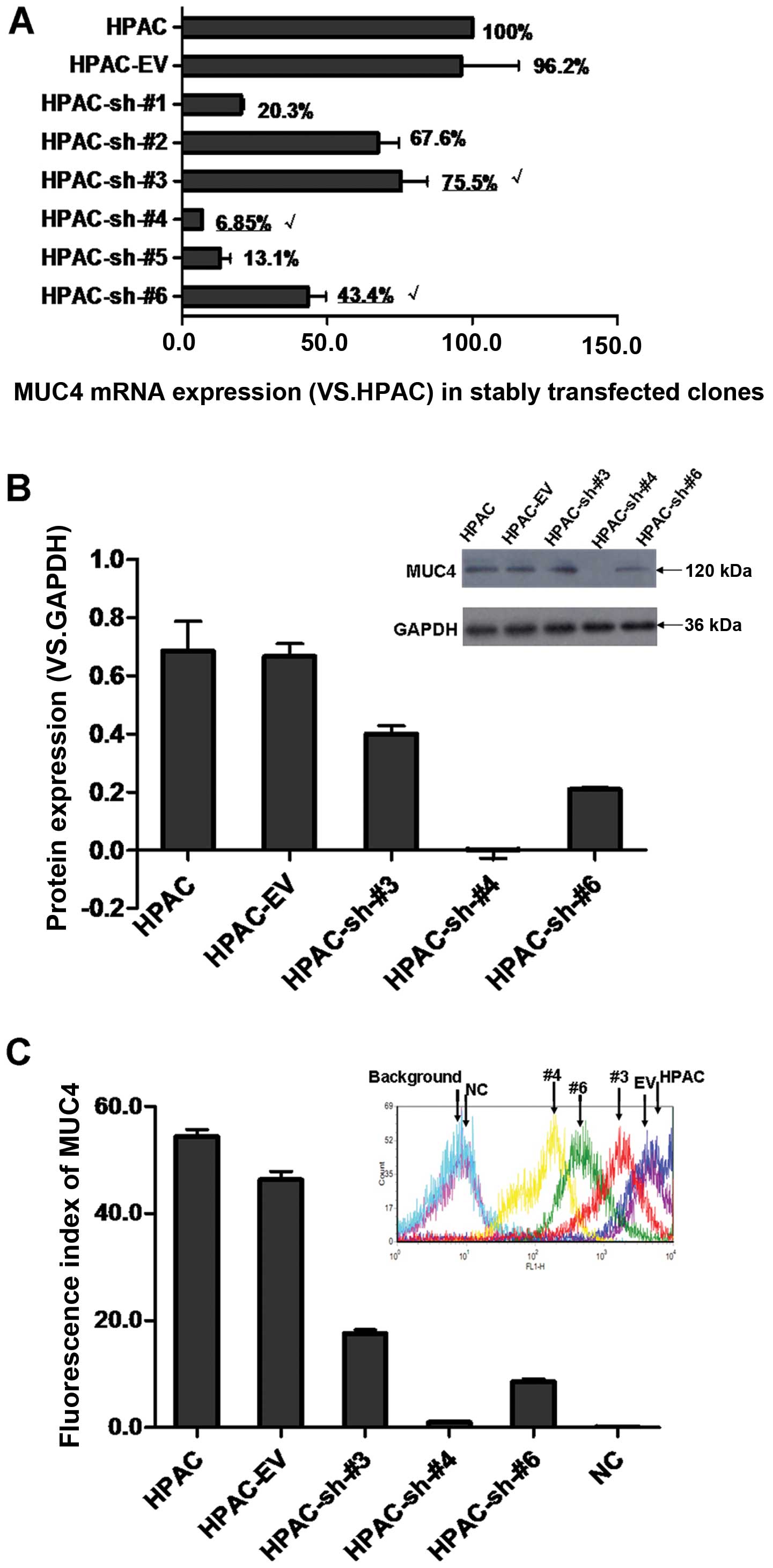
Selection of MUC4-shRNA HPAC sublines
with different MUC4 expression
Seven stably transfected clones were identified via
real-time RT-PCR. The expression level of MUC4 normalized to that
of GAPDH is shown in Fig. 3A.
Compared to HPAC (100%), the MUC4 expression levels were
96.23±19.76, 20.30±1.05, 67.59±7.16, 75.49±9.17, 6.85±0.17,
13.07±3.62 and 43.35±3.15% in the stably transfected empty vector
clone of HPAC (HPAC-EV), six stably transfected MUC4-shRNA clones
of HPAC, i.e. HPAC-sh-#1–6, respectively.
Three clones, i.e. HPAC-sh-#3, HPAC-sh-#4 and
HPAC-sh-#6, with a wide range of different MUC4 expression levels
were selected for subsequent confirmation via western blot analysis
and flow cytometry. Fig. 3B shows
that the relative protein expression levels of MUC4 (vs. GAPDH)
were 0.68±0.10, 0.66±0.04, 0.40±0.03, −0.0008±0.03 and 0.21±0.01 in
HPAC, HPAC-EV, HPAC-sh-#3, HPAC-sh-#4 and HPAC-sh-#6,
respectively.
Cell membrane surface expression of MUC4 was
determined by FACS analysis. Fig.
3C shows that the fluorescence index (FI) on behalf of relative
surface expression levels of MUC4 was 54.40±1.32, 46.32±1.53,
17.56±0.69, 0.97±0.09, 8.61±0.51 and 0.10±0.01 in HPAC, HPAC-EV,
HPAC-sh-#3, HPAC-sh-#4, HPAC-sh-#6 and NC (negative control),
respectively. This is consistent with the results of the western
blot analysis. These results demonstrated higher, medium and lower
reduction of MUC4 expression in HPAC-sh-#4, HPAC-sh-#6 and
HPAC-sh-#3, and were therefore termed HPAC-sh-Higher (H-sh-H),
HPAC-sh-Medium (H-sh-M), HPAC-sh-Lower (H-sh-L), respectively.
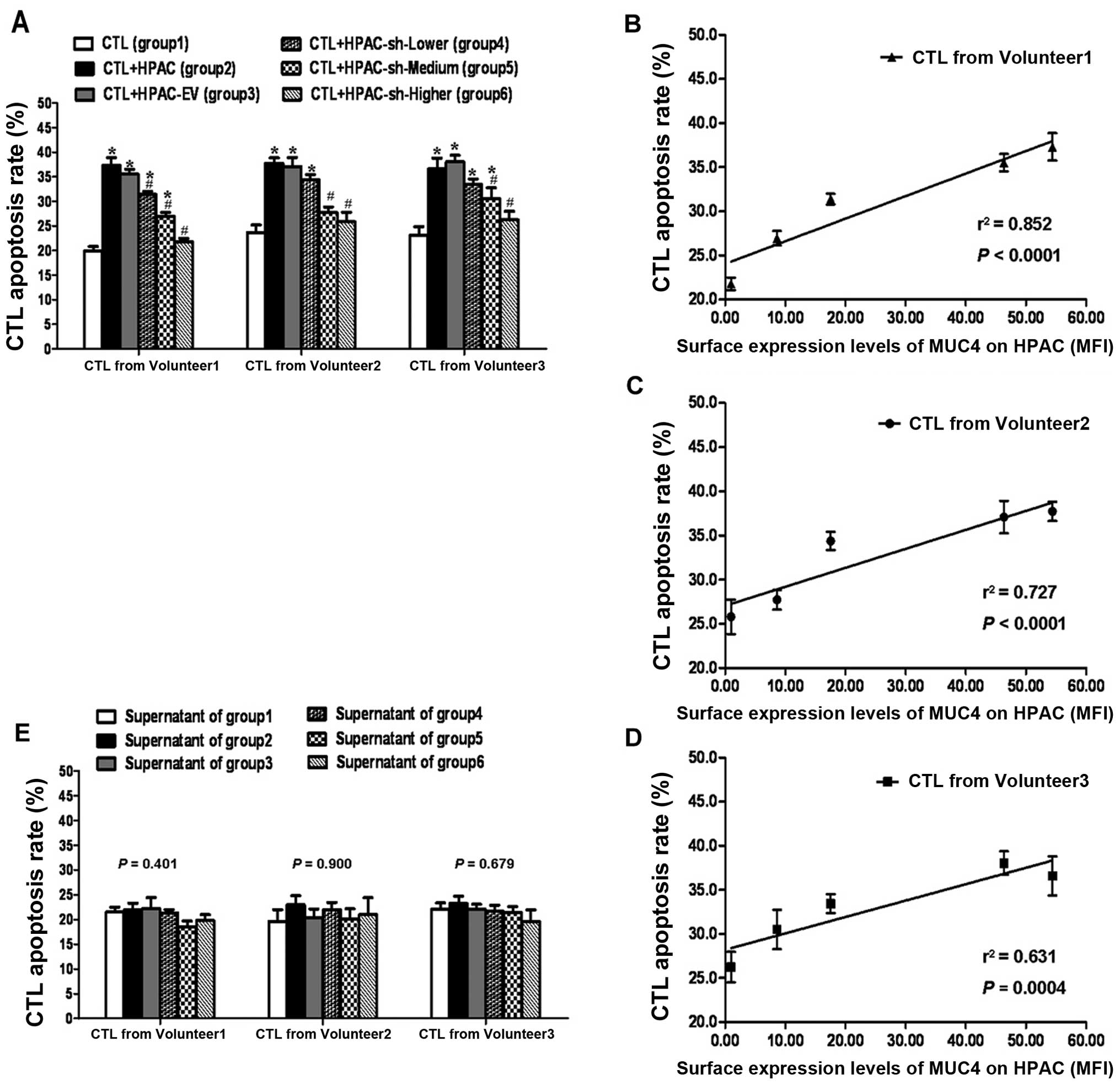
MUC4 expression on HPAC surface
contributes to MS-CTL apoptosis, while the influence of
supernatants does not significantly correlate with MS-CTL
apoptosis
Induction of MS-CTLs using blood sample from
volunteer 1: compared to background control (group 1), the
apoptosis rate of MS-CTLs in HPAC group (group 2), HPAC-EV group
(group 3), H-sh-L group (group 4), and H-sh-M (group 5) increased
significantly, P<0.05, respectively (Fig. 4A). Compared to group 2, MS-CTL
apoptosis rate in groups 4, 5 and H-sh-H (group 6) decreased
significantly, P<0.05, respectively (Fig. 4A). Meanwhile, as shown in Fig. 4B, a strong positive correlation
(r2=0.852, P<0.0001) was observed between CTL
apoptosis rate and the surface expression levels of MUC4 on HPAC
sublines indicated by mean fluorescence index (MFI). Using blood
samples from volunteers 2 and 3, the variation of data presented a
similar general trend, although the changing extent was different
(Fig. 4B and D). Therefore, these
results demonstrated that surface expression of MUC4 on HPACs
contributed to MS-CTL apoptosis.
After MS-CTLs were re-cultured using the
supernatants of groups 1–6 with fixed basic condition, one-way
ANOVA demonstrated MS-CTL apoptosis had no statistically
significant differences (P>0.05) among distinct groups (Fig. 4E). The result showed that the total
effects of supernatants which might hold various soluble factors
were not clearly produced on MS-CTL apoptosis.
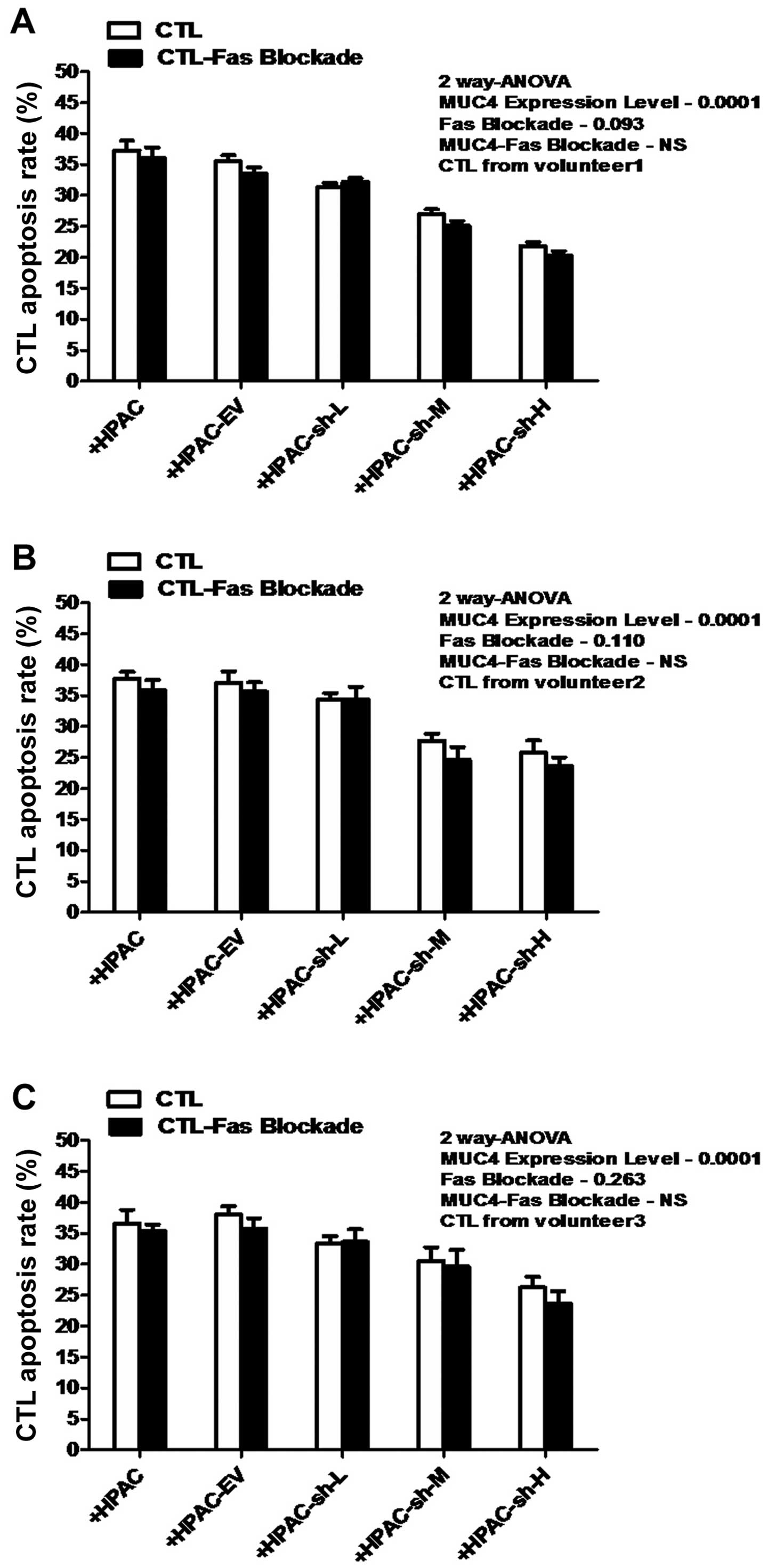
Fas-blockade has no significant effect on
MUC4-mediated MS-CTL apoptosis
Although high levels of MUC4 expression on HPAC
surface were verified to have an effect on the significant increase
of apoptosis of MS-CTLs during tumor cell-CTL interaction, whether
the classical Fas-FasL pathway is actively involved in this process
or not remains to be determined. As shown in Fig. 5, two-way ANOVA indicated that
Fas-blockade/non had no statistically significant effect on MS-CTL
apoptosis (P>0.05). At the same time, the effect of interaction
of the factors (Fas-blockade and MUC4-expression) was
non-significant. These results indicated that the Fas-FasL pathway
did not participate in the process of MUC4-mediated MS-CTL
apoptosis.
Collectively, the results suggested that during the
cytolytic process, MUC4-bearing tumor cells could counterattack
MS-CTLs via cell surface MUC4-mediated MS-CTL apoptosis, indicating
a novel Fas-independent counterattack pathway, although the
underlying mechanism may be diverse.
Discussion
As a class of molecules with significant roles in
cell-cell communication, tumor-associated mucins, including human
mucin4 (MUC4), might affect the immune response in several ways:
they might provide an impenetrable barrier for immune effector
cells, preventing an antitumor response; they might inactivate
immune effector cells through receptor-ligand interactions; or they
might sequester cytokines or other compounds that suppress a tumor
immune response (19).
Although the exact mechanisms of immune regulation
by MUC4 are not yet clear, our observations support the hypothesis
that MUC4 may be employed by MUC4-bearing cancer cells to
counterattack immune cells for their enhanced survival. Our data
clearly indicate that the apoptosis of MS-CTLs can be mediated by
membrane-associated MUC4 on the surface of pancreatic cancer cells
during the cytolytic process. However, our data showed that
supernatants (which could contain various soluble factors generated
by the interaction of cancer cells and CTLs) have non-significant
effects on the apoptosis of MS-CTLs. Moreover, in light of previous
reports, three soluble factors, TNF-α (CD120a,b) (26,27),
TNF-related apoptosis-inducing ligand (TRAIL) (28,29)
and secreted forms of MUC4 (sMUC4) (30), could be involved in the apoptosis
induction and function inhibition of activated T cells. We also
quantitatively determined the levels of these three soluble
apoptosis-related factors in supernatants with standard ELISA kits.
The concentrations of three factors appeared to be in the picomolar
or nanomolar ranges, respectively, but there was no significant
difference in TNF-α, TRAIL or sMUC4 levels among distinct groups
and MS-CTL apoptosis did not correlate with these soluble factors
(unpublished data). Thus, these two aspects clearly show that
MUC4-mediated apoptosis of MS-CTLs is contact-dependent of effector
cell with target cell.
Currently, it is considered that the Fas-FasL
pathway can result in T-cell suicide and fratricide, known as
activation-induced cell death (AICD) (31–33).
In the present study, a small degree of apoptosis (~20%) of
background control (CTLs without target cells added) was observed,
which could occur related with that it took two weeks to induce
MUC4 specific CTLs from peripheral blood in vitro and
cell-culture medium containing IL-2 might induce the FasL
expression on CTL surface and contribute to the AICD effect. FasL
expression on tumor cells is considered the ‘counterattack’ of
tumor to Fas-expressing effector T lymphocytes, which leads to the
occurrence of immune evasion (33–35).
However, our data demonstrated that blockade of Fas receptor by
pretreatment with the antagonistic Fas antibody (ZB4) did not
significantly inhibit the occurrence of MS-CTL apoptosis during the
control experiments and the parallel processing systems, suggesting
that mainly the cell death stimulus is initiated through a
Fas-independent pathway; it might be possible that the interaction
of MUC4 (functionally characterized domains) with other receptors
on T cells results in a series of apoptosis signal
transductions.
Additionally, we utilized CTL self control group or
CTL added with wild tumor cell group to rule out the possible
effect of intrinsic activated cell-autonomous death (ACAD)
(32) and analogous
antigen-dependent apoptosis of CTL (ADAC) (36) in our study system, which gave
indirect evidence to the localization of extrinsic MUC4-mediated
apoptosis of MS-CTLs. Generally, the pathway of the T-cell
apoptosis is considered the intrinsic and extrinsic apoptotic
pathway, meanwhile, the absence of appropriate survival signals can
cause ACAD (32). On the other
hand, ADAC appears only when there is both excessive antigen
density and a sufficiently high avidity in the TCR-MHC/peptide
interaction (36). However, in the
present study, MS-CTL apoptosis did not agree with ACAD and ADAC,
since the induction process of MS-CTL is fixed and standard by
using appropriate amounts of MUC4 epitope peptide. Moreover, the
presence of extrinsic MUC4-mediated apoptosis of MS-CTLs can be
verified by implementing the appropriate controls.
In addition, regulatory T cells (Tregs,
CD4+ CD25+) are considered to suppress
effector T-cell activation, proliferation, and cytokine production
(37,38). In view of the fact that Treg cells
are capable of killing autologous CD8+ T cells,
CD8+ T lymphocytes were isolated from the PBMCs before
the induction and amplification of MS-CTLs in order to eliminate
the effect of Tregs in this study.
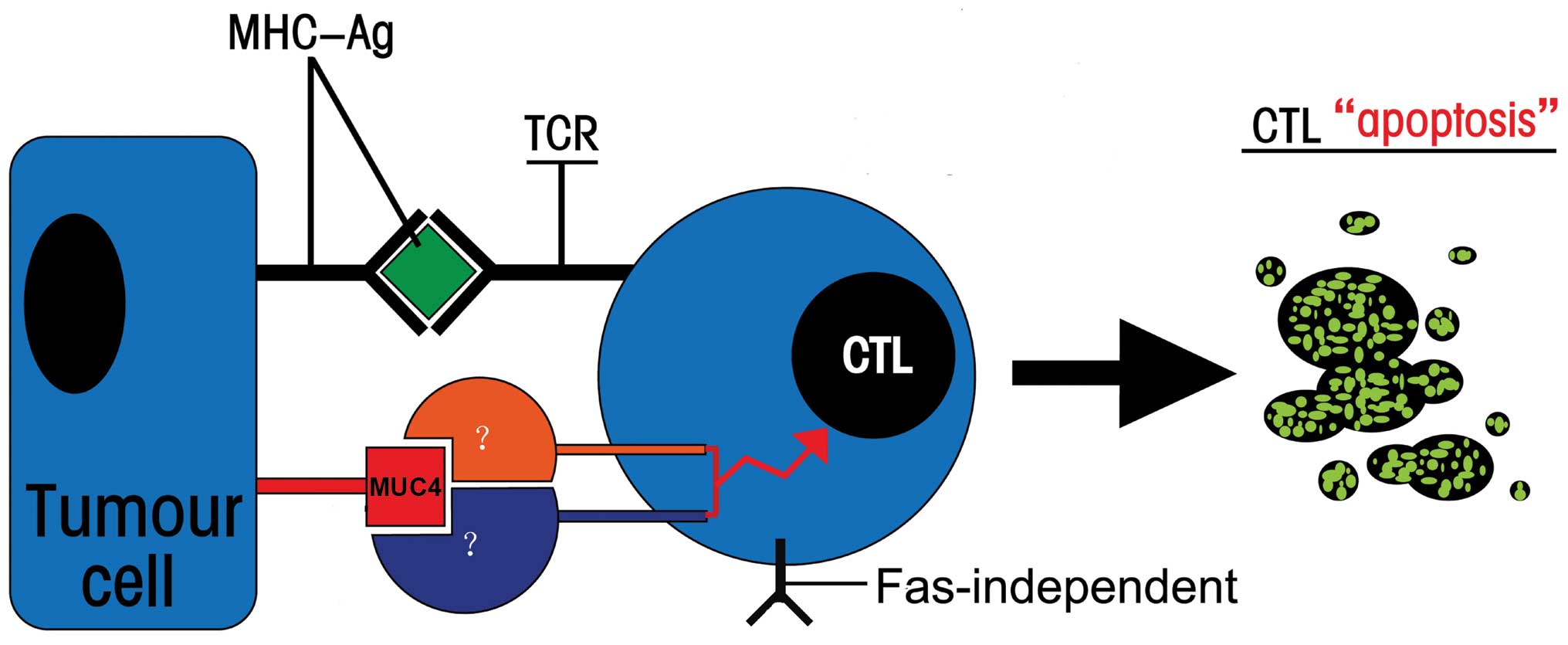
As shown in Fig. 6,
the present study may provide a first step towards the elucidation
of this mechanism in the apoptosis of MUC4-specific CTLs,
suggesting that high levels of MUC4 molecules on cancer cell
surface as a ligand might induce apoptosis of MS-CTLs by binding to
undiscovered receptor on T cells to initiate a signal transduction
cascade of apoptosis. Muc4/MUC4 was confirmed to be a
ligand/modulator for the receptor tyrosine kinase ErbB2, regulating
its phosphorylation and the phosphorylation of its partner ErbB3,
with or without the involvement of the ErbB3 ligand neuregulin,
which induces signaling related to growth, motility, or
differentiation properties of the cell (39–41).
It has been speculated that the EGF-like domain of MUC4 specific
domains plays a role in the above receptor-ligand interactions
(42,43). Meanwhile, the other unique domains
present in the MUC4 mucin but not found in other membrane-bound
mucins are NIDO, AMOP, and vWD domains (44). Although there is no direct evidence
in the literature yet, the homology and evolution analysis hints at
a role for both NIDO and AMOP domain in cell-cell interaction and
adhesion to the extracellular matrix (45–48).
Whether MUC4 itself can establish novel interactions with receptor
protein on the surface of CTLs via functionally characterized
domain is yet to be confirmed. Further studies on the signaling
pathways downstream of CTL apoptosis are presently being carried
out by means of construction of functionally primed human
CD8+ MUC4-specific CTL clones model system, target
molecule screening, domain deletion mutant, function-blocking and
other techniques in our laboratory.
In summary, several lines of evidence suggest that
there may be a novel counterattack pathway of pancreatic cancer
cells, which is a MUC4-mediated, cell contact-dependent and
Fas-independent process, to induce CTL apoptosis and attenuate the
antitumor effect of CTLs (Fig.
6).
MUC4 is aberrantly expressed in a variety of human
epithelial malignancies, particularly in pancreatic cancer
(48), hence, the development and
implications of anti-MUC4-specific immunotherapeutic strategies are
important. Therefore, further exploration and understanding of the
potential counterattack mechanisms and the intention to develop
strategies to prevent or bypass apoptosis of CTLs is highly
advantageous in enhancing the efficacy of MUC4-specific tumor
vaccines.
Acknowledgements
The present study was partially supported by the
National Natural Science Foundation of China (81101802, 81001079),
the Natural Science Foundation of Jiangsu Province (BK2011845), the
Program for Development of Innovative Research Team in the First
Affiliated Hospital of NJMU, the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD,
JX10231801), and the Research Special Fund for Public Welfare
Industry of Health (201202007).
References
|
1
|
Wong HH and Lemoine NR: Biological
approaches to therapy of pancreatic cancer. Pancreatology.
8:431–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Merl MY, Chabot J and Saif MW:
Updates of adjuvant therapy in pancreatic cancer: where are we and
where are we going? In: Highlights from the ‘2010 ASCO Annual
Meeting’; Chicago, IL, USA. June 4–8, 2010; JOP. 11. pp. 310–312.
2010, PubMed/NCBI
|
|
3
|
Porchet N, Nguyen VC, Dufosse J, et al:
Molecular cloning and chromosomal localization of a novel human
tracheo-bronchial mucin cDNA containing tandemly repeated sequences
of 48 base pairs. Biochem Biophys Res Commun. 175:414–422. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moniaux N, Nollet S, Porchet N, Degand P,
Laine A and Aubert JP: Complete sequence of the human mucin MUC4: a
putative cell membrane-associated mucin. Biochem J. 338:325–333.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rachagani S, Torres MP, Moniaux N and
Batra SK: Current status of mucins in the diagnosis and therapy of
cancer. Biofactors. 35:509–527. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torres MP, Chakraborty S, Souchek J and
Batra SK: Mucin-based targeted pancreatic cancer therapy. Curr
Pharm Des. 18:2472–2481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andrianifahanana M, Moniaux N, Schmied BM,
et al: Mucin (MUC) gene expression in human pancreatic
adenocarcinoma and chronic pancreatitis: a potential role of MUC4
as a tumor marker of diagnostic significance. Clin Cancer Res.
7:4033–4040. 2001.
|
|
8
|
Jhala N, Jhala D, Vickers SM, et al:
Biomarkers in diagnosis of pancreatic carcinoma in fine-needle
aspirates. Am J Clin Pathol. 126:572–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Zhang JJ, Zhu R, et al: The
increase in the expression and hypomethylation of MUC4 gene with
the progression of pancreatic ductal adenocarcinoma. Med Oncol.
28(Suppl 1): S175–S184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saitou M, Goto M, Horinouchi M, et al:
MUC4 expression is a novel prognostic factor in patients with
invasive ductal carcinoma of the pancreas. J Clin Pathol.
58:845–852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bafna S, Kaur S, Momi N and Batra SK:
Pancreatic cancer cells resistance to gemcitabine: the role of MUC4
mucin. Br J Cancer. 101:1155–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaturvedi P, Singh AP, Moniaux N, et al:
MUC4 mucin potentiates pancreatic tumor cell proliferation,
survival, and invasive properties and interferes with its
interaction to extracellular matrix proteins. Mol Cancer Res.
5:309–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh AP, Moniaux N, Chauhan SC, Meza JL
and Batra SK: Inhibition of MUC4 expression suppresses pancreatic
tumor cell growth and metastasis. Cancer Res. 64:622–630. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rachagani S, Macha MA, Ponnusamy MP, et
al: MUC4 potentiates invasion and metastasis of pancreatic cancer
cells through stabilization of fibroblast growth factor receptor 1.
Carcinogenesis. 33:1953–1964. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei J, Gao W, Wu J, et al: Dendritic cells
expressing a combined PADRE/MUC4-derived polyepitope DNA vaccine
induce multiple cytotoxic T-cell responses. Cancer Biother
Radiopharm. 23:121–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Wei J, Meng K, et al: Identification
of an HLA-A*0201-restrictive CTL epitope from MUC4 for applicable
vaccine therapy. Immunopharmacol Immunotoxicol. 31:468–476.
2009.
|
|
17
|
Gao WT, Zhang JJ, Zhu Y, et al: Pentamer
guided HLA-restricted epitope identification for mucoprotein 4
antigen of pancreatic ductal adenocarcinoma. Zhonghua Wai Ke Za
Zhi. 48:1416–1424. 2010.(In Chinese).
|
|
18
|
Singh AP, Chaturvedi P and Batra SK:
Emerging roles of MUC4 in cancer: a novel target for diagnosis and
therapy. Cancer Res. 67:433–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin Qin P, Su F, Xiao Yan W, et al:
Distribution of human leucocyte antigen-A, -B and -DR alleles and
haplotypes at high resolution in the population from Jiangsu
province of China. Int J Immunogenet. 38:475–481. 2011.PubMed/NCBI
|
|
21
|
Nagy P, Friedlander E, Tanner M, et al:
Decreased accessibility and lack of activation of ErbB2 in JIMT-1,
a herceptin-resistant, MUC4-expressing breast cancer cell line.
Cancer Res. 65:473–482. 2005.PubMed/NCBI
|
|
22
|
Workman HC, Sweeney C and Carraway KL III:
The membrane mucin Muc4 inhibits apoptosis induced by multiple
insults via ErbB2-dependent and ErbB2-independent mechanisms.
Cancer Res. 69:2845–2852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moffat J, Grueneberg DA, Yang X, et al: A
lentiviral RNAi library for human and mouse genes applied to an
arrayed viral high-content screen. Cell. 124:1283–1298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma C, Zhang J, Durrin LK, et al: The BCL2
major breakpoint region (mbr) regulates gene expression. Oncogene.
26:2649–2657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim GG, Donnenberg VS, Donnenberg AD,
Gooding W and Whiteside TL: A novel multiparametric flow
cytometry-based cytotoxicity assay simultaneously immunophenotypes
effector cells: comparisons to a 4 h 51Cr-release assay.
J Immunol Methods. 325:51–66. 2007. View Article : Google Scholar
|
|
26
|
Sedgwick JD, Riminton DS, Cyster JG and
Korner H: Tumor necrosis factor: a master-regulator of leukocyte
movement. Immunol Today. 21:110–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moss ML, Jin SL, Milla ME, et al: Cloning
of a disintegrin metalloproteinase that processes precursor
tumour-necrosis factor-α. Nature. 385:733–736. 1997.PubMed/NCBI
|
|
28
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Komatsu M, Yee L and Carraway KL:
Overexpression of sialomucin complex, a rat homologue of MUC4,
inhibits tumor killing by lymphokine-activated killer cells. Cancer
Res. 59:2229–2236. 1999.PubMed/NCBI
|
|
31
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krammer PH, Arnold R and Lavrik IN: Life
and death in peripheral T cells. Nat Rev Immunol. 7:532–542. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maher S, Toomey D, Condron C and
Bouchier-Hayes D: Activation-induced cell death: the controversial
role of Fas and Fas ligand in immune privilege and tumour
counterattack. Immunol Cell Biol. 80:131–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rivoltini L, Carrabba M, Huber V, et al:
Immunity to cancer: attack and escape in T lymphocyte-tumor cell
interaction. Immunol Rev. 188:97–113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Igney FH, Behrens CK and Krammer PH: CD95L
mediates tumor counterattack in vitro but induces
neutrophil-independent tumor rejection in vivo. Int J
Cancer. 113:78–87. 2005.PubMed/NCBI
|
|
36
|
Derby MA, Snyder JT, Tse R,
Alexander-Miller MA and Berzofsky JA: An abrupt and concordant
initiation of apoptosis: antigen-dependent death of CD8+
CTL. Eur J Immunol. 31:2951–2959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khazaie K and von Boehmer H: The impact of
CD4+CD25+ Treg on tumor specific
CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol.
16:124–136. 2006.
|
|
38
|
Miyara M and Sakaguchi S: Natural
regulatory T cells: mechanisms of suppression. Trends Mol Med.
13:108–116. 2007. View Article : Google Scholar
|
|
39
|
Carraway KL, Perez A, Idris N, et al:
Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer
and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol
Biol. 71:149–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jepson S, Komatsu M, Haq B, et al:
Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces
specific phosphorylation of ErbB2 and enhances expression of
p27kip, but does not activate mitogen-activated kinase
or protein kinaseB/Akt pathways. Oncogene. 21:7524–7532. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chaturvedi P, Singh AP, Chakraborty S, et
al: MUC4 mucin interacts with and stabilizes the HER2 oncoprotein
in human pancreatic cancer cells. Cancer Res. 68:2065–2070. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carraway KL, Ramsauer VP, Haq B and
Carothers Carraway CA: Cell signaling through membrane mucins.
Bioessays. 25:66–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carraway CA and Carraway KL: Sequestration
and segregation of receptor kinases in epithelial cells:
implications for ErbB2 oncogenesis. Sci STKE.
2007.re32007.PubMed/NCBI
|
|
44
|
Desseyn JL, Tetaert D and Gouyer V:
Architecture of the large membrane-bound mucins. Gene. 410:215–222.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Timpl R: Structure and biological activity
of basement membrane proteins. Eur J Biochem. 180:487–502. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mayer U, Kohfeldt E and Timpl R:
Structural and genetic analysis of laminin-nidogen interaction. Ann
NY Acad Sci. 857:130–142. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ciccarelli FD, Doerks T and Bork P: AMOP,
a protein module alternatively spliced in cancer cells. Trends
Biochem Sci. 27:113–115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chaturvedi P, Singh AP and Batra SK:
Structure, evolution, and biology of the MUC4 mucin. FASEB J.
22:966–981. 2008. View Article : Google Scholar : PubMed/NCBI
|